Abstract
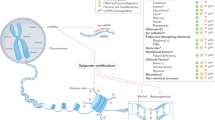
Amyotrophic lateral sclerosis (ALS) is a fatal disease of motor neuron degeneration with typical survival of only 2–5 years from diagnosis. The causes of ALS are multifactorial: known genetic mutations account for only around 70% of cases of familial ALS and 15% of sporadic cases, and heritability estimates range from 8% to 61%, indicating additional causes beyond genetics. Consequently, interest has grown in environmental contributions to ALS risk and progression. The gene–time–environment hypothesis posits that ALS onset occurs through an interaction of genes with environmental exposures during ageing. An alternative hypothesis, the multistep model of ALS, suggests that several hits, at least some of which could be environmental, are required to trigger disease onset, even in the presence of highly penetrant ALS-associated mutations. Studies have sought to characterize the ALS exposome — the lifetime accumulation of environmental exposures that increase disease risk and affect progression. Identifying the full scope of environmental toxicants that enhance ALS risk raises the prospect of preventing disease by eliminating or mitigating exposures. In this Review, we summarize the evidence for an ALS exposome, discussing the strengths and limitations of epidemiological studies that have identified contributions from various sources. We also consider potential mechanisms of exposure-mediated toxicity and suggest future directions for ALS exposome research.
Key points
-
Amyotrophic lateral sclerosis (ALS) is a fatal disease of motor neuron degeneration, with both genetic and environmental factors contributing to the risk and rate of disease progression.
-
The gene–time–environment hypothesis of ALS posits that disease arises from an interaction of genetic burden with environmental burden over the life course.
-
The multistep model of ALS posits that multiple ‘hits’, some of which are presumed to be environmental in origin, trigger disease onset, even in carriers of highly penetrant mutations.
-
Epidemiological studies suggest potential contributions to the ALS exposome from pesticides, occupational exposures, sports and physical activity, metals, air pollution, trauma, electromagnetic fields, the gut microbiome, diet and lifestyle factors.
-
The mechanisms underlying the effects of environmental factors on ALS risk remain incompletely understood but might involve neurotoxicity from specific environmental toxins, microbiome-mediated changes, epigenetic restructuring, systemic and central inflammation and excitotoxicity.
-
Most studies of the ALS exposome have a retrospective design using questionnaires and are, therefore, prone to recall bias and other limitations. Future studies will require prospective, longitudinal designs that include quantification of exposures in biosamples in addition to questionnaires.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Feldman, E. L. et al. Amyotrophic lateral sclerosis. Lancet 400, 1363–1380 (2022).
Brown, R. H. & Al-Chalabi, A. Amyotrophic lateral sclerosis. N. Engl. J. Med. 377, 162–172 (2017).
Goutman, S. A. et al. Recent advances in the diagnosis and prognosis of amyotrophic lateral sclerosis. Lancet Neurol. 21, 480–493 (2022).
Goutman, S. A. et al. Emerging insights into the complex genetics and pathophysiology of amyotrophic lateral sclerosis. Lancet Neurol. 21, 465–479 (2022).
Al-Chalabi, A. & Hardiman, O. The epidemiology of ALS: a conspiracy of genes, environment and time. Nat. Rev. Neurol. 9, 617–628 (2013).
Chio, A. et al. The multistep hypothesis of ALS revisited: the role of genetic mutations. Neurology 91, e635–e642 (2018).
Wild, C. P. Complementing the genome with an ‘exposome’: the outstanding challenge of environmental exposure measurement in molecular epidemiology. Cancer Epidemiol. Biomark. Prev. 14, 1847–1850 (2005).
Vineis, P. et al. What is new in the exposome? Environ. Int. 143, 105887 (2020).
Vermeulen, R., Schymanski, E. L., Barabási, A. L. & Miller, G. W. The exposome and health: where chemistry meets biology. Science 367, 392–396 (2020).
Jiang, C. et al. Dynamic human environmental exposome revealed by longitudinal personal monitoring. Cell 175, 277–291.e231 (2018).
Benatar, M. et al. A roadmap to ALS prevention: strategies and priorities. J. Neurol. Neurosurg. Psychiatry 94, 399–402 (2023).
Marin, B. et al. Variation in worldwide incidence of amyotrophic lateral sclerosis: a meta-analysis. Int. J. Epidemiol. 46, 57–74 (2017).
Chernoff, N. et al. A critical review of the postulated role of the non-essential amino acid, β-N-methylamino-l-alanine, in neurodegenerative disease in humans. J. Toxicol. Environ. Health B Crit. Rev. 20, 1–47 (2017).
Spencer, P. S. Hypothesis: etiologic and molecular mechanistic leads for sporadic neurodegenerative diseases based on experience with Western Pacific ALS/PDC. Front. Neurol. 10, 754 (2019).
Luker, J., Woodman, R. & Schultz, D. The incidence and prevalence of motor neurone disease in South Australia. Amyotroph. Lateral Scler. Frontotemporal Degener. 24, 195–202 (2023).
Newell, M. E., Adhikari, S. & Halden, R. U. Systematic and state-of the science review of the role of environmental factors in amyotrophic lateral sclerosis (ALS) or Lou Gehrig’s disease. Sci. Total Environ. 817, 152504 (2022).
Schwartz, G. G., Rundquist, B. C., Simon, I. J. & Swartz, S. E. Geographic distributions of motor neuron disease mortality and well water use in U.S. counties. Amyotroph. Lateral Scler. Frontotemporal Degener. 18, 279–283 (2017).
Torbick, N., Hession, S., Stommel, E. & Caller, T. Mapping amyotrophic lateral sclerosis lake risk factors across northern New England. Int. J. Health Geogr. 13, 1 (2014).
Brown, C. A., Lally, C., Kupelian, V. & Flanders, W. D. Estimated prevalence and incidence of amyotrophic lateral sclerosis and SOD1 and C9orf72 genetic variants. Neuroepidemiology 55, 342–353 (2021).
Elden, A. C. et al. Ataxin-2 intermediate-length polyglutamine expansions are associated with increased risk for ALS. Nature 466, 1069–1075 (2010).
Julian, T. H. et al. Physical exercise is a risk factor for amyotrophic lateral sclerosis: convergent evidence from Mendelian randomisation, transcriptomics and risk genotypes. eBioMedicine 68, 103397 (2021).
Mandrioli, J. et al. Elevated levels of selenium species in cerebrospinal fluid of amyotrophic lateral sclerosis patients with disease-associated gene mutations. Neurodegener. Dis. 17, 171–180 (2017).
McCann, E. P. et al. Evidence for polygenic and oligogenic basis of Australian sporadic amyotrophic lateral sclerosis. J. Med. Genet. https://doi.org/10.1136/jmedgenet-2020-106866 (2020).
Bandres-Ciga, S. et al. Shared polygenic risk and causal inferences in amyotrophic lateral sclerosis. Ann. Neurol. 85, 470–481 (2019).
van Rheenen, W. et al. Genome-wide association analyses identify new risk variants and the genetic architecture of amyotrophic lateral sclerosis. Nat. Genet. 48, 1043–1048 (2016).
Al-Chalabi, A. et al. An estimate of amyotrophic lateral sclerosis heritability using twin data. J. Neurol. Neurosurg. Psychiatry 81, 1324–1326 (2010).
McComas, A. J., Upton, A. R. M. & Sica, R. E. P. Motoneurone disease and ageing. Lancet 302, 1477–1480 (1973).
Al-Chalabi, A. et al. Analysis of amyotrophic lateral sclerosis as a multistep process: a population-based modelling study. Lancet Neurol. 13, 1108–1113 (2014).
Vucic, S. et al. Amyotrophic lateral sclerosis as a multi-step process: an Australia population study. Amyotroph. Lateral Scler. Frontotemporal Degener. 20, 532–537 (2019).
Vucic, S. et al. ALS is a multistep process in South Korean, Japanese, and Australian patients. Neurology 94, e1657–e1663 (2020).
Armitage, P. & Doll, R. The age distribution of cancer and a multi-stage theory of carcinogenesis. Br. J. Cancer 8, 1–12 (1954).
Dardiotis, E. et al. Genetic polymorphisms in amyotrophic lateral sclerosis: evidence for implication in detoxification pathways of environmental toxicants. Environ. Int. 116, 122–135 (2018).
Vasta, R., Chia, R., Traynor, B. J. & Chiò, A. Unraveling the complex interplay between genes, environment, and climate in ALS. eBioMedicine 75, 103795 (2022).
Spencer, P. S. Parkinsonism and motor neuron disorders: lessons from Western Pacific ALS/PDC. J. Neurol. Sci. 433, 120021 (2022).
Wang, M. D., Little, J., Gomes, J., Cashman, N. R. & Krewski, D. Identification of risk factors associated with onset and progression of amyotrophic lateral sclerosis using systematic review and meta-analysis. Neurotoxicology 61, 101–130 (2017).
Gunnarsson, L. G. & Bodin, L. Amyotrophic lateral sclerosis and occupational exposures: a systematic literature review and meta-analyses. Int. J. Environ. Res. Public Health 15, 2371 (2018).
Su, F. C. et al. Association of environmental toxins with amyotrophic lateral sclerosis. JAMA Neurol. 73, 803–811 (2016).
Vinceti, M. et al. Pesticides, polychlorinated biphenyls and polycyclic aromatic hydrocarbons in cerebrospinal fluid of amyotrophic lateral sclerosis patients: a case–control study. Environ. Res. 155, 261–267 (2017).
Vinceti, M. et al. Pesticide exposure assessed through agricultural crop proximity and risk of amyotrophic lateral sclerosis. Environ. Health 16, 91 (2017).
Andrew, A. et al. Pesticides applied to crops and amyotrophic lateral sclerosis risk in the U.S. Neurotoxicology 87, 128–135 (2021).
Andrew, A. et al. Airborne lead and polychlorinated biphenyls (PCBs) are associated with amyotrophic lateral sclerosis (ALS) risk in the U.S. Sci. Total Environ. 819, 153096 (2022).
Goutman, S. A. et al. Associations of self-reported occupational exposures and settings to ALS: a case–control study. Int. Arch. Occup. Environ. Health 95, 1567–1586 (2022).
Goutman, S. A. et al. Occupational history associates with ALS survival and onset segment. Amyotroph. Lateral Scler. Frontotemporal Degener. 24, 219–229 (2022).
Filippini, T. et al. Environmental and occupational risk factors of amyotrophic lateral sclerosis: a population-based case–control study. Int. J. Environ. Res. Public Health 17, 2882 (2020).
Andrew, A. S. et al. Risk factors for amyotrophic lateral sclerosis: a regional United States case–control study. Muscle Nerve 63, 52–59 (2021).
Andrew, A. S. et al. Environmental and occupational exposures and amyotrophic lateral sclerosis in New England. Neurodegener. Dis. 17, 110–116 (2017).
Peters, T. L. et al. Occupational exposures and the risk of amyotrophic lateral sclerosis. Occup. Environ. Med. 74, 87–92 (2017).
Dickerson, A. S., Hansen, J., Gredal, O. & Weisskopf, M. G. Study of occupational chromium, iron, and nickel exposure and amyotrophic lateral sclerosis in Denmark. Int. J. Environ. Res. Public Health 17, 8086 (2020).
Dickerson, A. S., Hansen, J., Specht, A. J., Gredal, O. & Weisskopf, M. G. Population-based study of amyotrophic lateral sclerosis and occupational lead exposure in Denmark. Occup. Environ. Med. 76, 208–214 (2019).
Mitsumoto, H. et al. Case–control study in ALS using the National ALS Registry: lead and agricultural chemicals are potential risk factors. Amyotroph. Lateral Scler. Frontotemporal Degener. 23, 190–202 (2022).
Visser, A. E. et al. Multicentre, population-based, case–control study of particulates, combustion products and amyotrophic lateral sclerosis risk. J. Neurol. Neurosurg. Psychiatry 90, 854–860 (2019).
Dickerson, A. S., Hansen, J., Gredal, O. & Weisskopf, M. G. Amyotrophic lateral sclerosis and exposure to diesel exhaust in a Danish cohort. Am. J. Epidemiol. 187, 1613–1622 (2018).
Seals, R. M., Kioumourtzoglou, M. A., Gredal, O., Hansen, J. & Weisskopf, M. G. Occupational formaldehyde and amyotrophic lateral sclerosis. Eur. J. Epidemiol. 32, 893–899 (2017).
McKay, K. A. et al. Military service and related risk factors for amyotrophic lateral sclerosis. Acta Neurol. Scand. 143, 39–50 (2021).
Cragg, J. J., Johnson, N. J. & Weisskopf, M. G. Military service and amyotrophic lateral sclerosis in a population-based cohort: extended follow-up 1979-2011. Epidemiology 28, e15–e16 (2017).
Sagiraju, H. K. R. et al. Amyotrophic lateral sclerosis among veterans deployed in support of post-9/11 U.S. conflicts. Mil. Med. 185, e501–e509 (2020).
Xu, L. et al. Global variation in prevalence and incidence of amyotrophic lateral sclerosis: a systematic review and meta-analysis. J. Neurol. 267, 944–953 (2020).
Bellomo, G. et al. A systematic review on the risk of neurodegenerative diseases and neurocognitive disorders in professional and varsity athletes. Neurol. Sci. 43, 6667–6691 (2022).
Daneshvar, D. H. et al. Incidence of and mortality from amyotrophic lateral sclerosis in National Football League athletes. JAMA Netw. Open 4, e2138801 (2021).
Pupillo, E. et al. Increased risk and early onset of ALS in professional players from Italian Soccer Teams. Amyotroph. Lateral Scler. Frontotemporal Degener. 21, 403–409 (2020).
Russell, E. R. et al. Association of field position and career length with risk of neurodegenerative disease in male former professional soccer players. JAMA Neurol. 78, 1057–1063 (2021).
Fang, F. et al. Amyotrophic lateral sclerosis among cross-country skiers in Sweden. Eur. J. Epidemiol. 31, 247–253 (2016).
Chapman, L., Cooper-Knock, J. & Shaw, P. J. Physical activity as an exogenous risk factor for amyotrophic lateral sclerosis: a review of the evidence. Brain 146, 1745–1757 (2023).
Figueroa-Romero, C. et al. Early life metal dysregulation in amyotrophic lateral sclerosis. Ann. Clin. Transl. Neurol. 7, 872–882 (2020).
Andrew, A. S. et al. Toenail mercury levels are associated with amyotrophic lateral sclerosis risk. Muscle Nerve https://doi.org/10.1002/mus.26055 (2018).
Andrew, A. S. et al. Keratinous biomarker of mercury exposure associated with amyotrophic lateral sclerosis risk in a nationwide U.S. study. Amyotroph. Lateral Scler. Frontotemporal Degener. 21, 420–427 (2020).
Peters, S. et al. Blood metal levels and amyotrophic lateral sclerosis risk: a prospective cohort. Ann. Neurol. 89, 125–133 (2021).
Vinceti, M. et al. Lead, cadmium and mercury in cerebrospinal fluid and risk of amyotrophic lateral sclerosis: a case–control study. J. Trace Elem. Med. Biol. 43, 121–125 (2017).
Guidotti, T. L., Audette, R. J. & Martin, C. J. Interpretation of the trace metal analysis profile for patients occupationally exposed to metals. Occup. Med. 47, 497–503 (1997).
Rooney, J. et al. No association between soil constituents and amyotrophic lateral sclerosis relative risk in Ireland. Environ. Res. 147, 102–107 (2016).
Parks, R. M. et al. Long-term traffic-related air pollutant exposure and amyotrophic lateral sclerosis diagnosis in Denmark: a Bayesian hierarchical analysis. Epidemiology 33, 757–766 (2022).
Yu, Z. et al. Long-term exposure to ultrafine particles and particulate matter constituents and the risk of amyotrophic lateral sclerosis. Environ. Health Perspect. 129, 97702 (2021).
Seelen, M. et al. Long-term air pollution exposure and amyotrophic lateral sclerosis in Netherlands: a population-based case–control study. Environ. Health Perspect. 125, 097023 (2017).
Malek, A. M. et al. Long-term air pollution and risk of amyotrophic lateral sclerosis mortality in the Women’s Health Initiative cohort. Environ. Res. 216, 114510 (2022).
Filippini, T. et al. Risk of amyotrophic lateral sclerosis and exposure to particulate matter from vehicular traffic: a case–control study. Int. J. Environ. Res. Public Health 18, 973 (2021).
Nunez, Y. et al. PM(2.5) composition and disease aggravation in amyotrophic lateral sclerosis: an analysis of long-term exposure to components of fine particulate matter in New York State. Environ. Epidemiol. 6, e204 (2022).
Myung, W., Lee, H. & Kim, H. Short-term air pollution exposure and emergency department visits for amyotrophic lateral sclerosis: a time-stratified case-crossover analysis. Environ. Int. 123, 467–475 (2019).
Thomsen, G. M. et al. A model of recurrent concussion that leads to long-term motor deficits, CTE-like tauopathy and exacerbation of an ALS phenotype. J. Trauma Acute Care Surg. 81, 1070–1079 (2016).
Anderson, E. N. et al. Traumatic injury induces stress granule formation and enhances motor dysfunctions in ALS/FTD models. Hum. Mol. Genet. 27, 1366–1381 (2018).
Walt, G. S. et al. Chronic traumatic encephalopathy within an amyotrophic lateral sclerosis brain bank cohort. J. Neuropathol. Exp. Neurol. 77, 1091–1100 (2018).
Blecher, R. et al. Contact sports as a risk factor for amyotrophic lateral sclerosis: a systematic review. Glob. Spine J. 9, 104–118 (2019).
Iverson, G. L. et al. Examining later-in-life health risks associated with sport-related concussion and repetitive head impacts: a systematic review of case–control and cohort studies. Br. J. Sports Med. 57, 810–821 (2023).
Filippini, T. et al. Clinical and lifestyle factors and risk of amyotrophic lateral sclerosis: a population-based case–control study. Int. J. Environ. Res. Public Health 17, 857 (2020).
Pupillo, E. et al. Trauma and amyotrophic lateral sclerosis: a European population-based case–control study from the EURALS consortium. Amyotroph. Lateral Scler. Frontotemporal Degener. 19, 118–125 (2018).
Gu, D. et al. Trauma and amyotrophic lateral sclerosis: a systematic review and meta-analysis. Amyotroph. Lateral Scler. Frontotemporal Degener. 22, 170–185 (2021).
Levitt, B. B., Lai, H. C. & Manville, A. M. Effects of non-ionizing electromagnetic fields on flora and fauna, part 1. Rising ambient EMF levels in the environment. Rev. Environ. Health 37, 81–122 (2022).
Hu, C., Zuo, H. & Li, Y. Effects of radiofrequency electromagnetic radiation on neurotransmitters in the brain. Front. Public Health 9, 691880 (2021).
Luna, J. et al. Residential exposure to ultra high frequency electromagnetic fields emitted by Global system for mobile (GSM) antennas and amyotrophic lateral sclerosis incidence: a geo-epidemiological population-based study. Environ. Res. 176, 108525 (2019).
Peters, S. et al. Associations of electric shock and extremely low-frequency magnetic field exposure with the risk of amyotrophic lateral sclerosis. Am. J. Epidemiol. 188, 796–805 (2019).
Koeman, T. et al. Occupational exposure and amyotrophic lateral sclerosis in a prospective cohort. Occup. Environ. Med. 74, 578–585 (2017).
Vinceti, M. et al. Magnetic fields exposure from high-voltage power lines and risk of amyotrophic lateral sclerosis in two Italian populations. Amyotroph. Lateral Scler. Frontotemporal Degener. 18, 583–589 (2017).
Filippini, T., Hatch, E. E. & Vinceti, M. Residential exposure to electromagnetic fields and risk of amyotrophic lateral sclerosis: a dose–response meta-analysis. Sci. Rep. 11, 11939 (2021).
Boddy, S. L. et al. The gut microbiome: a key player in the complexity of amyotrophic lateral sclerosis (ALS). BMC Med. 19, 13 (2021).
Nicholson, K. et al. The human gut microbiota in people with amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. Frontotemporal Degener. 22, 186–194 (2021).
Di Gioia, D. et al. A prospective longitudinal study on the microbiota composition in amyotrophic lateral sclerosis. BMC Med. 18, 153 (2020).
Hertzberg, V. S. et al. Gut microbiome differences between amyotrophic lateral sclerosis patients and spouse controls. Amyotroph. Lateral Scler. Frontotemporal Degener. 23, 91–99 (2022).
Niccolai, E. et al. The gut microbiota–immunity axis in ALS: a role in deciphering disease heterogeneity? Biomedicines 9, 753 (2021).
Kim, H. S. et al. Gut- and oral-dysbiosis differentially impact spinal- and bulbar-onset ALS, predicting ALS severity and potentially determining the location of disease onset. BMC Neurol. 22, 62 (2022).
Yu, H., Kim, S. H., Noh, M. Y., Lee, S. & Park, Y. Relationship between dietary fiber intake and the prognosis of amyotrophic lateral sclerosis in Korea. Nutrients 12, 3420 (2020).
Ngo, S. T. et al. Progression and survival of patients with motor neuron disease relative to their fecal microbiota. Amyotroph. Lateral Scler. Frontotemporal Degener. 21, 549–562 (2020).
Goutman, S. A. et al. Metabolomics identifies shared lipid pathways in independent amyotrophic lateral sclerosis cohorts. Brain 145, 4425–4439 (2022).
Zeng, Q. et al. The alteration of gut microbiome and metabolism in amyotrophic lateral sclerosis patients. Sci. Rep. 10, 12998 (2020).
Gong, Z. et al. Gut microbiota links with cognitive impairment in amyotrophic lateral sclerosis: a multi-omics study. J. Biomed. Res. 37, 125–137 (2022).
Zhang, L., Zhuang, Z., Zhang, G., Huang, T. & Fan, D. Assessment of bidirectional relationships between 98 genera of the human gut microbiota and amyotrophic lateral sclerosis: a 2-sample Mendelian randomization study. BMC Neurol. 22, 8 (2022).
Ning, J. et al. Investigating casual associations among gut microbiota, metabolites, and neurodegenerative diseases: a Mendelian randomization study. J. Alzheimers Dis. 87, 211–222 (2022).
Castanedo-Vazquez, D., Bosque-Varela, P., Sainz-Pelayo, A. & Riancho, J. Infectious agents and amyotrophic lateral sclerosis: another piece of the puzzle of motor neuron degeneration. J. Neurol. 266, 27–36 (2019).
Levine, K. S. et al. Virus exposure and neurodegenerative disease risk across national biobanks. Neuron 111, 1086–1093.e2 (2023).
French, P. W., Ludowyke, R. & Guillemin, G. J. Fungal neurotoxins and sporadic amyotrophic lateral sclerosis. Neurotox. Res. 35, 969–980 (2019).
Pierce, E. S. How did Lou Gehrig get Lou Gehrig’s disease? Mycobacterium avium subspecies paratuberculosis in manure, soil, dirt, dust and grass and amyotrophic lateral sclerosis (motor neurone disease) clusters in football, rugby and soccer players. Med. Hypotheses 119, 1–5 (2018).
Pape, J. A. & Grose, J. H. The effects of diet and sex in amyotrophic lateral sclerosis. Rev. Neurol. 176, 301–315 (2020).
D’Amico, E. et al. Metabolic abnormalities, dietary risk factors and nutritional management in amyotrophic lateral sclerosis. Nutrients 13, 2273 (2021).
Huisman, M. H. et al. Effect of presymptomatic body mass index and consumption of fat and alcohol on amyotrophic lateral sclerosis. JAMA Neurol. 72, 1155–1162 (2015).
Westeneng, H. J. et al. Associations between lifestyle and amyotrophic lateral sclerosis stratified by C9orf72 genotype: a longitudinal, population-based, case-control study. Lancet Neurol. 20, 373–384 (2021).
Goutman, S. A. et al. Body mass index associates with amyotrophic lateral sclerosis survival and metabolomic profiles. Muscle Nerve 67, 208–216 (2023).
Fitzgerald, K. C. et al. Dietary ω-3 polyunsaturated fatty acid intake and risk for amyotrophic lateral sclerosis. JAMA Neurol. 71, 1102–1110 (2014).
Michels, S. et al. Association of blood lipids with onset and prognosis of amyotrophic lateral sclerosis: results from the ALS Swabia registry. J. Neurol. 270, 3082–3090 (2023).
Hop, P. J. et al. Genome-wide study of DNA methylation shows alterations in metabolic, inflammatory, and cholesterol pathways in ALS. Sci. Transl. Med. 14, eabj0264 (2022).
Pupillo, E. et al. Amyotrophic lateral sclerosis and food intake. Amyotroph. Lateral Scler. Frontotemporal Degener. 19, 267–274 (2018).
Jin, Y. et al. Dietary intake of fruits and beta-carotene is negatively associated with amyotrophic lateral sclerosis risk in Koreans: a case–control study. Nutr. Neurosci. 17, 104–108 (2014).
Okamoto, K. et al. Fruit and vegetable intake and risk of amyotrophic lateral sclerosis in Japan. Neuroepidemiology 32, 251–256 (2009).
Petimar, J. et al. Coffee, tea, and caffeine intake and amyotrophic lateral sclerosis mortality in a pooled analysis of eight prospective cohort studies. Eur. J. Neurol. 26, 468–475 (2019).
Goncharova, P. S. et al. Nutrient effects on motor neurons and the risk of amyotrophic lateral sclerosis. Nutrients 13, 3804 (2021).
Xia, K. et al. Dietary-derived essential nutrients and amyotrophic lateral sclerosis: a two-sample Mendelian randomization study. Nutrients 14, 920 (2022).
Joo, J., Williamson, S. A., Vazquez, A. I., Fernandez, J. R. & Bray, M. S. The influence of 15-week exercise training on dietary patterns among young adults. Int. J. Obes. 43, 1681–1690 (2019).
Julian, T. H. et al. A review of Mendelian randomization in amyotrophic lateral sclerosis. Brain 145, 832–842 (2022).
Henry, K. A., Fagliano, J., Jordan, H. M., Rechtman, L. & Kaye, W. E. Geographic variation of amyotrophic lateral sclerosis incidence in New Jersey, 2009–2011. Am. J. Epidemiol. 182, 512–519 (2015).
Lian, L. et al. Environmental risk factors and amyotrophic lateral sclerosis (ALS): a case–control study of ALS in China. J. Clin. Neurosci. 66, 12–18 (2019).
Beaudin, M., Salachas, F., Pradat, P. F. & Dupré, N. Environmental risk factors for amyotrophic lateral sclerosis: a case–control study in Canada and France. Amyotroph. Lateral Scler. Frontotemporal Degener. 23, 592–600 (2022).
Korner, S. et al. Influence of environment and lifestyle on incidence and progress of amyotrophic lateral sclerosis in a German ALS population. Aging Dis. 10, 205–216 (2019).
Peters, S. et al. Effect modification of the association between total cigarette smoking and ALS risk by intensity, duration and time-since-quitting: Euro-MOTOR. J. Neurol. Neurosurg. Psychiatry 91, 33–39 (2020).
Keren, N. et al. Evidence of an environmental effect on survival in ALS. Amyotroph. Lateral Scler. Frontotemporal Degener. 15, 528–533 (2014).
Rooney, J. et al. Survival analysis of geospatial factors in the Irish ALS cohort. Amyotroph. Lateral Scler. Frontotemporal Degener. 17, 555–560 (2016).
Goutman, S. A. et al. High plasma concentrations of organic pollutants negatively impact survival in amyotrophic lateral sclerosis. J. Neurol. Neurosurg. Psychiatry 90, 907–912 (2019).
Farrugia Wismayer, M. et al. Occupation and amyotrophic lateral sclerosis risk: a case–control study in the isolated island population of Malta. Amyotroph. Lateral Scler. Frontotemporal Degener. 22, 528–534 (2021).
Furby, A., Beauvais, K., Kolev, I., Rivain, J. G. & Sébille, V. Rural environment and risk factors of amyotrophic lateral sclerosis: a case–control study. J. Neurol. 257, 792–798 (2010).
Chio, A. et al. ALS in Italian professional soccer players: the risk is still present and could be soccer-specific. Amyotroph. Lateral Scler. 10, 205–209 (2009).
Chiò, A., Benzi, G., Dossena, M., Mutani, R. & Mora, G. Severely increased risk of amyotrophic lateral sclerosis among Italian professional football players. Brain 128, 472–476 (2005).
Gotkine, M., Friedlander, Y. & Hochner, H. Triathletes are over-represented in a population of patients with ALS. Amyotroph. Lateral Scler. Frontotemporal Degener. 15, 534–536 (2014).
Gamez, J. & Carmona, F. Confirmation of early non-bulbar onset of amyotrophic lateral sclerosis in Spanish league soccer players. J. Neurol. Sci. 428, 117586 (2021).
Benatar, M. et al. Preventing amyotrophic lateral sclerosis: insights from pre-symptomatic neurodegenerative diseases. Brain 145, 27–44 (2022).
Wills, A. M. et al. Hypercaloric enteral nutrition in patients with amyotrophic lateral sclerosis: a randomised, double-blind, placebo-controlled phase 2 trial. Lancet 383, 2065–2072 (2014).
Ludolph, A. C. et al. Effect of high-caloric nutrition on survival in amyotrophic lateral sclerosis. Ann. Neurol. 87, 206–216 (2020).
Dorst, J. et al. Effect of high-caloric nutrition on serum neurofilament light chain levels in amyotrophic lateral sclerosis. J. Neurol. Neurosurg. Psychiatry 91, 1007–1009 (2020).
De Marchi, F. et al. Study protocol on the safety and feasibility of a normocaloric ketogenic diet in people with amyotrophic lateral sclerosis. Nutrition 94, 111525 (2022).
Paganoni, S. & Wills, A. M. High-fat and ketogenic diets in amyotrophic lateral sclerosis. J. Child Neurol. 28, 989–992 (2013).
De Marchi, F., Venkatesan, S., Saraceno, M., Mazzini, L. & Grossini, E. Acetyl-l-carnitine and amyotrophic lateral sclerosis: current evidence and potential use. CNS Neurol. Disord. Drug. Targets https://doi.org/10.2174/1871527322666230330083757 (2023).
Wong, C. et al. Clinical trials in amyotrophic lateral sclerosis: a systematic review and perspective. Brain Commun. 3, fcab242 (2021).
Blacher, E. et al. Potential roles of gut microbiome and metabolites in modulating ALS in mice. Nature 572, 474–480 (2019).
Burberry, A. et al. C9orf72 suppresses systemic and neural inflammation induced by gut bacteria. Nature 582, 89–94 (2020).
Mandrioli, J. et al. FETR-ALS study protocol: a randomized clinical trial of fecal microbiota transplantation in amyotrophic lateral sclerosis. Front. Neurol. 10, 1021 (2019).
Lu, G., Wen, Q., Cui, B., Li, Q. & Zhang, F. Washed microbiota transplantation stopped the deterioration of amyotrophic lateral sclerosis: the first case report and narrative review. J. Biomed. Res. 37, 69–76 (2022).
Paoli, A. et al. Ketogenic diet and microbiota: friends or enemies? Genes 10, 534 (2019).
Rawat, K., Singh, N., Kumari, P. & Saha, L. A review on preventive role of ketogenic diet (KD) in CNS disorders from the gut microbiota perspective. Rev. Neurosci. 32, 143–157 (2021).
Wosiski-Kuhn, M., Lyon, M. S., Caress, J. & Milligan, C. Inflammation, immunity, and amyotrophic lateral sclerosis: II. immune-modulating therapies. Muscle Nerve 59, 23–33 (2019).
Johnson, F. O. et al. Exposure to an environmental neurotoxicant hastens the onset of amyotrophic lateral sclerosis-like phenotype in human Cu2+/Zn2+ superoxide dismutase 1 G93A mice: glutamate-mediated excitotoxicity. J. Pharmacol. Exp. Ther. 338, 518–527 (2011).
Bailey, J. M., Colón-Rodríguez, A. & Atchison, W. D. Evaluating a gene–environment interaction in amyotrophic lateral sclerosis: methylmercury exposure and mutated SOD1. Curr. Environ. Health Rep. 4, 200–207 (2017).
Ash, P. E. A. et al. Heavy metal neurotoxicants induce ALS-linked TDP-43 pathology. Toxicol. Sci. 167, 105–115 (2019).
Koski, L., Ronnevi, C., Berntsson, E., Wärmländer, S. & Roos, P. M. Metals in ALS TDP-43 pathology. Int. J. Mol. Sci. 22, 12193 (2021).
Minj, E., Upadhayay, S. & Mehan, S. Nrf2/HO-1 signaling activator acetyl-11-keto-beta boswellic acid (AKBA)-mediated neuroprotection in methyl mercury-induced experimental model of ALS. Neurochem. Res. 46, 2867–2884 (2021).
Iqubal, A. et al. Environmental neurotoxic pollutants: review. Environ. Sci. Pollut. Res. Int. 27, 41175–41198 (2020).
Kisby, G. E. & Spencer, P. S. Genotoxic damage during brain development presages prototypical neurodegenerative disease. Front. Neurosci. 15, 752153 (2021).
Lagrange, E. et al. An amyotrophic lateral sclerosis hot spot in the French Alps associated with genotoxic fungi. J. Neurol. Sci. 427, 117558 (2021).
Costa, L. G. et al. Neurotoxicity of traffic-related air pollution. Neurotoxicology 59, 133–139 (2017).
Pan, Y. & Nicolazzo, J. A. Altered blood–brain barrier and blood–spinal cord barrier dynamics in amyotrophic lateral sclerosis: impact on medication efficacy and safety. Br. J. Pharmacol. 179, 2577–2588 (2022).
Murdock, B. J., Goutman, S. A., Boss, J., Kim, S. & Feldman, E. L. Amyotrophic lateral sclerosis survival associates with neutrophils in a sex-specific manner. Neurol. Neuroimmunol. Neuroinflamm. 8, e953 (2021).
Mostafalou, S. & Abdollahi, M. The link of organophosphorus pesticides with neurodegenerative and neurodevelopmental diseases based on evidence and mechanisms. Toxicology 409, 44–52 (2018).
Kulick, D. et al. Amyotrophic lateral sclerosis-associated persistent organic pollutant cis-chlordane causes GABA(A)-independent toxicity to motor neurons, providing evidence toward an environmental component of sporadic amyotrophic lateral sclerosis. ACS Chem. Neurosci. 13, 3567–3577 (2022).
de Vos, W. M., Tilg, H., Van Hul, M. & Cani, P. D. Gut microbiome and health: mechanistic insights. Gut 71, 1020–1032 (2022).
Wu, S., Yi, J., Zhang, Y. G., Zhou, J. & Sun, J. Leaky intestine and impaired microbiome in an amyotrophic lateral sclerosis mouse model. Physiol. Rep. 3, e12356 (2015).
Zhang, Y. G. et al. Target intestinal microbiota to alleviate disease progression in amyotrophic lateral sclerosis. Clin. Ther. 39, 322–336 (2017).
Koh, A., De Vadder, F., Kovatcheva-Datchary, P. & Bäckhed, F. From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell 165, 1332–1345 (2016).
Zhang, Y., Ogbu, D., Garrett, S., Xia, Y. & Sun, J. Aberrant enteric neuromuscular system and dysbiosis in amyotrophic lateral sclerosis. Gut Microbes 13, 1996848 (2021).
Trabjerg, M. S. et al. Dysregulation of metabolic pathways by carnitine palmitoyl-transferase 1 plays a key role in central nervous system disorders: experimental evidence based on animal models. Sci. Rep. 10, 15583 (2020).
Trabjerg, M. S. et al. Downregulating carnitine palmitoyl transferase 1 affects disease progression in the SOD1 G93A mouse model of ALS. Commun. Biol. 4, 509 (2021).
Figueroa-Romero, C. et al. Temporal evolution of the microbiome, immune system and epigenome with disease progression in ALS mice. Dis. Model Mech. 13, dmm041947 (2019).
Cox, L. M. et al. The microbiota restrains neurodegenerative microglia in a model of amyotrophic lateral sclerosis. Microbiome 10, 47 (2022).
Xiao, L. et al. Associations of heavy metals with activities of daily living disability: an epigenome-wide view of DNA methylation and mediation analysis. Environ. Health Perspect. 130, 87009 (2022).
Hoang, T. T. et al. Epigenome-wide DNA methylation and pesticide use in the agricultural lung health study. Environ. Health Perspect. 129, 97008 (2021).
Grova, N., Schroeder, H., Olivier, J. L. & Turner, J. D. Epigenetic and neurological impairments associated with early life exposure to persistent organic pollutants. Int. J. Genomics 2019, 2085496 (2019).
Shock, T., Badang, L., Ferguson, B. & Martinez-Guryn, K. The interplay between diet, gut microbes, and host epigenetics in health and disease. J. Nutr. Biochem. 95, 108631 (2021).
Paez-Colasante, X., Figueroa-Romero, C., Sakowski, S. A., Goutman, S. A. & Feldman, E. L. Amyotrophic lateral sclerosis: mechanisms and therapeutics in the epigenomic era. Nat. Rev. Neurol. 11, 266–279 (2015).
Paez-Colasante, X. et al. Cytoplasmic TDP43 binds microRNAs: new disease targets in amyotrophic lateral sclerosis. Front. Cell. Neurosci. 14, 117 (2020).
Young, P. E., Kum Jew, S., Buckland, M. E., Pamphlett, R. & Suter, C. M. Epigenetic differences between monozygotic twins discordant for amyotrophic lateral sclerosis (ALS) provide clues to disease pathogenesis. PLoS ONE 12, e0182638 (2017).
Ruf, W. P. et al. Methylome analysis of ALS patients and presymptomatic mutation carriers in blood cells. Neurobiol. Aging 116, 16–24 (2022).
Zhang, M. et al. Combined epigenetic/genetic study identified an ALS age of onset modifier. Acta Neuropathol. Commun. 9, 75 (2021).
Benatar, M., Wuu, J., Andersen, P. M., Lombardi, V. & Malaspina, A. Neurofilament light: a candidate biomarker of presymptomatic amyotrophic lateral sclerosis and phenoconversion. Ann. Neurol. 84, 130–139 (2018).
Bjornevik, K. et al. Prediagnostic plasma metabolomics and the risk of amyotrophic lateral sclerosis. Neurology 92, e2089–e2100 (2019).
Bjornevik, K. et al. Pre-diagnostic plasma lipid levels and the risk of amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. Frontotemporal Degener. 22, 133–143 (2021).
Price, E. J. et al. Merging the exposome into an integrated framework for ‘omics’ sciences. iScience 25, 103976 (2022).
Harlan, B. A. et al. Evaluation of the NAD(+) biosynthetic pathway in ALS patients and effect of modulating NAD(+) levels in hSOD1-linked ALS mouse models. Exp. Neurol. 327, 113219 (2020).
Liguori, F., Amadio, S. & Volonté, C. Where and why modeling amyotrophic lateral sclerosis. Int. J. Mol. Sci. 22, 3977 (2021).
Giacomelli, E. et al. Human stem cell models of neurodegeneration: from basic science of amyotrophic lateral sclerosis to clinical translation. Cell Stem Cell 29, 11–35 (2022).
Acknowledgements
The authors are grateful to the patients attending the Pranger ALS Clinic at the University of Michigan, along with their families, whose interest in clinical research and participation in studies have made advances in amyotrophic lateral sclerosis (ALS) exposome science possible. The authors also acknowledge the faculty and staff at the ALS Center of Excellence for their help in performing these studies and thank E.J. Koubek for help with the supplementary information. S.A.G. and E.L.F. acknowledge funding from the National ALS Registry/CDC/ATSDR (1R01TS000289, R01TS000327); National ALS Registry/CDC/ATSDR CDCP-DHHS-US (CDC/ATSDR 200-2013-56856); the ALS Association (20-IIA-532); NIEHS (K23ES027221, R01ES030049), NINDS (R01NS127188, R01NS120926); the NeuroNetwork for Emerging Therapies; the NeuroNetwork Therapeutic Discovery Fund; the Peter R. Clark Fund for Amyotrophic Lateral Sclerosis Research; the Sinai Medical Staff Foundation; Scott L. Pranger and the University of Michigan.
Author information
Authors and Affiliations
Contributions
S.A.G., M.G.S., J.H. and E.L.F. contributed substantially to discussion of the article content. All authors wrote the article and reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
S.A.G., D.-G.J. and E.L.F. are employees of the University of Michigan. M.G.S. and J.H. are employees of the University of North Dakota. S.A.G. and E.L.F. are listed as inventors on a patent, issue number US10660895, held by the University of Michigan, entitled ‘Methods for treating amyotrophic lateral sclerosis’ that targets immune pathways for use in amyotrophic lateral sclerosis (ALS) therapeutics. S.A.G. has served on a Data and Safety Monitoring Board and as a medical adviser for an ALS documentary. E.L.F. has consulted for Biogen.
Peer review
Peer review information
Nature Reviews Neurology thanks J. Mandrioli, S. Vucic and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Review criteria
We searched PubMed for articles in the English language, using with the following terms in addition to ‘amyotrophic lateral sclerosis’: ‘agriculture risk’, ‘air pollution’, ‘clinical trial diet’, ‘detoxifying gene’, ‘detoxifying SNP’, ‘diet risk’, ‘education polygenic’, ‘electromagnetic’, ‘environment’, ‘exposure’, ‘faecal microbiota transplant’, ‘FMT’, ‘gene environment’, ‘gene exposome’, ‘geographic distribution’, ‘head trauma’, ‘immunity environment’, ‘immunity exposome’, and ‘immunity exposure’, ‘infectious agent’, ‘ketogenic diet’, ‘mechanism’, ‘metals’, ‘metals blood’, ‘metals plasma’, ‘microbiome’, ‘microbiome risk’, ‘military’, ‘mutation environment’, ‘mutation exposome’, ‘neuroinflammation environment’, ‘neuroinflammation exposome’, ‘neuroinflammation exposure’, ‘occupational exposure’, ‘persistent organic pollutant’, ‘persistent organic pollutant blood’, ‘persistent organic pollutant plasma’, ‘pesticide’, ‘pesticide blood’, ‘pesticide plasma’, ‘pollutant’, ‘probiotic’, ‘professional sports’, ‘polygenic environment’, ‘polygenic exposome’, ‘socioeconomic’, ‘spatial clustering’, ‘traffic’, ‘trauma’. The search focused on articles published from 1st January 2017 to 23rd November 2022; however, older seminal papers were also considered. In addition, articles from the authors’ personal reference lists were included. Articles were selected on the basis of relevance to this Review.
Supplementary information
Glossary
- Dual hit models
-
Models incorporating both genetic mutations and environmental exposures.
- Epigenetic age
-
A composite measure of DNA methylation across specific CpG sites that is associated with chronological age.
- Familial ALS
-
Heritable amyotrophic lateral sclerosis occurring in individuals with a family history of the illness.
- Heritability
-
The extent to which a trait can be explained by inheritance.
- Mendelian randomization
-
A method that examines causality of a modifiable exposure on a disease by using measured variation in genes with characterized functions.
- Microbiome
-
A community of microorganisms that dwells within a specific habitat. In the context of the human microbiome, the habitat compromises organs, for example, the gut microbiome or the skin microbiome.
- Microbiota
-
All of the living microorganisms within a microbiome.
- Monogenic
-
A mode of inheritance in which a trait is attributable to a single gene.
- Penetrant
-
Penetrance is defined as the extent to which a genetic trait manifests in an individual. Mutations that are highly penetrant are more likely to manifest phenotypically than are those with low penetrance.
- Polygenic
-
A mode of inheritance in which a trait is attributable to several genes.
- Sporadic ALS
-
Amyotrophic lateral sclerosis occurring in individuals without a family history of the illness.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Goutman, S.A., Savelieff, M.G., Jang, DG. et al. The amyotrophic lateral sclerosis exposome: recent advances and future directions. Nat Rev Neurol 19, 617–634 (2023). https://doi.org/10.1038/s41582-023-00867-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41582-023-00867-2
This article is cited by
-
Quantitative association between lead exposure and amyotrophic lateral sclerosis: a Bayesian network-based predictive study
Environmental Health (2024)
-
Immunological aspects of central neurodegeneration
Cell Discovery (2024)
-
Computational screening of damaging nsSNPs in human SOD1 genes associated with amyotrophic lateral sclerosis identifies destabilising effects of G38R and G42D mutations through in silico evaluation
In Silico Pharmacology (2024)