Abstract
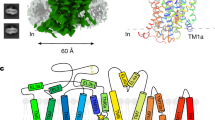
γ-Aminobutyric acid (GABA), an important inhibitory neurotransmitter in the central nervous system, is recycled through specific GABA transporters (GATs). GAT1, which is mainly expressed in the presynaptic terminals of axons, is a potential drug target of neurological disorders due to its essential role in GABA transport. Here we report four cryogenic electron microscopy structures of human GAT1, at resolutions of 2.2–3.2 Å. GAT1 in substrate-free form or in complex with the antiepileptic drug tiagabine exhibits an inward-open conformation. In the presence of GABA or nipecotic acid, inward-occluded structures are captured. The GABA-bound structure reveals an interaction network bridged by hydrogen bonds and ion coordination for GABA recognition. The substrate-free structure unwinds the last helical turn of transmembrane helix TM1a to release sodium ions and substrate. Complemented by structure-guided biochemical analyses, our studies reveal detailed mechanism of GABA recognition and transport, and elucidate mode of action of the inhibitors, nipecotic acid and tiagabine.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The UniProt accession codes for the sequences of human GAT1, GAT2, GAT3 and BGT1 are P30531, Q9NSD5, P48066 and P48065, respectively. Atomic coordinates of GAT1 in the substrate-free state, or the presence of GABA, or inhibitors in various conditions (GAT1A/G/N/T) have been deposited in the PDB (http://www.rcsb.org) under the accession codes 7Y7V (https://doi.org/10.2210/pdb7Y7V/pdb), 7Y7W (https://doi.org/10.2210/pdb7Y7W/pdb), 7Y7Y (https://doi.org/10.2210/pdb7Y7Y/pdb) and 7Y7Z (https://doi.org/10.2210/pdb7Y7Z/pdb), respectively. The corresponding EM maps have been deposited in the Electron Microscopy Data Bank (https://www.ebi.ac.uk/pdbe/emdb/), under the accession codes EMD-33671, EMD-33672, EMD-33674 and EMD-33675, respectively. Previously resolved structures used in this study are under the accession codes 4US3 (inward-occluded Trp-bound MhsT structure), 6XWM (inward-occluded Phe-bound LeuT structure), 7LIA (outward-open serotonin-bound SERT structure), 4XP1 (outward-open dopamine-bound dDAT structure), 7LI8 (inward-open apo SERT structure), 3TT3 (inward-open apo LeuT structure), 6ZPL (inward-open cmpd1-bound GlyT1 structure) and 7SK2 (inward-open tiagabine-bound GAT1 structure) in the PDB. Source data are provided with this paper.
References
Petroff, O. A. GABA and glutamate in the human brain. Neuroscientist 8, 562–573 (2002).
Ghit, A., Assal, D., Al-Shami, A. S. & Hussein, D. E. E. GABA(A) receptors: structure, function, pharmacology, and related disorders. J. Genet Eng. Biotechnol. 19, 123 (2021).
Evenseth, L. S. M., Gabrielsen, M. & Sylte, I. The GABA(B) receptor-structure, ligand binding and drug development. Molecules 25, 3093 (2020).
Ben-Ari, Y. Excitatory actions of GABA during development: the nature of the nurture. Nat. Rev. Neurosci. 3, 728–739 (2002).
Rivera, C. et al. The K+/Cl− co-transporter KCC2 renders GABA hyperpolarizing during neuronal maturation. Nature 397, 251–255 (1999).
Zhou, Y. & Danbolt, N. C. GABA and glutamate transporters in brain. Front. Endocrinol. 4, 165 (2013).
Guastella, J. et al. Cloning and expression of a rat brain GABA transporter. Science 249, 1303–1306 (1990).
Keynan, S., Suh, Y. J., Kanner, B. I. & Rudnick, G. Expression of a cloned gamma-aminobutyric acid transporter in mammalian cells. Biochemistry 31, 1974–1979 (1992).
Conti, F., Minelli, A. & Melone, M. GABA transporters in the mammalian cerebral cortex: localization, development and pathological implications. Brain Res. Brain Res. Rev. 45, 196–212 (2004).
Jin, X. T., Galvan, A., Wichmann, T. & Smith, Y. Localization and function of GABA transporters GAT-1 and GAT-3 in the basal ganglia. Front. Syst. Neurosci. 5, 63 (2011).
Melone, M., Ciappelloni, S. & Conti, F. A quantitative analysis of cellular and synaptic localization of GAT-1 and GAT-3 in rat neocortex. Brain Struct. Funct. 220, 885–897 (2015).
Benarroch, E. What is the role of GABA transporters in seizures? Neurology 97, 580–584 (2021).
Ramamoorthi, K. & Lin, Y. The contribution of GABAergic dysfunction to neurodevelopmental disorders. Trends Mol. Med. 17, 452–462 (2011).
Fuhrer, T. E. et al. Impaired expression of GABA transporters in the human Alzheimer’s disease hippocampus, subiculum, entorhinal cortex and superior temporal gyrus. Neuroscience 351, 108–118 (2017).
Lyu, S. et al. Blockade of GABA transporter-1 and GABA transporter-3 in the lateral habenula improves depressive-like behaviors in a rat model of Parkinson’s disease. Neuropharmacology 181, 108369 (2020).
Mermer, F. et al. Common molecular mechanisms of SLC6A1 variant-mediated neurodevelopmental disorders in astrocytes and neurons. Brain 144, 2499–2512 (2021).
Bialer, M. et al. Progress report on new antiepileptic drugs: a summary of the Eigth Eilat Conference (EILAT VIII). Epilepsy Res. 73, 1–52 (2007).
Knutsen, L. J. et al. Synthesis of novel GABA uptake inhibitors. 3. Diaryloxime and diarylvinyl ether derivatives of nipecotic acid and guvacine as anticonvulsant agents. J. Med. Chem. 42, 3447–3462 (1999).
Adkins, J. C. & Noble, S. Tiagabine. A review of its pharmacodynamic and pharmacokinetic properties and therapeutic potential in the management of epilepsy. Drugs 55, 437–460 (1998).
Braestrup, C. et al. (R)-N-[4,4-bis(3-methyl-2-thienyl)but-3-en-1-yl]nipecotic acid binds with high affinity to the brain gamma-aminobutyric acid uptake carrier. J. Neurochem. 54, 639–647 (1990).
Borden, L. A. et al. Tiagabine, SK&F 89976-A, CI-966, and NNC-711 are selective for the cloned GABA transporter GAT-1. Eur. J. Pharmacol. 269, 219–224 (1994).
Kristensen, A. S. et al. SLC6 neurotransmitter transporters: structure, function, and regulation. Pharm. Rev. 63, 585–640 (2011).
Navratna, V. & Gouaux, E. Insights into the mechanism and pharmacology of neurotransmitter sodium symporters. Curr. Opin. Struct. Biol. 54, 161–170 (2019).
Zomot, E. et al. Mechanism of chloride interaction with neurotransmitter:sodium symporters. Nature 449, 726–730 (2007).
Forrest, L. R., Tavoulari, S., Zhang, Y. W., Rudnick, G. & Honig, B. Identification of a chloride ion binding site in Na+/Cl−-dependent transporters. Proc. Natl Acad. Sci. USA 104, 12761–12766 (2007).
Yamashita, A., Singh, S. K., Kawate, T., Jin, Y. & Gouaux, E. Crystal structure of a bacterial homologue of Na+/Cl−-dependent neurotransmitter transporters. Nature 437, 215–223 (2005).
Singh, S. K., Yamashita, A. & Gouaux, E. Antidepressant binding site in a bacterial homologue of neurotransmitter transporters. Nature 448, 952–956 (2007).
Singh, S. K., Piscitelli, C. L., Yamashita, A. & Gouaux, E. A competitive inhibitor traps LeuT in an open-to-out conformation. Science 322, 1655–1661 (2008).
Krishnamurthy, H. & Gouaux, E. X-ray structures of LeuT in substrate-free outward-open and apo inward-open states. Nature 481, 469–474 (2012).
Penmatsa, A., Wang, K. H. & Gouaux, E. X-ray structure of dopamine transporter elucidates antidepressant mechanism. Nature 503, 85–90 (2013).
Wang, K. H., Penmatsa, A. & Gouaux, E. Neurotransmitter and psychostimulant recognition by the dopamine transporter. Nature 521, 322–327 (2015).
Coleman, J. A., Green, E. M. & Gouaux, E. X-ray structures and mechanism of the human serotonin transporter. Nature 532, 334–339 (2016).
Coleman, J. A. & Gouaux, E. Structural basis for recognition of diverse antidepressants by the human serotonin transporter. Nat. Struct. Mol. Biol. 25, 170–175 (2018).
Coleman, J. A. et al. Serotonin transporter-ibogaine complexes illuminate mechanisms of inhibition and transport. Nature 569, 141–145 (2019).
Yang, D. & Gouaux, E. Illumination of serotonin transporter mechanism and role of the allosteric site. Sci. Adv. 7, eabl3857 (2021).
Shahsavar, A. et al. Structural insights into the inhibition of glycine reuptake. Nature 591, 677–681 (2021).
Motiwala, Z. et al. Structural basis of GABA reuptake inhibition. Nature 606, 820–826 (2022).
Yan, R., Zhao, X., Lei, J. & Zhou, Q. Structure of the human LAT1-4F2hc heteromeric amino acid transporter complex. Nature 568, 127–130 (2019).
Wang, N. et al. Structural basis of human monocarboxylate transporter 1 inhibition by anti-cancer drug candidates. Cell 184, 370–383.e13 (2021).
Parker, J. L. et al. Structural basis of antifolate recognition and transport by PCFT. Nature 595, 130–134 (2021).
Yuan, Y. et al. Cryo-EM structure of human glucose transporter GLUT4. Nat. Commun. 13, 2671 (2022).
Deng, D. et al. Molecular basis of ligand recognition and transport by glucose transporters. Nature 526, 391–396 (2015).
Jiang, X. et al. Structural basis for blocking sugar uptake into the malaria parasite Plasmodium falciparum. Cell 183, 258–268.e12 (2020).
Garcia, M. L., Viitanen, P., Foster, D. L. & Kaback, H. R. Mechanism of lactose translocation in proteoliposomes reconstituted with lac carrier protein purified from Escherichia coli. 1. Effect of pH and imposed membrane potential on efflux, exchange, and counterflow. Biochemistry 22, 2524–2531 (1983).
Punjani, A., Rubinstein, J. L., Fleet, D. J. & Brubaker, M. A. cryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods 14, 290–296 (2017).
Zhang, X. et al. An atomic structure of the human spliceosome. Cell 169, 918–929.e14 (2017).
Cai, G. et al. The role of N-glycosylation in the stability, trafficking and GABA-uptake of GABA-transporter 1. Terminal N-glycans facilitate efficient GABA-uptake activity of the GABA transporter. FEBS J. 272, 1625–1638 (2005).
Gotfryd, K. et al. X-ray structure of LeuT in an inward-facing occluded conformation reveals mechanism of substrate release. Nat. Commun. 11, 1005 (2020).
Malinauskaite, L. et al. A mechanism for intracellular release of Na+ by neurotransmitter/sodium symporters. Nat. Struct. Mol. Biol. 21, 1006–1012 (2014).
Bismuth, Y., Kavanaugh, M. P. & Kanner, B. I. Tyrosine 140 of the gamma-aminobutyric acid transporter GAT-1 plays a critical role in neurotransmitter recognition. J. Biol. Chem. 272, 16096–16102 (1997).
Kanner, B. I. Transmembrane domain I of the gamma-aminobutyric acid transporter GAT-1 plays a crucial role in the transition between cation leak and transport modes. J. Biol. Chem. 278, 3705–3712 (2003).
Johnston, G. A., Stephanson, A. L. & Twitchin, B. Uptake and release of nipecotic acid by rat brain slices. J. Neurochem. 26, 83–87 (1976).
Loo, D. D., Eskandari, S., Boorer, K. J., Sarkar, H. K. & Wright, E. M. Role of Cl− in electrogenic Na+-coupled cotransporters GAT1 and SGLT1. J. Biol. Chem. 275, 37414–37422 (2000).
Kovalev, G. I. & Raevskiĭ, K. S. Nipecotic acid, a competitive inhibitor of the net uptake of 3H-GABA by rat brain synaptosomes. Biull. Eksp. Biol. Med. 91, 692–694 (1981).
Pettersen, E. F. et al. UCSF ChimeraX: structure visualization for researchers, educators, and developers. Protein Sci. 30, 70–82 (2021).
Lei, J. & Frank, J. Automated acquisition of cryo-electron micrographs for single particle reconstruction on an FEI Tecnai electron microscope. J. Struct. Biol. 150, 69–80 (2005).
Zheng, S. Q. et al. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods 14, 331–332 (2017).
Jumper, J. et al. Highly accurate protein structure prediction with AlphaFold. Nature 596, 583–589 (2021).
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D 60, 2126–32 (2004).
Afonine, P. V. et al. Real-space refinement in PHENIX for cryo-EM and crystallography. Acta Crystallogr. D 74, 531–544 (2018).
Liebschner, D. et al. Macromolecular structure determination using X-rays, neutrons and electrons: recent developments in Phenix. Acta Crystallogr. D 75, 861–877 (2019).
Amunts, A. et al. Structure of the yeast mitochondrial large ribosomal subunit. Science 343, 1485–1489 (2014).
Acknowledgements
We thank F. Yang and X. Li for technical support during EM data collection. We thank the Tsinghua University Branch of China National Center for Protein Sciences (Beijing) for providing the cryo-EM facility support and the computational facility support. This work was funded by the National Key R&D Program of China (2020YFA0509301, C.Y.), Beijing Nova Program (Z201100006820039, C.Y.), Beijing Frontier Research Center for Biological Structure, Beijing Advanced Innovation Center for Structural Biology, State Key Laboratory of Membrane Biology, Tsinghua University Initiative Scientific Research Program, and Start-up funds from Tsinghua-Peking Center for Life Sciences and Tsinghua University.
Author information
Authors and Affiliations
Contributions
C.Y. and Y.Y. conceived the project. Y.Y., C.Y. and A.Z. designed all experiments. A.Z., J.H., Y.Y. and J.T. performed the experiments. A.Z., F.K., C.Y. and J.L. contributed to the structure determination. All authors analyzed the data and contributed to manuscript preparation. C.Y., A.Z. and Y.Y. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Structural & Molecular Biology thanks Gary Rudnick and Azadeh Shahsavar for their contribution to the peer review of this work. Primary Handling Editors: Florian Ullrich and Katarzyna Ciazynska, in collaboration with the Nature Structural & Molecular Biology team. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Biochemical characterization of recombinantly expressed human GAT1.
a, SEC purification of the human GAT1 in the presence of 0.02% (w/v) DDM from one independent experiment. b, Peak fractions of SEC purification were further examined by Coomassie blue staining of SDS–PAGE. GAT1 appeared in two smeared bands due to different extent of glycosylation. The experiments were repeated independently more than 3 times with consistent results. c, The time course analysis of the transport activity of GAT1. Data are presented as mean with SD of three independent experiments. d, Determination of the kinetic parameters of GAT1 for the transport of GABA. Data are presented as mean with SD of three independent experiments and were fitted using the Michaelis–Menten non-linear fitting method, yielding Km at 27.51 ± 3.86 μM and Vmax at 248.6 ± 9.33 nmol/mg/min. Source data are provided.
Extended Data Fig. 2 Data processing of different GAT1 datasets.
a, The flowchart for GAT1T data processing. Details can be found in Methods. b, The flowchart for GAT1A/G/N data processing. Details can be found in Methods.
Extended Data Fig. 3 Cryo-EM analysis of GAT1 in different states.
a, Gold-standard Fourier shell correlation (FSC) curve for the 3D refinement of the overall structure of GAT1A/G/N/T, respectively. b, Angular distribution of the particles used for the final reconstructions. c, FSC curves of the refined models versus the sharpen maps (black); of the models refined against the first half maps versus the same maps (purple); and of the models refined against the first half maps versus the second half maps (green). The small difference between the purple and green curves indicates that the refinement of the atomic coordinates did not suffer from overfitting. d, Local resolution of the sharpen maps shows in the front view and longitudinal section view. Red dashed lines label the location of GABA, nipecotic acid or tiagabine in the map of GAT1G/N/T, respectively.
Extended Data Fig. 4 Representative EM densities of GAT1.
a, Representative EM maps of GAT1A transmembrane helices and EM densities are contoured at 5 σ. b, EM maps of TM1a in GAT1A, GAT1G and GAT1T, respectively. EM densities are contoured at 4 σ. c, EM map of the disulfide bond between C164 and C173 in GAT1A and EM densities are contoured at 5 σ.
Extended Data Fig. 5 Sequence alignment of human GAT1-3 and BGT1.
Secondary structural elements of hGAT1 are indicated above the sequence alignment. Invariant and highly conserved amino acids are shaded yellow and grey, respectively. The residues that are hydrogen-bonded with GABA, or chelating Na+ at the Na1 and Na2 sites and Cl– in hGAT1 are indicated by orange star, magenta, red and green circles, respectively. The residues which form disulfide bond are indicated by brown triangles. The glycosylation site residues are indicated by cyan squares. The UniProt (https://www.uniprot.org) IDs for the aligned proteins are: hGAT1 (P30531), hGAT2 (Q9NSD5), hGAT3 (P48066) and hBGT1 (P48065). Sequences are aligned with ClustalW.
Extended Data Fig. 6 Water molecules determined in the structures of GAT1.
a, Water molecules in the structure of GAT1G in an inward-occluded state. Waters are shown as marine spheres. b, Waters in the structure of GAT1A in an inward-open state. c, The structural waters that mediated hydrogen bonds with GABA or GAT1 residues. d, Extracellular gating residues in GAT1G. The hydrogen bonds formed by Arg69, Gln291, Asp451 and Ser456 are indicated by gold dashed lines. Cation-π interaction is indicated by black dashed line. The hydrogen bonds formed by the water cluster are indicated by cyan dashed lines.
Extended Data Fig. 7 Structural comparison of GAT1G to substrate-bound prokaryotic NSS members.
a, Overall structural comparison between the cryo-EM structures of GABA-bound GAT1G, inward-occluded Trp-bound MhsT (PDB code: 4US3), inward-occluded Phe-bound LeuT (PDB code: 6XWM). The intracellular permeation pathway is occluded by TM1a colored marine among the three structures. TM5 colored maroon shows different geometry among the structures. b-d, Comparison of Na1 and Na2 site in GABA-bound GAT1G (b), Trp-bound MhsT (c) and Phe-bound LeuT (d). The sodium ions are colored magenta, and the ion coordination is colored maroon. e-g, Comparison of substrates binding mode in GABA-bound GAT1G (e), Trp-bound MhsT (PDB code: 4US3) (f) and Phe-bound LeuT (PDB code: 6XWM) (g). The yellow dashed lines indicate hydrogen bonds and ion coordination of the substrate.
Extended Data Fig. 8 Structural comparison of GAT1G to eukaryotic NSS members.
a-d, Comparison of Cl– binding site in GABA-bound GAT1G (a), serotonin-bound SERT (PDB code: 7LIA) (b), dopamine-bound dDAT (PDB code: 4XP1) (c) and Cmpd1-bound GlyT1 (PDB code: 6ZPL) (d). The chloride ions are colored green, and the ion coordination is colored dark green. The tetra-coordination of Cl– is strictly conserved among the four structures. e-g, Comparison of substrates binding mode in GABA-bound GAT1G (e, colored silver in f and g), serotonin-bound SERT (PDB code: 7LIA) (f) and dopamine-bound dDAT (PDB code: 4XP1) (g). Monoamine substrates like serotonin and dopamine are located in the cleft formed by TM3 and TM8 compared to GABA.
Extended Data Fig. 9 Structural comparison of inward-open NSS members.
a, Shown here are superimposed structures of inward-open substrate-free GAT1A (colored purple), inward-open tiagabine-bound GAT1T (colored yellow), inward-open apo SERT (PDB code: 7LI8) (colored salmon), inward-open apo LeuT (PDB code: 3TT3) (colored pink) and inward-open Cmpd1-bound GlyT1 (PDB code: 6ZPL) (colored marine). TM1a adopts a different orientation in different structures. b, Shown here are superimposed structures of inward-open substrate-free GAT1A (colored purple), inward-open tiagabine-bound GAT1T (colored yellow) and inward-open tiagabine-bound GAT1 reported by Gati’s group (PDB code: 7SK2) (colored cyan). The compound tiagabine is located in the same central pocket in both structures. Coordination of the nipecotic acid moiety is similar, while the two 3-methyl-2-thienyl rings cannot be accurately compared due to their relative flexibility (right panel).
Supplementary information
Source data
Source Data Figs. 3c and 5a,b and Extended Data Fig.1a,c–d
Statistical source data.
Source Data Extended Fig. 1b
Uncropped gel.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhu, A., Huang, J., Kong, F. et al. Molecular basis for substrate recognition and transport of human GABA transporter GAT1. Nat Struct Mol Biol 30, 1012–1022 (2023). https://doi.org/10.1038/s41594-023-00983-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41594-023-00983-z
This article is cited by
-
Ion and lipid orchestration of secondary active transport
Nature (2024)
-
GABA transport cycle: beyond a GAT feeling
Nature Structural & Molecular Biology (2023)