Abstract
Mitochondria are dynamic organelles that continually respond to cellular stress. Recent studies have demonstrated that mitochondrial stress is relayed from mitochondria to the cytosol by the release of a proteolytic fragment of DELE1 that binds to the eIF2α kinase HRI to initiate integrated stress response (ISR) signaling. We report the cryo-electron microscopy structure of the C-terminal cleavage product of human DELE1, which assembles into a high-order oligomer. The oligomer consists of eight DELE1 monomers that assemble with D4 symmetry via two sets of hydrophobic inter-subunit interactions. We identified the key residues involved in DELE1 oligomerization, and confirmed their role in stabilizing the octamer in vitro and in cells using mutagenesis. We further show that assembly-impaired DELE1 mutants are compromised in their ability to induce HRI-dependent ISR activation in cell culture models. Together, our findings provide molecular insights into the activity of DELE1 and how it signals to promote ISR activity following mitochondrial insult.
This is a preview of subscription content, access via your institution
Access options




Similar content being viewed by others
Data availability
The cryo-EM reconstruction and associated atomic model of DELE1CTD have been deposited to the Electron Microscopy Data Bank and PDB under accession numbers EMDB EMD-27269 and PDB 8D9X, respectively. All data needed to evaluate the conclusions in the paper are present in the paper and the supporting information. All the plasmids used in this study are available from the corresponding authors upon reasonable request. Source data are provided with this paper.
References
Palikaras, K., Lionaki, E. & Tavernarakis, N. Mechanisms of mitophagy in cellular homeostasis, physiology and pathology. Nat. Cell Biol. 20, 1013–1022 (2018).
Spinelli, J. B. & Haigis, M. C. The multifaceted contributions of mitochondria to cellular metabolism. Nat. Cell Biol. 20, 745–754 (2018).
Lee, S. S. et al. A systematic RNAi screen identifies a critical role for mitochondria in C. elegans longevity. Nat. Genet. 33, 40–48 (2003).
Moehle, E. A., Shen, K. & Dillin, A. Mitochondrial proteostasis in the context of cellular and organismal health and aging. J. Biol. Chem. 294, 5396–5407 (2019).
Pakos-Zebrucka, K. et al. The integrated stress response. EMBO Rep. 17, 1374–1395 (2016).
Guo, X. et al. Mitochondrial stress is relayed to the cytosol by an OMA1–DELE1–HRI pathway. Nature 579, 427–432 (2020).
Fessler, E. et al. A pathway coordinated by DELE1 relays mitochondrial stress to the cytosol. Nature 579, 433–437 (2020).
Ahola, S. et al. OMA1-mediated integrated stress response protects against ferroptosis in mitochondrial cardiomyopathy. Cell Metab. 34, 1875–1891 (2022).
Fessler, E., Krumwiede, L. & Jae, L. T. DELE1 tracks perturbed protein import and processing in human mitochondria. Nat. Commun. 13, 1853 (2022).
Jin, T. et al. Design of an expression system to enhance MBP-mediated crystallization. Sci. Rep. 7, 40991 (2017).
Krissinel, E. & Henrick, K. Inference of macromolecular assemblies from crystalline state. J. Mol. Biol. 372, 774–797 (2007).
Chen, T. et al. Chemical genetics identify eIF2α kinase heme-regulated inhibitor as an anticancer target. Nat. Chem. Biol. 7, 610–616 (2011).
Sekine, Y. et al. A mitochondrial iron-responsive pathway regulated by DELE1. Mol. Cell 83, 2059–2076.e6 (2023).
Suomalainen, A. & Battersby, B. J. Mitochondrial diseases: the contribution of organelle stress responses to pathology. Nat. Rev. Mol. Cell Biol. 19, 77–92 (2018).
Sun, N., Youle, R. J. & Finkel, T. The mitochondrial basis of aging. Mol. Cell 61, 654–666 (2016).
Shpilka, T. & Haynes, C. M. The mitochondrial UPR: mechanisms, physiological functions and implications in ageing. Nat. Rev. Mol. Cell Biol. 19, 109–120 (2018).
Costa-Mattioli, M. & Walter, P. The integrated stress response: from mechanism to disease. Science 368, eaat5314 (2020).
Dar, A. C., Dever, T. E. & Sicheri, F. Higher-order substrate recognition of eIF2α by the RNA-dependent protein kinase PKR. Cell 122, 887–900 (2005).
Dey, M. et al. Mechanistic link between PKR dimerization, autophosphorylation, and eIF2α substrate recognition. Cell 122, 901–913 (2005).
Zappa, F. et al. Signaling by the integrated stress response kinase PKR is fine-tuned by dynamic clustering. J. Cell Biol. 221, e202111100 (2022).
Mattson, M. P., Gleichmann, M. & Cheng, A. Mitochondria in neuroplasticity and neurological disorders. Neuron 60, 748–766 (2008).
Prisant, M. G., Williams, C. J., Chen, V. B., Richardson, J. S. & Richardson, D. C. New tools in MolProbity validation: CaBLAM for CryoEM backbone, UnDowser to rethink “waters”, and NGL Viewer to recapture online 3D graphics. Protein Sci. 29, 315–329 (2019).
Herzik, M. A. Jr. Setting up parallel illumination on the Talos Arctica for high-resolution data collection. Methods Mol. Biol. 2215, 125–144 (2021).
Herzik, M. A. Jr., Wu, M. & Lander, G. C. Achieving better-than-3-Å resolution by single-particle cryo-EM at 200 keV. Nat. Methods 14, 1075–1078 (2017).
Suloway, C. et al. Automated molecular microscopy: the new Leginon system. J. Struct. Biol. 151, 41–60 (2005).
Lander, G. C. et al. Appion: an integrated, database-driven pipeline to facilitate EM image processing. J. Struct. Biol. 166, 95–102 (2009).
Zheng, S. Q. et al. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods 14, 331–332 (2017).
Punjani, A., Rubinstein, J. L., Fleet, D. J. & Brubaker, M. A. cryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods 14, 290–296 (2017).
Tan, Y. Z. et al. Addressing preferred specimen orientation in single-particle cryo-EM through tilting. Nat. Methods 14, 793–796 (2017).
Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D 66, 213–221 (2010).
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D 66, 486–501 (2010).
Pettersen, E. F. et al. UCSF Chimera—a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004).
Pettersen, E. F. et al. UCSF ChimeraX: structure visualization for researchers, educators, and developers. Protein Sci. 30, 70–82 (2021).
Acknowledgements
We thank B. Anderson at the Scripps Research Electron Microscopy Facility for microscopy support. We thank C. Bowman and J.-C. Ducom at Scripps Research High Performance Computing core for computational support. We thank T. S. Xiao at Case Western Reserve University for kindly sharing the MBP-tagged vectors. We thank B. Basanta for his valuable comments on designing DELE1 mutants. This work is supported by the National Institutes of Health (NIH) (NS125674 to R.L.W. and NS095892 to R.L.W. and G.C.L). A.S.S. is supported by the NIH F31AG071162 and the Olson-King Endowed Skaggs Fellowship from Scripps Research. Cryo-EM data were acquired using shared instrumentation funded by NIH S10OD032467 to G.C.L. Computational analyses of EM data were performed using shared instrumentation funded by NIH S10OD021634 to G.C.L.
Author information
Authors and Affiliations
Contributions
J.Y. and G.C.L. conceptualized and led the project. J.Y. created all the constructs, purified the proteins, and performed all cryo-EM structure determination, model building and refinement, and mechanistic interpretation. K.R.B. and R.L.W. designed and performed the ATF4 immunoblotting and ATF4 luciferase assay. D.E.P. assisted in purifying the DELE1 mutant recombinant proteins. A.S. performed the sucrose gradient sedimentation assay. J.Y. and W.C. performed the co-IP assays. A.S.S. assisted in the western blot. X.G., G.A., and M.K. generated the ATF4-FLuc reporter cell line. X.G. performed the full-length DELE1 rescue assay. J.Y. wrote the original draft of the paper. G.C.L. and R.L.W. reviewed and edited the paper. All authors commented on the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Structural & Molecular Biology thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editor: Katarzyna Ciazynska, in collaboration with the Nature Structural & Molecular Biology team. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Mass photometry of recombinant MBP-DELE1 proteins.
Mass distribution histograms obtained using mass photometry for (a) full-length MBP-DELE1, (b) MBP-DELE1 CTD, and (c) MBP-DELE1 Δ244 truncation. The calculated molecular weight (MW) at the peak for each histogram is shown. BSA and thyroglobulin were used as MW standards for calibrations. Data shown are representative of experiments that were performed in triplicate or more.
Extended Data Fig. 2 Cryo-EM structure determination of DELE1.
a, Representative micrograph of cryo-EM data collection. A total of 13,164 micrographs were collected. The high background on the micrograph appears to be the monomeric or small oligomeric species of DELE1CTD, possibly caused by denaturing due to interaction with the air-water interface during cryo-freezing34,35, although there may be oligomeric heterogeneity within the sample prior to freezing. b, Cryo-EM data processing workflow using CryoSPARC software. The final 3D reconstruction map was used for model building and refinement. c, 3D Fourier Shell Correlation (3DFSC)29 of the final reconstruction reporting a global resolution at ~3.8 Å and high level of resolution isotropy, with the FSC at 0.143 denoted. The model-to-map FSC plot from Phenix real space refinement is overlaid. d, Final reconstruction filtered and colored by local resolution, as estimated in CryoSPARC. e, Euler angle distribution plot of the particles used in the final 3D reconstruction showing complete tomographic coverage of projections.
Extended Data Fig. 3 Inter-subunit interactions among the DELE1 oligomer.
a, A surface representation of the DELE1CTD monomer is shown in two orthogonal views, colored gray. The buried surface areas associated with oligomerization Interfaces I and II are colored orange and salmon, respectively. b-e, Detailed views of the Interface I and II interactions demonstrating the quality of the cryo-EM density, which is shown as transparent surface. The interacting residues are shown as sticks, and the coloring is consistent with that of Fig. 2.
Extended Data Fig. 4 AlphaFold2-predicted models of DELE1 and negative stain EM.
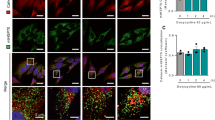
a, The AlphaFold2 predicted structure with the DELE1 CTD sequence harboring L239E, L242E, F250A, L251E, R403A, and F431S mutations. The mutated residues are denoted as yellow spheres. b, Structural alignment of the DELE1 cryo-EM structure and AlphaFold2-predicted monomeric structure in a, showing that the overall predicted structure of the mutant DELE1 is highly similar to the WT DELE1. c-e, Representative negative stain EM images of the WT DELE1, the Interface II mutant (R403A/F431S), and the Interface I truncation (Δ244). A total of 326, 257, and 392 images were collected, respectively. f, Fluorescence-detection size-exclusion chromatography (FSEC) analysis for WT DELE1CTD-GFP (labeled as WT in blue) and DELE1CTD-GFP Δ244 truncation (labeled as Δ244 in magenta) expressed for 24 hrs post transfection from HEK293T cells.
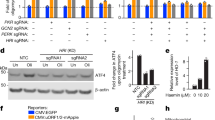
Extended Data Fig. 5 Mutant DELE1 expression, ATF4 reporter construct, HRI phosphorylation, and full length DELE1 oligomerization in cells.
a. Quantification of DELE1 mutants expressed in HEK293T cells. (mean ± SEM, n = 6 experimental repeats). One R403A/F431S outlier was excluded using the ROUT outlier test in PRISM. b. Description of the ATF4 reporter stable cell line, which contains the upstream open reading frames (uORF1 and uORF2) in the ATF4 5′ untranslated region (ATF4 5′ UTR). The ATF4 coding sequence was replaced by a luciferase gene (Fluc). c. Quantification of phosphorylated HRI in lysates prepared from HEK293T cells transfected with FLAG-HRI and the different DELE1 mutant constructs show in Fig. 4e. (Error bars show the range, n = 2 experimental repeats). d. Immunoblot of the full-length DELE1-mClover protein from sucrose gradient fractions. The 14 sucrose gradient fractions from light to heavy molecular weights (left to right) were blotted with an anti-GFP antibody to detect the DELE1-mClover protein. The upper and lower bands represent the full-length DELE1 and the cleaved DELE1, respectively. β tubulin was used as a control. Data shown are representative of two independent experiments.
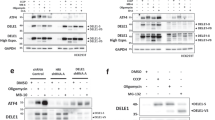
Extended Data Fig. 6 HRI NTD mediates its interactions with DELE1.
a. Co-immunoprecipitation of FLAG-tagged HRI and GFP tagged WT DELE1CTD (CTD), and different DELE1 mutants. Anti-GFP antibody and Anti-FLAG antibody were used to detect DELE1 and HRI, respectively. b. The AlphaFold2 model of HRI. The HRI N terminal domain (NTD: 1-160) is colored blue, and the HRI C terminal domain (CTD: 161- 630) is colored yellow. The disordered kinase insertion region (239-375) from the AlphaFold2 model was deleted and instead shown as dashed line. c. Structural alignment for the kinase domains of PKR and HRI. The crystal structure of PKR kinase domain (PDB: 2A19) is colored purple, and the HRI kinase domain is yellow. d. Schematic of DELE1 and HRI constructs used for co-immunoprecipitation (co-IP). e. Co-IP of GFP-tagged WT DELE1 C-terminal domain (CTD), and different Flag tagged HRI constructs including full-length HRI (FL), HRI N-terminal domain (NTD), and HRI C-terminal domain (CTD). An anti-GFP antibody and anti-FLAG antibody were used to detect DELE1 and HRI, respectively. Left: Immunoblotting of designated proteins from IP fractions. Right: Immunoblotting of designated proteins from whole cell lysis (WCL) fractions. In 6a and 6e, Data shown are representative of two independent experiments.
Supplementary information
Source data
Source Data Fig. 3
Unprocessed western blots, and Coomassie blue staining gels.
Source Data Fig. 4
Unprocessed western blots.
Source Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 5
Unprocessed western blots.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 6
Processed western blots.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yang, J., Baron, K.R., Pride, D.E. et al. DELE1 oligomerization promotes integrated stress response activation. Nat Struct Mol Biol 30, 1295–1302 (2023). https://doi.org/10.1038/s41594-023-01061-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41594-023-01061-0
This article is cited by
-
Stress response silencing by an E3 ligase mutated in neurodegeneration
Nature (2024)
-
Cytosolic retention of HtrA2 during mitochondrial protein import stress triggers the DELE1-HRI pathway
Communications Biology (2024)