Abstract
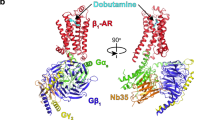
The β1-adrenergic receptor (β1-AR) can activate two families of G proteins. When coupled to Gs, β1-AR increases cardiac output, and coupling to Gi leads to decreased responsiveness in myocardial infarction. By comparative structural analysis of turkey β1-AR complexed with either Gi or Gs, we investigate how a single G-protein-coupled receptor simultaneously signals through two G proteins. We find that, although the critical receptor-interacting C-terminal α5-helices on Gαi and Gαs interact similarly with β1-AR, the overall interacting modes between β1-AR and G proteins vary substantially. Functional studies reveal the importance of the differing interactions and provide evidence that the activation efficacy of G proteins by β1-AR is determined by the entire three-dimensional interaction surface, including intracellular loops 2 and 4 (ICL2 and ICL4).
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The cryo-EM reconstructions of the β1-AR–Gi–isoproterenol complex and the β1-AR–Gi/s–isoproterenol complex have been deposited in the Election Microscopy Data Bank (EMDB) under ID codes EMD-24789 and EMD-24790, respectively. The corresponding atomic models have been deposited in the Protein Data Bank (PDB) under ID codes 7S0F and 7S0G, respectively. Source data are provided with this paper.
References
Benovic, J. L. Novel β2-adrenergic receptor signaling pathways. J. Allergy Clin. Immunol. 110, S229–S235 (2002).
Post, S. R., Hammond, H. K. & Insel, P. A. Beta-adrenergic receptors and receptor signaling in heart failure. Annu. Rev. Pharmacol. Toxicol. 39, 343–360 (1999).
Lohse, M. J., Engelhardt, S. & Eschenhagen, T. What is the role of β-adrenergic signaling in heart failure? Circ. Res. 93, 896–906 (2003).
Hilger, D., Masureel, M. & Kobilka, B. K. Structure and dynamics of GPCR signaling complexes. Nat. Struct. Mol. Biol. 25, 4–12 (2018).
Asano, T., Katada, T., Gilman, A. G. & Ross, E. M. Activation of the inhibitory GTP-binding protein of adenylate cyclase, Gi, by β-adrenergic receptors in reconstituted phospholipid vesicles. J. Biol. Chem. 259, 9351–9354 (1984).
Kompa, A. R., Gu, X. H., Evans, B. A. & Summers, R. J. Desensitization of cardiac β-adrenoceptor signaling with heart failure produced by myocardial infarction in the rat. Evidence for the role of Gi but not Gs or phosphorylating proteins. J. Mol. Cell. Cardiol. 31, 1185–1201 (1999).
Wang, J. et al. Gαi is required for carvedilol-induced β1 adrenergic receptor β-arrestin biased signaling. Nat. Commun. 8, 1706 (2017).
Dwivedi, H., Baidya, M. & Shukla, A. K. GPCR signaling: the interplay of Gαi and β-arrestin. Curr. Biol. 28, R324–R327 (2018).
Lukasheva, V. et al. Signal profiling of the β1AR reveals coupling to novel signalling pathways and distinct phenotypic responses mediated by β1AR and β2AR. Sci. Rep. 10, 8779 (2020).
Rubenstein, R. C., Linder, M. E. & Ross, E. M. Selectivity of the β-adrenergic receptor among Gs, Gi’s, and Go: assay using recombinant α subunits in reconstituted phospholipid vesicles. Biochemistry 30, 10769–10777 (1991).
Su, M. et al. Structural basis of the activation of heterotrimeric Gs-protein by isoproterenol-bound β1-adrenergic receptor. Mol. Cell 80, 59–71 (2020).
Huang, J., Chen, S., Zhang, J. J. & Huang, X. Y. Crystal structure of oligomeric β1-adrenergic G protein-coupled receptors in ligand-free basal state. Nat. Struct. Mol. Biol. 20, 419–425 (2013).
Wall, M. A. et al. The structure of the G protein heterotrimer Giα1β1γ2. Cell 83, 1047–1058 (1995).
Sprang, S. R., Chen, Z. & Du, X. Structural basis of effector regulation and signal termination in heterotrimeric Gα proteins. Adv. Protein Chem. 74, 1–65 (2007).
Noel, J. P., Hamm, H. E. & Sigler, P. B. The 2.2-Å crystal structure of transducin-α complexed with GTPγS. Nature 366, 654–663 (1993).
Coleman, D. E. et al. Structures of active conformations of Giα1 and the mechanism of GTP hydrolysis. Science 265, 1405–1412 (1994).
Rasmussen, S. G. et al. Crystal structure of the β2 adrenergic receptor–Gs protein complex. Nature 477, 549–555 (2011).
Kang, Y. et al. Cryo-EM structure of human rhodopsin bound to an inhibitory G protein. Nature 558, 553–558 (2018).
Wall, M. A., Posner, B. A. & Sprang, S. R. Structural basis of activity and subunit recognition in G protein heterotrimers. Structure 6, 1169–1183 (1998).
Aurora, R., Srinivasan, R. & Rose, G. D. Rules for α-helix termination by glycine. Science 264, 1126–1130 (1994).
Liu, X. et al. Structural insights into the process of GPCR-G protein complex formation. Cell 177, 1243–1251 (2019).
Sprang, S. R. G protein mechanisms: insights from structural analysis. Annu. Rev. Biochem. 66, 639–678 (1997).
Lee, E., Taussig, R. & Gilman, A. G. The G226A mutant of Gsα highlights the requirement for dissociation of G protein subunits. J. Biol. Chem. 267, 1212–1218 (1992).
Liang, Y. L. et al. Phase-plate cryo-EM structure of a class B GPCR–G-protein complex. Nature 546, 118–123 (2017).
Zhang, Y. et al. Cryo-EM structure of the activated GLP-1 receptor in complex with a G protein. Nature 546, 248–253 (2017).
Koehl, A. et al. Structure of the μ-opioid receptor–Gi protein complex. Nature 558, 547–552 (2018).
Krishna Kumar, K. et al. Structure of a signaling cannabinoid receptor 1-G protein complex. Cell 176, 448–458 e412 (2019).
Conklin, B. R., Farfel, Z., Lustig, K. D., Julius, D. & Bourne, H. R. Substitution of three amino acids switches receptor specificity of Gqα to that of Giα. Nature 363, 274–276 (1993).
Conklin, B. R. & Bourne, H. R. Structural elements of Gα subunits that interact with Gβγ, receptors, and effectors. Cell 73, 631–641 (1993).
Hisano, Y. et al. Lysolipid receptor cross-talk regulates lymphatic endothelial junctions in lymph nodes. J. Exp. Med. 216, 1582–1598 (2019).
Jelinek, V., Mosslein, N. & Bunemann, M. Structures in G proteins important for subtype selective receptor binding and subsequent activation. Commun. Biol. 4, 635 (2021).
Okashah, N. et al. Variable G protein determinants of GPCR coupling selectivity. Proc. Natl Acad. Sci. USA 116, 12054–12059 (2019).
Draper-Joyce, C. J. et al. Structure of the adenosine-bound human adenosine A1 receptor–Gi complex. Nature 558, 559–563 (2018).
Ma, X. et al. Analysis of β2AR-Gs and β2AR-Gi complex formation by NMR spectroscopy. Proc. Natl Acad. Sci. USA 117, 23096–23105 (2020).
Bos, J. L., Rehmann, H. & Wittinghofer, A. GEFs and GAPs: critical elements in the control of small G proteins. Cell 129, 865–877 (2007).
Boriack-Sjodin, P. A., Margarit, S. M., Bar-Sagi, D. & Kuriyan, J. The structural basis of the activation of Ras by Sos. Nature 394, 337–343 (1998).
Bourne, H. R., Sanders, D. A. & McCormick, F. The GTPase superfamily: conserved structure and molecular mechanism. Nature 349, 117–127 (1991).
Warne, T. et al. Structure of a β1-adrenergic G-protein-coupled receptor. Nature 454, 486–491 (2008).
Baker, J. G. A full pharmacological analysis of the three turkey β-adrenoceptors and comparison with the human β-adrenoceptors. PLoS ONE 5, e15487 (2010).
Baker, J. G., Proudman, R. G. & Tate, C. G. The pharmacological effects of the thermostabilising (m23) mutations and intra and extracellular (β36) deletions essential for crystallisation of the turkey β-adrenoceptor. Naunyn Schmiedebergs Arch. Pharm. 384, 71–91 (2011).
Carragher, B. et al. Leginon: an automated system for acquisition of images from vitreous ice specimens. J. Struct. Biol. 132, 33–45 (2000).
Rohou, A. & Grigorieff, N. CTFFIND4: fast and accurate defocus estimation from electron micrographs. J. Struct. Biol. 192, 216–221 (2015).
Scheres, S. H. Processing of structurally heterogeneous cryo-EM data in RELION. Methods Enzymol. 579, 125–157 (2016).
Punjani, A., Rubinstein, J. L., Fleet, D. J. & Brubaker, M. A. cryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods 14, 290–296 (2017).
Punjani, A., Zhang, H. & Fleet, D. J. Non-uniform refinement: adaptive regularization improves single particle cryo-EM reconstruction. Nat. Methods 17, 1214–1221 (2020).
Terwilliger, T. C., Ludtke, S. J., Read, R. J., Adams, P. D. & Afonine, P. V. Improvement of cryo-EM maps by density modification. Nat. Methods 17, 923–927 (2020).
Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 66, 213–221 (2010).
Pettersen, E. F. et al. UCSF Chimera—a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004).
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 (2004).
Chen, V. B. et al. MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr. D Biol. Crystallogr. 66, 12–21 (2010).
McEwen, D. P., Gee, K. R., Kang, H. C. & Neubig, R. R. Fluorescent BODIPY-GTP analogs: real-time measurement of nucleotide binding to G proteins. Anal. Biochem. 291, 109–117 (2001).
Wan, Y., Kurosaki, T. & Huang, X. Y. Tyrosine kinases in activation of the MAP kinase cascade by G-protein-coupled receptors. Nature 380, 541–544 (1996).
Ma, Y. C. & Huang, X. Y. Identification of the binding site for Gqα on its effector Bruton’s tyrosine kinase. Proc. Natl Acad. Sci. USA 95, 12197–12201 (1998).
Acknowledgements
We thank members of our research groups for helpful discussions and comments on the manuscript. We thank A. Inoue (Tohoku University, Japan) for the Gα-depleted HEK293 cells. This work was supported by NIH grant no. GM138676 (X.-Y.H.), the Josie Robertson Investigators Program (R.K.H.) and the Searle Scholars Program (R.K.H.). The Simons Electron Microscopy Center and the National Resource for Automated Molecular Microscopy located at the New York Structural Biology Center are supported by grants from the NIH National Institute of General Medical Sciences (GM103310), NYSTAR and the Simons Foundation (SF349247). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
K.O.A. expressed and purified β1-AR, Gαi, Gβ1γ2 and the protein complexes, made cryo-EM grids, performed cryo-EM screening, data collection, image processing, determined the EM density map and performed the functional studies. N.P. made cryo-EM grids, performed data collection, image processing, EM density map determination and model building. M.S. purified β1-AR and the complex of β1-AR–Gi, provided the β1-AR–Gs structural information and prepared some figures. J.-S.L. performed the functional studies. J.H. generated the β1-AR and G-protein constructs. K.D.J. and E.T.E. performed negative-stain EM and data collection. J.R.M. performed image processing. R.K.H. supervised the project and performed image processing, EM density map determination, model building and data interpretation. X.-Y.H. supervised the project, interpreted data and wrote the manuscript. All authors contributed to the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Structural & Molecular Biology thanks Qing Fan and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available. Florian Ullrich was the primary editor on this article and managed its editorial process and peer review in collaboration with the rest of the editorial team.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Summary of cryo-EM data processing.
Details are found in the methods. In short, 2680 micrographs were collected on a Krios equipped with Gatan K2 direct electron detector. Heterogeneous refinement was used to remove junk, with 2D classification as a form of verification. Once an ideal particle stack was identified, subsequent rounds of heterogeneous refinement were combined with local CTF refinement and Bayesian polishing to further improve the map. Local refinements of b1-AR and Gi helped bring out features in the periphery of the structure. The model was built into the consensus and local refinements, and then used to generate a composite map that was used for the final round of real-space refinement. Overall, even in the consensus structure, almost all of the model was well represented by density. The alpha-helical domain was less well resolved due to flexibility, but intermediate resolution rigid-body docking was possible.
Extended Data Fig. 2 Per residue model-map correlation coefficient plots.
Per residue model-map correlation coefficient was calculated using phenix.real_space_refine with maps pre (red line) and post (black line) density modification. In both β1-AR–Gi complex and β1-AR–Gi/s complex models, chain A is the Ras-like domain of Gα, Chain B is Gβ1, Chain G is Gγ2, Chain HD is the helical domain of Gα, and Chain R is β1-AR. Comparing model-map correction coefficient pre and post density modification, especially for β1-AR–Gi complex, density modification shows a clear improvement across all chains.
Extended Data Fig. 3 Comparison of the inactive β1-AR receptor with the activated β1-AR in complex with Gi.
(a-c) Side (left), extracellular (top right) and cytoplasmic (bottom right) views of the inactive β1-AR (grey)(PDB code 4GPO) compared to the activated β1-AR in complex with Gi. (d) Structural comparison of the Gi α5-helix binding region to show the major conformational changes of β1-AR. (e) The ionic lock between Arg139 and Glu285 is broken in the active state. (f) Tyr343 packs against Leu132 and Ile135 in the active state. (g) Cryo-EM density map of the Gi α5-helix binding region (contoured at 1.2 σ level).
Extended Data Fig. 4 Isoproterenol occupancy in orthosteric binding pocket of β1-AR.
(a,b) Side view (a) and oblique view (b) of activated β1-AR to highlight the location of the agonist isoproterenol bound in the orthosteric binding pocket at the extracellular side of the receptor. β1-AR is shown in cartoon in red and isoproterenol is shown in green. Experimental density carved around ligand shown at 13σ, supporting complete occupancy. (c) Close-up view of the orthosteric binding pocket with model docked into 8σ experimental density to highlight agonist coordination. Isoproterenol is colored in green and residues lining the binding pocket are in red.
Extended Data Fig. 5 Rotational opening of the α-helical domain during G-protein activation by GPCRs.
(a) Structure of Gαi in the complex of β1-AR–Gi shows the rotational opening of the α-helical domain away from the Ras-like domain. (b) Comparison of the structures of Gαi in the complex of β1-AR–Gi (in green and orange) and in the inactive GDP-bound Gi crystal structure (in gray; PDB: 1GG2). (c) View from the receptor towards the cytoplasmic end shows the rotation of the α-helical domain from the position in inactive Gi (in gray) to the location in the β1-AR–Gi complex (in orange). (d) View from Gβγ towards the Ras-like domain shows the position of the α-helical domain relative to Gβ. (e and f) Comparisons of the locations of the α-helical domains in the complexes of β2-AR–Gs (PDB: 3SN6), β1-AR–Gs (PDB: 7JJO), rhodopsin–Gi (PDB: 6CMO), and β1-AR–Gi (this paper).
Extended Data Fig. 6 Conformational changes of the GDP/GTP-binding pocket of Gαi after β1-AR interaction.
(a) Comparison of the β1 strand, α1-helix and the β1-α1 loop of the Ras-like domains from β1-AR–Gi (in green) and from Gαi1Gβ1Gγ2 (PDB: 1GG2; in gray) when the Ras-like domains are superimposed. (b) Comparison of Switch II region from β1-AR–Gi and from Gαi1Gβ1Gγ2. (c) Comparison of Switch III region from β1-AR–Gi and from Gαi1Gβ1Gγ2. (d) Comparison of the regions from αG to α5-helix from β1-AR–Gi and from Gαi1Gβ1Gγ2. (E) Comparison of all GDP-interacting residues of the Ras-like domains from β1-AR–Gi and from Gαi1Gβ1Gγ2.
Extended Data Fig. 7 Structural changes of G-proteins during the activation.
Separation of the Ras-like domains of Gi (a) and Gs (b) from Gβγ subunits in the complex of β1-AR–Gi (a) and β1-AR–Gs (b). Gβγ subunits are superimposed.
Extended Data Fig. 8 Conformational changes of the GDP-binding pocket of Gαs after β1-AR interaction.
(a) Comparison of the β1 strand, α1-helix and the β1-α1 loop of the Ras-like domains from β1-AR–Gs (in violet) and from GαsGβ1Gγ2 (PDB: 6EG8; in teal) when the Ras-like domains are superimposed. (b) Comparison of Switch II region from β1-AR–Gs and from GαsGβ1Gγ2. (c) Comparison of Switch III region from β1-AR–Gs and from GαsGβ1Gγ2. (d) Comparison of the regions from αG to α5-helix from β1-AR–Gs and from GαsGβ1Gγ2. (e) Comparison of all GDP-interacting residues of the Ras-like domains from β1-AR–Gs and from GαsGβ1Gγ2.(f) Disruptions of intra-molecular interactions of Gαs during Gs activation by β1-AR. An interaction between the sidechain of His373 in the α5-helix and the backbone of Arg38 in the αN-helix is broken. An interacting network involving the sidechain of Gln59 in the α1-helix, the backbone carbonyl of Ala352 in the β6-α5 loop, and the sidechain of Thr355 in the α5-helix is disrupted.
Extended Data Fig. 9 Summary of cryo-EM data processing.
Details are found in the methods. In short, 3,285 micrographs were collected on a Krios equipped with Gatan K2 direct electron detector. Heterogeneous refinement was used to remove junk, with 2D classification as a form of verification. Once an ideal particle stack was identified, subsequent rounds of heterogeneous refinement were combined with local CTF refinement and Bayesian polishing to further improve the map. Local refinement of Gi/s helped bring out features in the periphery of the structure. The model was built into the consensus and local refinements, and then used to generate a composite map that was used for the final round of real-space refinement.
Extended Data Fig. 10 Structural comparisons of the β1-AR–Gi complex with other GPCR–Gi complexes.
(a) The structure of β1-AR–Gi1 (in green) is superimposed with rhodopsin–Gi1 (PDB 6CMO, in magenta), cannabinoid receptor 1–Gi1 (PDB 6N4B, in cyan), and mu-opioid receptor–Gi1 (PDB 6DDE, in yellow). (b) Comparisons of the Gαi1Gβγ trimer structures in the four complexes (superimposed by Gβ subunits). (c) Comparisons of the interactions between ICL2s of the receptors and the αN-helices of Gαi1. (d) Comparisons of the interactions between the α5-helices of Gαi1 and the receptors. (e) Structural comparison of the regions around G203 of Gαi1 in the four structures.
Supplementary information
Supplementary Information
Supplementary Discussion, Figs. 1–3 and the full scan images for Supplementary Fig. 1b.
Source data
Source Data Fig. 3
Statistical source data.
Source Data Fig. 7
Statistical source data.
Rights and permissions
About this article
Cite this article
Alegre, K.O., Paknejad, N., Su, M. et al. Structural basis and mechanism of activation of two different families of G proteins by the same GPCR. Nat Struct Mol Biol 28, 936–944 (2021). https://doi.org/10.1038/s41594-021-00679-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41594-021-00679-2
This article is cited by
-
Binding kinetics drive G protein subtype selectivity at the β1-adrenergic receptor
Nature Communications (2024)
-
Structural titration reveals Ca2+-dependent conformational landscape of the IP3 receptor
Nature Communications (2023)
-
Structural basis of agonist specificity of α1A-adrenergic receptor
Nature Communications (2023)
-
Ligand recognition and activation of neuromedin U receptor 2
Nature Communications (2022)
-
Differential activation mechanisms of lipid GPCRs by lysophosphatidic acid and sphingosine 1-phosphate
Nature Communications (2022)