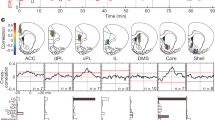
Abstract
The nervous system uses fast- and slow-adapting sensory detectors in parallel to enable neuronal representations of external states and their temporal dynamics. It is unknown whether this dichotomy also applies to internal representations that have no direct correlation in the physical world. Here we find that two distinct dopamine (DA) neuron subtypes encode either a state or its rate-of-change. In mice performing a reward-seeking task, we found that the animal’s behavioral state and rate-of-change were encoded by the sustained activity of DA neurons in medial ventral tegmental area (VTA) DA neurons and transient activity in lateral VTA DA neurons, respectively. The neural activity patterns of VTA DA cell bodies matched DA release patterns within anatomically defined mesoaccumbal pathways. Based on these results, we propose a model in which the DA system uses two parallel lines for proportional–differential encoding of a state variable and its temporal dynamics.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Source data are provided with this paper. All other data are available upon request from the corresponding author.
Code availability
Custom code used for the processing of raw in vivo electrophysiology or fiber photometry data is available on GitHub at https://github.com/handejong/post-kilosort (electrophysiology) and https://github.com/handejong/Fipster (fiber photometry). Code used for model fitting is available at https://github.com/lammellab/model_fitting.
References
Młynarski, W. F. & Hermundstad, A. M. Efficient and adaptive sensory codes. Nat. Neurosci. 24, 998–1009 (2021).
Kim, A. J., Lazar, A. A. & Slutskiy, Y. B. Projection neurons in Drosophila antennal lobes signal the acceleration of odor concentrations. eLife 4, e06651 (2015).
Luo, J., Shen, W. L. & Montell, C. TRPA1 mediates sensation of the rate of temperature change in Drosophila larvae. Nat. Neurosci. 20, 34–41 (2017).
Adrian, E. D. & Zotterman, Y. The impulses produced by sensory nerve endings: part 3. Impulses set up by touch and pressure. J. Physiol. 61, 465–483 (1926).
Schultz, W. Dopamine reward prediction-error signalling: a two-component response. Nat. Rev. Neurosci. 17, 183–195 (2016).
Gershman, S. J. & Uchida, N. Believing in dopamine. Nat. Rev. Neurosci. 20, 703–714 (2019).
Watabe-Uchida, M., Eshel, N. & Uchida, N. Neural circuitry of reward prediction error. Annu. Rev. Neurosci. 40, 373–394 (2017).
Schultz, W. & Dickinson, A. Neuronal coding of prediction errors. Annu. Rev. Neurosci. 23, 473–500 (2000).
Schultz, W., Dayan, P. & Montague, P. R. A neural substrate of prediction and reward. Science 275, 1593–1599 (1997).
Mohebi, A. et al. Dissociable dopamine dynamics for learning and motivation. Nature 570, 65–70 (2019).
Hamid, A. A. et al. Mesolimbic dopamine signals the value of work. Nat. Neurosci. 19, 117–126 (2016).
Wise, R. A. Dopamine, learning and motivation. Nat. Rev. Neurosci. 5, 483–494 (2004).
Salamone, J. D. & Correa, M. The mysterious motivational functions of mesolimbic dopamine. Neuron 76, 470–485 (2012).
Sun, F. et al. A genetically encoded fluorescent sensor enables rapid and specific detection of dopamine in flies, fish, and mice. Cell 174, 481–496 (2018).
Tsutsui-Kimura, I. et al. Distinct temporal difference error signals in dopamine axons in three regions of the striatum in a decision-making task. eLife 9, e62390 (2020).
Berke, J. D. What does dopamine mean? Nat. Neurosci. 21, 787–793 (2018).
Kim, H. R. et al. A unified framework for dopamine signals across timescales. Cell 183, 1600–1616 (2020).
Coddington, L. T. & Dudman, J. T. Learning from action: reconsidering movement signaling in midbrain dopamine neuron activity. Neuron 104, 63–77 (2019).
Dayan, P. & Berridge, K. C. Model-based and model-free Pavlovian reward learning: revaluation, revision, and revelation. Cogn. Affect. Behav. Neurosci. 14, 473–492 (2014).
Gardner, M. P. H., Schoenbaum, G. & Gershman, S. J. Rethinking dopamine as generalized prediction error. Proc. Biol. Sci. 285, 20181645 (2018).
Howe, M. W., Tierney, P. L., Sandberg, S. G., Phillips, P. E. M. & Graybiel, A. M. Prolonged dopamine signalling in striatum signals proximity and value of distant rewards. Nature 500, 575–579 (2013).
Lammel, S. et al. Unique properties of mesoprefrontal neurons within a dual mesocorticolimbic dopamine system. Neuron 57, 760–773 (2008).
De Jong, J. W. et al. A neural circuit mechanism for encoding aversive stimuli in the mesolimbic dopamine system. Neuron 101, 133–151 (2019).
Engelhard, B. et al. Specialized coding of sensory, motor and cognitive variables in VTA dopamine neurons. Nature 570, 509–513 (2019).
Poulin, J.-F., Gaertner, Z., Moreno-Ramos, O. A. & Awatramani, R. Classification of midbrain dopamine neurons using single-cell gene expression profiling approaches. Trends Neurosci. 43, 155–169 (2020).
Ekstrand, M. I. et al. Molecular profiling of neurons based on connectivity. Cell 157, 1230–1242 (2014).
De Jong, J. W., Fraser, K. M. & Lammel, S. Mesoaccumbal dopamine heterogeneity: what do dopamine firing and release have to do with it?. Annu. Rev. Neurosci. 45, 109–129 (2022).
Steinmetz, N. A., Zatka-Haas, P., Carandini, M. & Harris, K. D. Distributed coding of choice, action and engagement across the mouse brain. Nature 576, 266–273 (2019).
Cohen, J. Y., Haesler, S., Vong, L., Lowell, B. B. & Uchida, N. Neuron-type-specific signals for reward and punishment in the ventral tegmental area. Nature 482, 85–88 (2012).
Starkweather, C. K., Babayan, B. M., Uchida, N. & Gershman, S. J. Dopamine reward prediction errors reflect hidden-state inference across time. Nat. Neurosci. 20, 581–589 (2017).
Beier, K. T. et al. Circuit architecture of VTA dopamine neurons revealed by systematic input-output mapping. Cell 162, 622–634 (2015).
Wassum, K. M., Ostlund, S. B. & Maidment, N. T. Phasic mesolimbic dopamine signaling precedes and predicts performance of a self-initiated action sequence task. Biol. Psychiatry 71, 846–854 (2012).
Phillips, P. E. M., Stuber, G. D., Heien, M. L. A. V., Wightman, R. M. & Carelli, R. M. Subsecond dopamine release promotes cocaine seeking. Nature 422, 614–618 (2003).
Roitman, M. F. Dopamine operates as a subsecond modulator of food seeking. J. Neurosci. 24, 1265–1271 (2004).
Verharen, J. P. H., Kentrop, J., Vanderschuren, L. J. M. J. & Adan, R. A. H. Reinforcement learning across the rat estrous cycle. Psychoneuroendocrinology 100, 27–31 (2019).
Verharen, J. P. H., Adan, R. A. H. & Vanderschuren, L. J. M. J. Differential contributions of striatal dopamine D1 and D2 receptors to component processes of value-based decision making. Neuropsychopharmacology 44, 2195–2204 (2019).
Rescorla, R. A. ‘Configural’ conditioning in discrete-trial bar pressing. J. Comp. Physiol. Psychol. 79, 307–317 (1972).
Langdon, A. J., Sharpe, M. J., Schoenbaum, G. & Niv, Y. Model-based predictions for dopamine. Curr. Opin. Neurobiol. 49, 1–7 (2018).
Cox, J. & Witten, I. B. Striatal circuits for reward learning and decision-making. Nat. Rev. Neurosci. 20, 482–494 (2019).
Badrinarayan, A. et al. Aversive stimuli differentially modulate real-time dopamine transmission dynamics within the nucleus accumbens core and shell. J. Neurosci. 32, 15779–15790 (2012).
Kutlu, M. G. et al. Dopamine signaling in the nucleus accumbens core mediates latent inhibition. Nat. Neurosci. 25, 1071–1081 (2022).
Threlfell, S. et al. Striatal dopamine release is triggered by synchronized activity in cholinergic interneurons. Neuron 75, 58–64 (2012).
Liu, C. et al. An action potential initiation mechanism in distal axons for the control of dopamine release. Science 375, 1378–1385 (2022).
Syed, E. C. J. et al. Action initiation shapes mesolimbic dopamine encoding of future rewards. Nat. Neurosci. 19, 34–36 (2016).
Soares, S., Atallah, B. V. & Paton, J. J. Midbrain dopamine neurons control judgment of time. Science 354, 1273–1277 (2016).
Sadacca, B. F., Jones, J. L. & Schoenbaum, G. Midbrain dopamine neurons compute inferred and cached value prediction errors in a common framework. eLife 5, e13665 (2016).
Niv, Y., Daw, N. D., Joel, D. & Dayan, P. Tonic dopamine: opportunity costs and the control of response vigor. Psychopharmacology (Berl.) 191, 507–520 (2007).
Niv, Y. & Schoenbaum, G. Dialogues on prediction errors. Trends Cogn. Sci. 12, 265–272 (2008).
Takahashi, Y. K. et al. Dopamine neurons respond to errors in the prediction of sensory features of expected rewards. Neuron 95, 1395–1405 (2017).
Castro, D. C. & Bruchas, M. R. A motivational and neuropeptidergic hub: anatomical and functional diversity within the nucleus accumbens shell. Neuron 102, 529–552 (2019).
Ikemoto, S. Dopamine reward circuitry: two projection systems from the ventral midbrain to the nucleus accumbens–olfactory tubercle complex. Brain Res. Rev. 56, 27–78 (2007).
Zahm, D. S. & Brog, J. S. On the significance of subterritories in the ‘accumbens’ part of the rat ventral striatum. Neuroscience 50, 751–767 (1992).
Franklin, K. & Paxinos, G. Paxinos and Franklin’s the Mouse Brain in Stereotaxic Coordinates, Compact 5th edn (Academic Press, 2019).
Paxinos, G. & Watson, C. The Rat Brain in Stereotaxic Coordinates. Sixth Edition (Academic Press, 2007).
Cerniauskas, I. et al. Chronic stress induces activity, synaptic, and transcriptional remodeling of the lateral habenula associated with deficits in motivated behaviors. Neuron 104, 899–915 (2019).
Kim, C. K. et al. Simultaneous fast measurement of circuit dynamics at multiple sites across the mammalian brain. Nat. Methods 13, 325–328 (2016).
Virtanen, P. et al. SciPy 1.0: fundamental algorithms for scientific computing in Python. Nat. Methods 17, 261–272 (2020)
JASP Team. JASP (version 0.16.1). JASP https://jasp-stats.org (2022).
Acknowledgements
We thank everyone who provided advice throughout the study and on this manuscript—J. Roeper, R. Malenka, R. Awatramani, D. Dombeck and M. Yartsev. We thank the UNC vector core for AAVs, M. Kleinman and the Foster Lab at UC Berkeley for providing rats and assistance with tracing experiments. We thank J. Mankel for technical assistance with immunohistochemistry experiments. S.L. is a John P. Stock Faculty Fellow, Weill Neurohub Investigator and Rita Allen Scholar. This work was supported by the National Institutes of Health (grants R01-DA042889 and R01-MH123246 to S.L.), the Tobacco-Related Disease Research Program (T32IR5075 to S.L.), the One Mind Foundation (047483 to S.L.), the Weill Neurohub (to S.L.), the Rita Allen Foundation (to S.L.), the McKnight Foundation (to S.L.), the Wayne and Gladys Valley Foundation (to S.L.) and a NARSAD Young Investigator Award (27936 to J.W.d.J.).
Author information
Authors and Affiliations
Contributions
Stereotaxic injections were performed by Y.L. and J.W.d.J. Neuropixels recordings were performed by J.W.d.J. Immunohistochemistry was performed by Y.L. Computational modeling was performed by J.W.d.J. and J.P.H.V. Fiber photometry was performed by J.W.d.J., Y.L. and J.P.H.V. Behavior experiments were performed by J.W.d.J., Y.L. and J.P.H.V. Data were analyzed by J.W.d.J. and J.P.H.V. The study was designed by J.W.d.J. and S.L. The manuscript was written by J.W.d.J., K.M.F. and S.L. and edited by all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Neuroscience thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Proportional-differential encoding in the peripheral and central nervous systems.
(a) Schematic showing proportional and rate-of-change encoding of a stimulus (for example, pressure on skin) in the peripheral nervous system. (b) Schematic showing state value and rate-of-change encoding in the central nervous system. The state value and its rate-of-change during a Pavlovian conditioning task in which a reward is partially predicted (for example, as in the behavioral task used for experiments in Figs. 1 and 2). Note that in this schematic the ‘state value’ refers to both expected reward as well as the reward itself.
Extended Data Fig. 2 Neuropixels-based opto-tagging of dopamine neurons.
(a) Anatomical locations of the recorded VTA cells. Although Neuropixels probes allowed to record more than n = 186 cells (n = 6 mice), we only included cells that were simultaneously recorded on the same or neighboring electrodes as the opto-tagged cells. Thus, only VTA cells and not cells from adjacent brain regions were included. Different colors indicate locations of recorded cells in mVTA or lVTA and whether the cells were identified as dopaminergic (‘tagged’) or non-dopaminergic (‘non-tagged’). (b) Representative single-channel recording showing 3 action potentials (APs) of the same unit (red arrows). Two of these APs are induced by a 1 ms light pulse (blue). Inset shows how the AP, and the light pulse are separated in time (scale bar 10 ms). (c) Mean delay, mean delay-variation to the first evoked AP, and waveform correlation between evoked and spontaneous APs for n = 67 opto-tagged cells (n = 6 mice). (d) Peri-event histogram of an opto-tagged cell following a 1 ms light pulse. ‘P’ indicates firing fidelity. Total firing fidelity is the sum of the bins between 0 and 5 ms. (e) Peri-event histogram of the same cell as in (d) but following a 2 ms light pulse, which increased firing fidelity, but at the risk of adulterating the waveforms with a light-offset artifact. (f) Evoked (blue) and spontaneous (black) waveforms for all opto-tagged cells. The waveform on the channel with the highest amplitude was chosen. (g) Peri-event histogram of an opto-tagged cell following a light pulse at 1% of the max laser power (max laser power: 25 mW at tip of optical fiber at a distance of ~1 mm from the recording site). (h) Same unit as in (g), but laser power was set to 10% of max. (i) Same unit as in (g, h), but following a 1 ms light pulse with laser at max intensity. Note, increase in firing fidelity when comparing (g, h, i). Firing fidelity was always < 1 (that is, one light pulse (1 ms) did not evoke more than a single AP). (j) AP width and baseline firing frequency (FF) for all recorded cells (n = 186 cells). Inset: Sample APs showing calculation of waveform width (that is, difference between AP peak and hyperpolarization peak). (k) Mean FF (left) and mean waveform widths for dopamine (tagged; n = 67 cells) and non-dopamine cells (non-tagged; n = 119 cells). Individual data points are presented in (j). Significance was calculated by means of two-sided Welch t-test; frequency ***P < 0.001. Opto-tagged cells had a significantly lower baseline FF and a longer waveform width compared to non-opto-tagged cells. (l) Mean FF (left) and mean waveform widths for dopamine neurons located in mVTA (n = 40 cells; orange) and lVTA (n = 27 cells; blue). Individual data points are shown in (j). Significance was calculated by means of two-sided Welch t-test. All data are represented as mean ± SEM.
Extended Data Fig. 3 Four distinct activity patterns describe neural activity of VTA cells during behavior.
(a) Sparce PCA embedding of all recorded VTA cells (n = 186 cells; n = 6 mice); color coding indicates that four different activity patterns were obtained using k-means clustering. (b) Graph showing action potential (AP) width and baseline firing frequency of all recorded VTA cells (n = 186 cells); color coding indicates cluster identity (cluster 1: gray, cluster 2: green, cluster 3: blue, cluster 4: red). (c) Bar graphs showing mean baseline firing frequency (left) and action potential width (right) for all recorded VTA cells (n = 186 cells) based on cluster identity. Significance was calculated by means of 1 way ANOVA. The annotations ‡ and # indicate significant difference in Tukey’s post hoc testing; ***P < 0.001. Data represented as mean ± SEM. (d) Percentage of non-opto-tagged (that is, non-dopaminergic neurons) neurons in lVTA (left) and mVTA (right) based on cluster identity. (e) Box plot (median, quantiles and outliers) depicting the anatomical location of all opto-tagged (that is, DA) neurons along a 45° (ventro-medial to dorso-lateral) axis through the VTA. Cluster 2 DA neurons were significantly more likely to be located in dorso-lateral part of the VTA compared to DA neurons in the other clusters. Cluster 1: n = 100 cells, Cluster 2: n = 33 cells, Cluster 3: n = 35 cells, Cluster 4: n = 18 cells. Significance was calculated by means of 1 way ANOVA; ***P < 0.001. Data are represented as median, interquartile range (shading) and total range (error bars).
Extended Data Fig. 4 VTA DA cell body activity is sufficient to explain DA release patterns in distinct NAc subregions.
(a) AAV-DIO-ChR2-mCherry was injected bilaterally into the VTA of DAT-Cre mice. The same mice were injected with dLight into NAcLat and NAcMed. Optical fibers were implanted dorsal to the VTA, in the NALat and NAcMed (optical fibers in NAcMed and NAcLat were counterbalanced across all animals, n = 6 mice). (b) Hundred trials of 1 ms 470 nm light stimulation of VTA DA neurons were used to calculate DA release (auROC) in NAcLat (left) and NAcMed (right). Blue line indicates 1 ms light pulse in VTA. (c) Response kernels in (b) were used to convolute single-unit recordings. Top: Sample unit, each vertical line denotes a single action potential. Bottom: Corresponding inferred dLight trace when the sequence of action potentials is convoluted with the NAcLat kernel in (b). (d) Mean normalized (auROC) single-unit recordings of all cluster 2 neurons in the lVTA; color code indicates random interval length. CS+ onset was at t = 0 sec. (e) Activity traces of the neurons in (d) were convoluted as in (c). Trials were normalized (auROC) and averaged over all cells as in (d). (f) Recorded NAcLat DA release patterns normalized (auROC) and averaged across all mice (n = 6 mice). The 2 sec interval (orange) is the same as in Fig. 2j. Note the qualitative and quantitative similarities with (e), including the below-baseline reduction in the interval between CS+ onset and reward delivery. (g–i) Same as in (d–f), but for NAcMed. Note that this experiment does not unequivocally prove that mVTA cluster 3 single-unit activity is responsible for NAcMed DA release patterns. However, it does suggest that mVTA cluster 3 single-unit activity is sufficient to explain NAcMed DA release patterns. All data are represented as mean ± SEM (shading).
Extended Data Fig. 5 A linear model to predict dopamine transients across all NAc subregions.
(a) Each NAc recording was normalized, and a linear regression model was fitted to the discrete task events to predict NAc DA release. Left: Animal behavior was binned to 100 ms and shifted in time (1 sec in both directions, 20 coefficients were fitted for each task event). Task events were encoded as ‘1’ or ‘0’ depending on occurrence in any given time bin except for the trial duration, which was coded from 0 to 1 over the 0-2500 ms interval. Right: Data were split into a training (80%) and test (20%) set. The task events in the training set were fitted to the dLight data using linear regression. The sample trace shows a high overlap between recorded response (blue) and model prediction (orange). Bar graph shows model performance on training and test set (mean ± SEM, n = 29 recordings). Note that the model performed notably better in NAcLat, probably because it performed well at fitting the large transients at CS+ onset and reward delivery. Lower right: Recorded data (left) and model prediction (right); color coded based on trial duration (averaged over all trials). (b) Task-event kernels used to fit the behavior to the fiber photometry signal. (c) AUCs of the kernels in (b), hexagons denote individual recording locations. The color map was selected to highlight the direction (up or down) of the kernels (aca: anterior commissure). (d) Because the anatomical location of individual recordings was histologically verified, we used Gaussian interpolation to calculate the task-event kernels for each pixel in a coronal section of the NAc. Note the gradient from NAcMed to NAcLat with NAcMed showing positive AUC for kernels related to reward seeking, reward consumption and trial duration. Conversely, NAcLat kernels were specifically tuned to trial start (CS+ onset) and reward delivery. The NAc core represents an intermediate structure with signals tuned either toward NAcMed or NAcLat based on the position more medial or more lateral to the aca, respectively. All data are represented as mean ± SEM (error bars or shading).
Extended Data Fig. 6 Topography of the mesoaccumbal dopamine system in mice and rats.
(a) Schematics showing representative fluorescent retrobeads locations (green) in different NAc subregions (NAcMed, NAc core, NAcLat) along the ventro-medial to dorso-lateral axis for different mice (n = 3 mice for each injection location; aca: anterior commissure). (b) Corresponding sample fluorescent images of coronal brain sections from the NAc (tyrosine hydroxylase (TH): red). (c) Sample fluorescent images of coronal midbrain sections from the same mice shown in (a,b). (d) Corresponding schematics highlighting locations of retrogradely labeled (that is, beads-positive, green) neurons in the medial VTA (mVTA) and lateral VTA (lVTA) (IF: interfascicular nucleus, PN: paranigral nucleus, PBP: parabrachial pigmented nucleus, IPN: interpeduncular nucleus; scale bars (b,c) 500 µm). (e) Schematic showing location of injection sites for fluorescent retrobeads (red) in the NAcLat and FluoroGold (gray) in the NAc core (n = 3 rats; CPu: Caudate Putamen). (f) Sample fluorescent images showing coronal brain sections of the NAcLat and NAc core at two different anterior-posterior coordinates (TH: blue, beads: red, FluoroGold: white; Scale bar 1 mm). (g) Fluorescent images showing coronal brain sections of the VTA from a rat that was injected with fluorescent retrobeads (red) into the NAcLat and FluoroGold (white) into the NAc core (scale bar 500 µm). Note, that mice and rats show a similar anatomical topography of NAcLat- and NAc core-projecting DA neurons (compare panels (c) and (g)). In both cases, NAcLat-projecting DA neurons are located in the lVTA, while NAc core-projecting DA neurons are located in the mVTA).
Extended Data Fig. 7 Bayesian model selection showing that the behavior of the animals can be best described by a Q learning model with a single learning rate.
(a) Results of Bayesian model selection. Model 2, a Q learning model with a single learning rate, has the highest protected exceedance probability and is, therefore, the best descriptor of the data of the three given models. Model 1 is a random choice model (in which every choice has a probability of 0.5); model 3 is a model with separate learning rates for learning from reward versus reward omission (n = 10 mice). (b) Best-fit model parameters for the experimental mice are shown in Fig. 3; each dot indicates an individual mouse/session. The two panels show the relation between learning rate ɑ (left) and explore/exploit parameter β (right) versus the number of achieved reversal per 100 trials; lines indicate least-squares linear fit and its 95% confidence intervals; significance was tested by means of two-sided t-test.
Extended Data Fig. 8 Dopamine release in NAcLat, but not NAcMed, encodes negative RPE.
(a) Graphs showing licking behavior of a representative sample animal during the task. Note that licking generally started ~500 ms after CS+ onset with only minimal variability. Inset shows schematic of experimental design: Head fixed mice were trained on a behavioral assay, in which the presence of a CS+ predicted the availability of a reward (3 μl of a 1% sucrose solution). The reward was delivered to the animal after one single lick (that is, it assumes minimal effort to the animal to obtain a reward). After training, mice were subjected to a session in which 10% of the trials were not rewarded (omission trials). DA release was measured simultaneously in the NAcLat and NAcMed using dLight1.2. (b) Top: Heatmaps showing auROC normalized dLight responses in the NAcLat and NAcMed for all animals (n = 9 mice) in standard trials in which reward delivery occurred after the first lick during CS+ presentation. Bottom: mean DA response in NAcLat (blue) and NAcMed (orange) as well as the mean lick rate (dashed line) for all mice. Note that NAcLat DA release tracks CS+ onset and reward delivery, while NAcMed DA tracks licking behavior, including reward consumption. (c) Same as in panel (b) but for omission trials in which no reward was delivered. Note that NAcMed DA release returns to baseline concurrent with the termination of licking behavior. Conversely, NAcLat DA release showed a negative deflection below baseline level during reward omission, which reflects a negative RPE. (d) Quantification (box plot, median and quantiles) of panels (b) and (c). Significance was calculated by means of a two-sided Students t-test; ***P < 0.001, n = 9 mice. All data, except panel (d), are represented as mean ± SEM (shading).
Extended Data Fig. 9 Dopamine release patterns for all mice used in experiment shown in Fig. 2g–o.
NAcLat (left), NAcMed (middle) DA release was z-score normalized and trials were sorted by interval length. The derivative of NAcMed DA release (dNAcMed/dt, right) was calculated by determining the slope of a line that was fitted (linear regression) over a 500 ms interval at each time point. Mouse 4 is the sample animal shown in Fig. 2i; mouse 1 is the sample animal shown in Fig. 2o. Although there is substantive similarity in the pattern of DA release in the NAcLat with the temporal derivative of DA release in the NAcMed, there are two important limitations with such a comparison. First, the release of DA in the NAc reflects a complex response to activity in DA cell bodies in the VTA. As shown in Extended Data Fig. 4, NAc DA release is a convolution of VTA cell body activity. Second, there is significant between animal variation. For example, mouse 6 lacks a transient increase in the dNAcMed/dt trace in response to reward delivery. Additionally, while most mice show a dip in NAcLat DA release below the baseline between the onset of CS+ and reward delivery, only mouse 2 and mouse 5 exhibit a downward slope in NAcMed DA release during the same period (see also Fig. 2j,n).
Extended Data Fig. 10 Dopamine release patterns in NAcMed and NAcLat in response to aversive stimuli can be conceptualized in the context of state and rate-of-change encoding.
(a) Hypothesis: the decrease in NAcLat DA release in response to a mild electric tail shock reflects the derivative of the downward slope in NAcMed DA release. If this is true, a sustained decrease in NAcLat DA release should be preceded by a brief increase that reflects the derivative of the onset of DA release in the NAcMed. (b) Experimental data: Head-fixed mice (n = 12 mice) were subjected to 10 unpredictable tail shocks while DA release in NAcMed and NAcLat was recorded using dLight 1.3. Left: Sustained increase in DA release in the NAcMed. Right: Sharp increase at shock onset that is followed by sustained below-baseline reduction of NAcLat DA release. While the gradual downward slope in the NAcMed and the transient increase in the NAcLat at shock onset suggest that our hypothesis is correct, there are two limitations that may be due to variability between experimental animals. First, the NAcMed DA response appears to be delayed compared to the NAcLat DA increase. Second, there is a sharp dip in NAcLat DA release that did not directly correspond to a sharp decrease in NAcMed DA release. All data are represented as mean ± SEM (shading).
Supplementary information
Source data
Source Data Fig. 1
Source data.
Source Data Fig. 2
Source data.
Source Data Fig. 3
Source data.
Source Data Fig. 4
Source data.
Source Data Extended Data Fig. 2
Source data.
Source Data Extended Data Fig. 3
Source data.
Source Data Extended Data Fig. 4
Source data.
Source Data Extended Data Fig. 5
Source data.
Source Data Extended Data Fig. 7
Source data.
Source Data Extended Data Fig. 8
Source data.
Source Data Extended Data Fig. 9
Source data.
Source Data Extended Data Fig. 10
Source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
de Jong, J.W., Liang, Y., Verharen, J.P.H. et al. State and rate-of-change encoding in parallel mesoaccumbal dopamine pathways. Nat Neurosci 27, 309–318 (2024). https://doi.org/10.1038/s41593-023-01547-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41593-023-01547-6