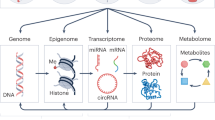
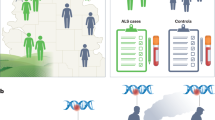
Abstract
Over the past few decades, numerous environmental chemicals from solvents to pesticides have been suggested to be involved in the development and progression of neurodegenerative diseases. Most of the evidence has accumulated from occupational or cohort studies in humans or laboratory research in animal models, with a range of chemicals being implicated. What has been missing is a systematic approach analogous to genome-wide association studies, which have identified dozens of genes involved in Alzheimer’s disease, Parkinson’s disease and other neurodegenerative diseases. Fortunately, it is now possible to study hundreds to thousands of chemical features under the exposome framework. This Perspective explores how advances in mass spectrometry make it possible to generate exposomic data to complement genomic data and thereby better understand neurodegenerative diseases.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Livingston, G. et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 396, 413–446 (2020).
Ascherio, A. & Schwarzschild, M. A. The epidemiology of Parkinson’s disease: risk factors and prevention. Lancet Neurol. 15, 1257–1272 (2016).
Schneider, L. A resurrection of aducanumab for Alzheimer’s disease. Lancet Neurol. 19, 111–112 (2020).
van Dyck, C. H. et al. Lecanemab in early Alzheimer’s disease. N. Engl. J. Med. 388, 9–21 (2023).
Gan, L., Cookson, M. R., Petrucelli, L. & La Spada, A. R. Converging pathways in neurodegeneration, from genetics to mechanisms. Nat. Neurosci. 21, 1300–1309 (2018).
Nussbaum, R. L. & Ellis, C. E. Alzheimer’s disease and Parkinson’s disease. N. Engl. J. Med. 348, 1356–1364 (2003).
Kunkle, B. W. et al. Genetic meta-analysis of diagnosed Alzheimer’s disease identifies new risk loci and implicates Aβ, tau, immunity and lipid processing. Nat. Genet. 51, 414 (2019).
Keller, M. F. et al. Using genome-wide complex trait analysis to quantify ‘missing heritability’ in Parkinson’s disease. Hum. Mol. Genet. 21, 4996–5009 (2012).
Rappaport, S. M. Genetic factors are not the major causes of chronic diseases. PLoS ONE 11, e0154387 (2016).
INSERM Collective Expertise Centre. Effects of Pesticides on Health: New Data https://doi.org/10.1051/978-2-7598-2721-3 (EDP Sciences, 2022).
Goldman, S. M. et al. Risk of Parkinson disease among service members at Marine Corps Base Camp Lejeune. JAMA Neurol. https://doi.org/10.1001/jamaneurol.2023.1168 (2023).
Landrigan, P. J. et al. The Lancet Commission on pollution and health. Lancet 391, 462–512 (2018).
Jokanović, M., Oleksak, P. & Kuca, K. Multiple neurological effects associated with exposure to organophosphorus pesticides in man. Toxicology 484, 153407 (2023).
Woodruff, T. J., Zota, A. R. & Schwartz, J. M. Environmental chemicals in pregnant women in the United States: NHANES 2003–2004. Environ. Health Perspect. 119, 878–885 (2011).
Govarts, E. et al. Harmonized human biomonitoring in European children, teenagers and adults: EU-wide exposure data of 11 chemical substance groups from the HBM4EU Aligned Studies (2014–2021). Int. J. Hyg. Environ. Health 249, 114119 (2023).
Wild, C. P. Complementing the genome with an ‘exposome’: the outstanding challenge of environmental exposure measurement in molecular epidemiology. Cancer Epidemiol. Biomarkers Prev. 14, 1847–1850 (2005).
Gunnarsson, L. -G. & Bodin, L. Occupational exposures and neurodegenerative diseases-a systematic literature review and meta-analyses. Int. J. Environ. Res. Public Health 16, 337 (2019).
Brouwer, M. et al. Environmental exposure to pesticides and the risk of Parkinson’s disease in the Netherlands. Environ. Int. 107, 100–110 (2017).
Paul, K. C. et al. A pesticide and iPSC dopaminergic neuron screen identifies and classifies Parkinson-relevant pesticides. Nat. Commun. 14, 2803 (2023).
Richardson, J. R. et al. Elevated serum pesticide levels and risk for Alzheimer disease. JAMA Neurol. 71, 284–290 (2014).
Ross, G. W. et al. Association of brain heptachlor epoxide and other organochlorine compounds with lewy pathology. Mov. Disord. 34, 228–235 (2019).
Weisskopf, M. G. et al. Persistent organochlorine pesticides in serum and risk of Parkinson disease. Neurology 74, 1055–1061 (2010).
Medehouenou, T. C. M. et al. Exposure to polychlorinated biphenyls and organochlorine pesticides and risk of dementia, Alzheimer’s disease and cognitive decline in an older population: a prospective analysis from the Canadian Study of Health and Aging. Environ. Health 18, 57 (2019).
Cicero, C. E. et al. Metals and neurodegenerative diseases. A systematic review. Environ. Res. 159, 82–94 (2017).
Zhao, Y., Ray, A., Portengen, L., Vermeulen, R. & Peters, S. Metal exposure and risk of Parkinson’s disease: a systematic review and meta-analysis. Am. J. Epidemiol. https://doi.org/10.1093/aje/kwad082 (2023).
Goldman, S. M. et al. Solvent exposures and Parkinson disease risk in twins. Ann. Neurol. 71, 776–784 (2012).
Nielsen, S. S. et al. Solvent exposed occupations and risk of Parkinson disease in Finland. Clin. Park. Relat. Disord. 4, 100092 (2021).
Koeman, T. et al. Occupational exposures and risk of dementia-related mortality in the prospective Netherlands Cohort Study. Am. J. Ind. Med. 58, 625–635 (2015).
Letellier, N. et al. Association between occupational solvent exposure and cognitive performance in the French CONSTANCES study. Occup. Environ. Med. 77, 223–230 (2020).
Hu, C. -Y. et al. Association between ambient air pollution and Parkinson’s disease: systematic review and meta-analysis. Environ. Res. 168, 448–459 (2019).
Gong, Y. et al. Global ambient particulate matter pollution and neurodegenerative disorders: a systematic review of literature and meta-analysis. Environ. Sci. Pollut. Res. 30, 39418–39430 (2023).
Weuve, J. et al. Exposure to air pollution in relation to risk of dementia and related outcomes: an updated systematic review of the epidemiological literature. Environ. Health Perspect. 129, 096001 (2021).
Best, E. A., Juarez-Colunga, E., James, K., LeBlanc, W. G. & Serdar, B. Biomarkers of exposure to polycyclic aromatic hydrocarbons and cognitive function among elderly in the United States (National Health and Nutrition Examination Survey: 2001–2002). PLoS ONE 11, e0147632 (2016).
Park, S. K., Ding, N. & Han, D. Perfluoroalkyl substances and cognitive function in older adults: should we consider non-monotonic dose-responses and chronic kidney disease? Environ. Res. 192, 110346 (2021).
Weng, X. et al. Association between mixed exposure of phthalates and cognitive function among the US elderly from NHANES 2011–2014: three statistical models. Sci. Total Environ. 828, 154362 (2022).
Shi, Y. et al. Association between exposure to phenols and parabens and cognitive function in older adults in the United States: a cross-sectional study. Sci. Total Environ. 858, 160129 (2023).
Rock, K. D. & Patisaul, H. B. Environmental mechanisms of neurodevelopmental toxicity. Curr. Environ. Health Rep. 5, 145–157 (2018).
Vrijheid, M., Casas, M., Gascon, M., Valvi, D. & Nieuwenhuijsen, M. Environmental pollutants and child health—a review of recent concerns. Int. J. Hyg. Environ. Health 219, 331–342 (2016).
Modgil, S., Lahiri, D. K., Sharma, V. L. & Anand, A. Role of early life exposure and environment on neurodegeneration: implications on brain disorders. Transl. Neurodegener. 3, 9 (2014).
Tanner, C. M., Goldman, S. M., Ross, G. W. & Grate, S. J. The disease intersection of susceptibility and exposure: chemical exposures and neurodegenerative disease risk. Alzheimers Dement. 10, S213–S225 (2014).
Shi, J.-Q. et al. NLRP3 inflammasome: a potential therapeutic target in fine particulate matter-induced neuroinflammation in Alzheimer’s disease. J. Alzheimers Dis. 77, 923–934 (2020).
Cresto, N. et al. Pesticides at brain borders: impact on the blood–brain barrier, neuroinflammation, and neurological risk trajectories. Chemosphere 324, 138251 (2023).
Nougadère, A. et al. Total diet study on pesticide residues in France: levels in food as consumed and chronic dietary risk to consumers. Environ. Int. 45, 135–150 (2012).
Turusov, V., Rakitsky, V. & Tomatis, L. Dichlorodiphenyltrichloroethane (DDT): ubiquity, persistence, and risks. Environ. Health Perspect. 110, 125–128 (2002).
Costas-Ferreira, C. & Faro, L. R. F. Neurotoxic Effects of neonicotinoids on mammals: what is there beyond the activation of nicotinic acetylcholine receptors?—a systematic review. Int. J. Mol. Sci. 22, 8413 (2021).
Andersen, H. R. et al. Pyrethroids and developmental neurotoxicity—a critical review of epidemiological studies and supporting mechanistic evidence. Environ. Res. 214, 113935 (2022).
Wan, F. et al. The pyrethroids metabolite 3-phenoxybenzoic acid induces dopaminergic degeneration. Sci. Total Environ. 838, 156027 (2022).
Jiao, Z., Wu, Y. & Qu, S. Fenpropathrin induces degeneration of dopaminergic neurons via disruption of the mitochondrial quality control system. Cell Death Discov. 6, 78 (2020).
Loser, D. et al. Functional alterations by a subgroup of neonicotinoid pesticides in human dopaminergic neurons. Arch. Toxicol. 95, 2081–2107 (2021).
Gan, W., Manning, K. J., Cleary, E. G., Fortinsky, R. H. & Brugge, D. Exposure to ultrafine particles and cognitive decline among older people in the United States. Environ. Res. 227, 115768 (2023).
Oberdörster, G., Elder, A. & Rinderknecht, A. Nanoparticles and the brain: cause for concern? J. Nanosci. Nanotechnol. 9, 4996–5007 (2009).
Prüst, M., Meijer, J. & Westerink, R. H. S. The plastic brain: neurotoxicity of micro- and nanoplastics. Part. Fibre Toxicol. 17, 24 (2020).
Shan, S., Zhang, Y., Zhao, H., Zeng, T. & Zhao, X. Polystyrene nanoplastics penetrate across the blood–brain barrier and induce activation of microglia in the brain of mice. Chemosphere 298, 134261 (2022).
Cryan, J. F., O’Riordan, K. J., Sandhu, K., Peterson, V. & Dinan, T. G. The gut microbiome in neurological disorders. Lancet Neurol. 19, 179–194 (2020).
Connell, E. et al. Microbial-derived metabolites as a risk factor of age-related cognitive decline and dementia. Mol. Neurodegener. 17, 43 (2022).
Kelly, G. C., Watase, C. K. & Ho, D. H. in Biomarkers in Toxicology https://doi.org/10.1007/978-3-030-87225-0_36-1 (eds. Patel, V. B. et al.) 1–25 (Springer, 2022).
Nicholson, S., Baccarelli, A. & Prada, D. Role of brain extracellular vesicles in air pollution-related cognitive impairment and neurodegeneration. Environ. Res. 204, 112316 (2022).
Ardeshir, R. A., Zolgharnein, H., Movahedinia, A., Salamat, N. & Zabihi, E. Comparison of waterborne and intraperitoneal exposure to fipronil in the Caspian white fish (Rutilus frisii) on acute toxicity and histopathology. Toxicol. Rep. 4, 348–357 (2017).
Meijer, M., Hamers, T. & Westerink, R. H. S. Acute disturbance of calcium homeostasis in PC12 cells as a novel mechanism of action for (sub)micromolar concentrations of organophosphate insecticides. Neurotoxicology 43, 110–116 (2014).
Loser, D. et al. Acute effects of the imidacloprid metabolite desnitro-imidacloprid on human nACh receptors relevant for neuronal signaling. Arch. Toxicol. 95, 3695–3716 (2021).
OECD. Considerations for Assessing the Risks of Combined Exposure to Multiple Chemicals https://doi.org/10.1787/ceca15a9-en (OECD, 2018).
Ashok, A., Rai, N. K., Tripathi, S. & Bandyopadhyay, S. Exposure to As-, Cd-, and Pb-mixture induces Aβ, amyloidogenic APP processing and cognitive impairments via oxidative stress-dependent neuroinflammation in young rats. Toxicol. Sci. 143, 64–80 (2015).
Fitzgerald, E. F. et al. Polybrominated diphenyl ethers (PBDEs), polychlorinated biphenyls (PCBs) and neuropsychological status among older adults in New York. Neurotoxicology 33, 8–15 (2012).
Xu, M. -Y., Wang, P., Sun, Y. -J., Yang, L. & Wu, Y. -J. Joint toxicity of chlorpyrifos and cadmium on the oxidative stress and mitochondrial damage in neuronal cells. Food Chem. Toxicol. 103, 246–252 (2017).
Yoon, J. et al. Urinary phthalate metabolites and slow walking speed in the Korean Elderly Environmental Panel II Study. Environ. Health Perspect. 131, 47005 (2023).
David, A. et al. Towards a comprehensive characterisation of the human internal chemical exposome: challenges and perspectives. Environ. Int. 156, 106630 (2021).
Orešič, M. et al. Metabolome in progression to Alzheimer’s disease. Transl. Psychiatry 1, tp201155 (2011).
Varma, V. R. et al. Brain and blood metabolite signatures of pathology and progression in Alzheimer disease: a targeted metabolomics study. PLoS Med. 15, e1002482 (2018).
Arnold, M. et al. Sex and APOE ε4 genotype modify the Alzheimer’s disease serum metabolome. Nat. Commun. 11, 1148 (2020).
Agin, A. et al. Environmental exposure to phthalates and dementia with Lewy bodies: contribution of metabolomics. J. Neurol. Neurosurg. Psychiatry 91, 968–974 (2020).
Chang, C.-W. et al. Monitoring long-term chemical exposome by characterizing the hair metabolome using a high-resolution mass spectrometry-based suspect screening approach. Chemosphere 332, 138864 (2023).
Niedzwiecki, M. M. et al. High-resolution metabolomic profiling of Alzheimer’s disease in plasma. Ann. Clin. Transl. Neurol. 7, 36–45 (2020).
Huber, C. et al. A large scale multi-laboratory suspect screening of pesticide metabolites in human biomonitoring: from tentative annotations to verified occurrences. Environ. Int. 168, 107452 (2022).
Paul, K. C., Horvath, S., Del Rosario, I., Bronstein, J. M. & Ritz, B. DNA methylation biomarker for cumulative lead exposure is associated with Parkinson’s disease. Clin. Epigenetics 13, 59 (2021).
Farooqui, Z. et al. Associations of cumulative Pb exposure and longitudinal changes in mini-mental status exam scores, global cognition and domains of cognition: The VA Normative Aging Study. Environ. Res. 152, 102–108 (2017).
Liu, W., Wang, B., Xiao, Y., Wang, D. & Chen, W. Secondhand smoking and neurological disease: a meta-analysis of cohort studies. Rev. Environ. Health 36, 271–277 (2021).
Ames, J. et al. Neurocognitive and physical functioning in the Seveso Women’s Health Study. Environ. Res. 162, 55–62 (2018).
Raffetti, E. et al. Polychlorinated biphenyls (PCBs) and risk of dementia and Parkinson disease: a population-based cohort study in a North Italian highly polluted area. Chemosphere 261, 127522 (2020).
Zhao, Y. et al. Association between organophosphorus flame retardants exposure and cognitive impairment among elderly population in southern China. Sci. Total Environ. https://doi.org/10.1016/j.scitotenv.2022.157763 (2022).
Mastrantonio, M. et al. Drinking water contamination from perfluoroalkyl substances (PFAS): an ecological mortality study in the Veneto Region, Italy. Eur. J. Public Health 28, 180–185 (2018).
Balali-Mood, M., Naseri, K., Tahergorabi, Z., Khazdair, M. R. & Sadeghi, M. Toxic mechanisms of five heavy metals: mercury, lead, chromium, cadmium, and arsenic. Front. Pharmacol. 12, 643972 (2021).
Eid, A. et al. Effects of DDT on amyloid precursor protein levels and amyloid beta pathology: mechanistic links to Alzheimer’s disease risk. Environ. Health Perspect. 130, 87005 (2022).
Oyovwi, M. O. et al. Repeated endosulfan exposure induces changes in neurochemicals, decreases ATPase transmembrane ionic-pumps, and increased oxidative/nitrosative stress in the brains of rats: reversal by quercetin. Pestic. Biochem. Physiol. 175, 104833 (2021).
Fernandes, L. S. et al. In vitro study of the neuropathic potential of the organophosphorus compounds trichlorfon and acephate. Toxicol. Vitr. 29, 522–528 (2015).
Taillebois, E., Cartereau, A., Jones, A. K. & Thany, S. H. Neonicotinoid insecticides mode of action on insect nicotinic acetylcholine receptors using binding studies. Pestic. Biochem. Physiol. 151, 59–66 (2018).
Farder-Gomes, C. F. et al. Harmful effects of fipronil exposure on the behavior and brain of the stingless bee Partamona helleri Friese (Hymenoptera: Meliponini). Sci. Total Environ. 794, 148678 (2021).
Hernández-Plata, I., Giordano, M., Díaz-Muñoz, M. & Rodríguez, V. M. The herbicide glyphosate causes behavioral changes and alterations in dopaminergic markers in male Sprague-Dawley rat. Neurotoxicology 46, 79–91 (2015).
Liu, C. et al. Exposure to the environmentally toxic pesticide maneb induces Parkinson’s disease-like neurotoxicity in mice: a combined proteomic and metabolomic analysis. Chemosphere 308, 136344 (2022).
Soares, M. V. et al. Neurotoxicity induced by toluene: In silico and in vivo evidences of mitochondrial dysfunction and dopaminergic neurodegeneration. Environ. Pollut. 298, 118856 (2022).
Wang, Y. et al. Exposure to PM2.5 aggravates Parkinson’s disease via inhibition of autophagy and mitophagy pathway. Toxicology 456, 152770 (2021).
Miyazaki, W., Fujiwara, Y. & Katoh, T. The effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin on the development and function of the blood–brain barrier. Neurotoxicology 52, 64–71 (2016).
Xia, Y. et al. Effects of subchronic exposure to benzo[a]pyrene (B[a]P) on learning and memory, and neurotransmitters in male Sprague–Dawley rat. Neurotoxicology 32, 188–198 (2011).
Park, E. -J. et al. Whole cigarette smoke condensates induce accumulation of amyloid beta precursor protein with oxidative stress in murine astrocytes. Toxics 9, 150 (2021).
Ono, K., Hasegawa, K., Yamada, M. & Naiki, H. Nicotine breaks down preformed Alzheimer’s beta-amyloid fibrils in vitro. Biol. Psychiatry 52, 880–886 (2002).
Costa, L. G., Pellacani, C., Dao, K., Kavanagh, T. J. & Roque, P. J. The brominated flame retardant BDE-47 causes oxidative stress and apoptotic cell death in vitro and in vivo in mice. Neurotoxicology 48, 68–76 (2015).
Moyano, P. et al. Bisphenol A single and repeated treatment increases HDAC2, leading to cholinergic neurotransmission dysfunction and SN56 cholinergic apoptotic cell death through AChE variants overexpression and NGF/TrkA/P75NTR signaling disruption. Food Chem. Toxicol. 157, 112614 (2021).
Huang, W. et al. Effects of di-(2-ethylhexyl) phthalate (DEHP) on behavior and dopamine signaling in zebrafish (Danio rerio). Environ. Toxicol. Pharmacol. 93, 103885 (2022).
Yu, Y. et al. Perfluorooctane sulfonate disrupts the blood brain barrier through the crosstalk between endothelial cells and astrocytes in mice. Environ. Pollut. 256, 113429 (2020).
Dührkop, K. et al. SIRIUS 4: a rapid tool for turning tandem mass spectra into metabolite structure information. Nat. Methods 16, 299–302 (2019).
Quinn, R. A. et al. Molecular networking as a drug discovery, drug metabolism, and precision medicine strategy. Trends Pharmacol. Sci. 38, 143–154 (2017).
Acknowledgements
S.L.-A., J.C., F.M., A.D. and C.S. were funded by a grant from the ‘Fondation pour la Recherche Medicale’ (ENV202003011520). They acknowledge L. Rieusset for her contribution to this work. C.S. also received research support from the ‘Fondation de France’ (00130155). C.S. acknowledges the Precision and global Vascular Brain Health Institute funded by the France 2030 investment plan (Instituts Hospitalo-Universitaires vague 3, IHU3 initiative). J.C., F.M., X.C., R.B. and A.D. acknowledge the research infrastructure France Exposome.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Neuroscience thanks Alice Chen-Plotkin and David Jett for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lefèvre-Arbogast, S., Chaker, J., Mercier, F. et al. Assessing the contribution of the chemical exposome to neurodegenerative disease. Nat Neurosci 27, 812–821 (2024). https://doi.org/10.1038/s41593-024-01627-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41593-024-01627-1