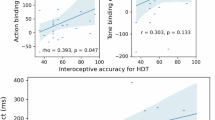
Abstract
Sensing internal bodily signals, or interoception, is fundamental to maintain life. However, interoception should not be viewed as an isolated domain, as it interacts with exteroception, cognition and action to ensure the integrity of the organism. Focusing on cardiac, respiratory and gastric rhythms, we review evidence that interoception is anatomically and functionally intertwined with the processing of signals from the external environment. Interactions arise at all stages, from the peripheral transduction of interoceptive signals to sensory processing and cortical integration, in a network that extends beyond core interoceptive regions. Interoceptive rhythms contribute to functions ranging from perceptual detection up to sense of self, or conversely compete with external inputs. Renewed interest in interoception revives long-standing issues on how the brain integrates and coordinates information in distributed regions, by means of oscillatory synchrony, predictive coding or multisensory integration. Considering interoception and exteroception in the same framework paves the way for biological modes of information processing specific to living organisms.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Sherrington, C. S. The Integrative Action of the Nervous System (Yale University Press, 1906).
Khalsa, S. S. et al. Interoception and mental health: a roadmap. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 3, 501–513 (2018).
Petzschner, F. H., Critchley, H. & Tallon-Baudry, C. Interoception. Scholarpedia https://doi.org/10.4249/scholarpedia.55569 (2022).
Berntson, G. G. & Khalsa, S. S. Neural circuits of interoception. Trends Neurosci. 44, 17–28 (2021).
Critchley, H. D. & Harrison, N. A. Visceral influences on brain and behavior. Neuron 77, 624–638 (2013).
Chen, W. G. et al. The emerging science of interoception: sensing, integrating, interpreting, and regulating signals within the self. Trends Neurosci. 44, 3–16 (2021).
Rinaman, L. & Schwartz, G. Anterograde transneuronal viral tracing of central viscerosensory pathways in rats. J. Neurosci. 24, 2782–2786 (2004).
Saper, C. B. & Loewy, A. D. Efferent connections of the parabrachial nucleus in the rat. Brain Res. 197, 291–317 (1980).
Cechetto, D. F. & Saper, C. B. Evidence for a viscerotopic sensory representation in the cortex and thalamus in the rat. J. Comp. Neurol. 262, 27–45 (1987).
Penzo, M. A. & Gao, C. The paraventricular nucleus of the thalamus: an integrative node underlying homeostatic behavior. Trends Neurosci. 44, 538–549 (2021).
Grady, F., Peltekian, L., Iverson, G. & Geerling, J. C. Direct parabrachial-cortical connectivity. Cereb. Cortex 30, 4811–4833 (2020).
Dum, R. P., Levinthal, D. J. & Strick, P. L. The spinothalamic system targets motor and sensory areas in the cerebral cortex of monkeys. J. Neurosci. 29, 14223–14235 (2009).
Amassian, V. E. Cortical representation of visceral afferents. J. Neurophysiol. 14, 433–444 (1951).
Bronk, D. W. & Stella, G. Afferent impulses in the carotid sinus nerve I. The relation of the discharge from single end organs to arterial blood pressure. J. Cell Comp. Physiol. 1, 113–130 (1932).
Paintal, A. S. Vagal sensory receptors and their reflex effects. Physiol. Rev. 53, 159–227 (1973).
Dampney, R. A., Polson, J. W., Potts, P. D., Hirooka, Y. & Horiuchi, J. Functional organization of brain pathways subserving the baroreceptor reflex: studies in conscious animals using immediate early gene expression. Cell Mol. Neurobiol. 23, 597–616 (2003).
Berntson, G. G., Quigley, K. S. & Lozano, D. in Handbook of Psychophysiology (eds. J. T. Cacioppo et al.) 898 (Cambridge University Press, 2007).
Bishop, V. S., Malliani, A. & Thoren, P. in Handbook of Physiology, Section 2: The Cardiovascular System Vol. III (eds. J. T. Shepherd & F. M. Abboud) 497–555 (Waverly Press, 1983).
Birznieks, I., Boonstra, T. W. & Macefield, V. G. Modulation of human muscle spindle discharge by arterial pulsations - functional effects and consequences. PLoS ONE 7, e35091 (2012).
Ford, T. W. & Kirkwood, P. A. Cardiac modulation of alpha motoneuron discharges. J. Neurophysiol. 119, 1723–1730 (2018).
Macefield, V. G. Cardiovascular and respiratory modulation of tactile afferents in the human finger pad. Exp. Physiol. 88, 617–625 (2003).
Kim, K. J., Ramiro Diaz, J., Iddings, J. A. & Filosa, J. A. Vasculo-neuronal coupling: retrograde vascular communication to brain neurons. J. Neurosci. 36, 12624–12639 (2016).
Marina, N. et al. Astrocytes monitor cerebral perfusion and control systemic circulation to maintain brain blood flow. Nature Commun. https://doi.org/10.1038/s41467-019-13956-y (2020).
Wang, J. & Hamill, O. P. Piezo2-peripheral baroreceptor channel expressed in select neurons of the mouse brain: a putative mechanism for synchronizing neural networks by transducing intracranial pressure pulses. J. Integr. Neurosci. 20, 825–837 (2021).
Kefauver, J. M., Ward, A. B. & Patapoutian, A. Discoveries in structure and physiology of mechanically activated ion channels. Nature 587, 567–576 (2020).
Moore, C. I. & Cao, R. The hemo-neural hypothesis: on the role of blood flow in information processing. J. Neurophysiol. 99, 2035–2047 (2008).
Beissner, F., Meissner, K., Bar, K. J. & Napadow, V. The autonomic brain: an activation likelihood estimation meta-analysis for central processing of autonomic function. J. Neurosci. 33, 10503–10511 (2013).
Kim, K. et al. Resting-state neural firing rate is linked to cardiac cycle duration in the human cingulate and parahippocampal cortices. J. Neurosci. https://doi.org/10.1523/JNEUROSCI.2291-18.2019 (2019).
Vila, J. et al. Cardiac defense: from attention to action. Int. J. Psychophysiol. 66, 169–182 (2007).
Klein, A. S., Dolensek, N., Weiand, C. & Gogolla, N. Fear balance is maintained by bodily feedback to the insular cortex in mice. Science 374, 1010–1015 (2021).
Lacey, B. C. & Lacey, J. I. Presidential address, 1979. Cognitive modulation of time-dependent primary bradycardia. Psychophysiology 17, 209–221 (1980).
Crone, E. A., Bunge, S. A., de Klerk, P. & van der Molen, M. W. Cardiac concomitants of performance monitoring: context dependence and individual differences. Brain Res. Cogn. Brain Res. 23, 93–106 (2005).
Park, H. D., Correia, S., Ducorps, A. & Tallon-Baudry, C. Spontaneous fluctuations in neural responses to heartbeats predict visual detection. Nat. Neurosci. 17, 612–618 (2014).
Raimondo, F. et al. Brain-heart interactions reveal consciousness in noncommunicating patients. Ann. Neurol. 82, 578–591 (2017).
Motyka, P. et al. Interactions between cardiac activity and conscious somatosensory perception. Psychophysiology 56, e13424 (2019).
Grund, M. et al. Respiration, heartbeat, and conscious tactile perception. J. Neurosci. 42, 643–656 (2022).
Banellis, L. & Cruse, D. Skipping a beat: heartbeat-evoked potentials reflect predictions during interoceptive-exteroceptive integration. Cereb. Cortex Commun. 1, tgaa060 (2020).
Marshall, A. C., Gentsch-Ebrahimzadeh, A. & Schütz-Bosbach, S. From the inside out: interoceptive feedback facilitates the integration of visceral signals for efficient sensory processing. Neuroimage 251, 119011 (2022).
van Elk, M., Lenggenhager, B., Heydrich, L. & Blanke, O. Suppression of the auditory N1-component for heartbeat-related sounds reflects interoceptive predictive coding. Biol. Psychol. 99, 172–182 (2014).
Salomon, R. et al. The insula mediates access to awareness of visual stimuli presented synchronously to the heartbeat. J. Neurosci. 36, 5115–5127 (2016).
Maister, L., Tang, T. & Tsakiris, M. Neurobehavioral evidence of interoceptive sensitivity in early infancy. Elife 6, e25318 (2017).
Charbonneau, J. A., Maister, L., Tsakiris, M. & Bliss-Moreau, E. Rhesus monkeys have an interoceptive sense of their beating hearts. Proc. Natl Acad. Sci. USA 119, e2119868119 (2022).
Suzuki, K., Garfinkel, S. N., Critchley, H. D. & Seth, A. K. Multisensory integration across exteroceptive and interoceptive domains modulates self-experience in the rubber-hand illusion. Neuropsychologia 51, 2909–2917 (2013).
Sel, A., Azevedo, R. T. & Tsakiris, M. Heartfelt self: cardio-visual integration affects self-face recognition and interoceptive cortical processing. Cereb. Cortex 27, 5144–5155 (2017).
Aspell, J. E. et al. Turning body and self inside out: visualized heartbeats alter bodily self-consciousness and tactile perception. Psychol. Sci. 24, 2445–2453 (2013).
Seth, A. K., Suzuki, K. & Critchley, H. D. An interoceptive predictive coding model of conscious presence. Front. Psychol. 2, 395 (2011).
Park, H. D. & Tallon-Baudry, C. The neural subjective frame: from bodily signals to perceptual consciousness. Philos. Trans. R. Soc. Lond. B Biol. Sci. 369, 20130208 (2014).
Park, H. D. & Blanke, O. Coupling inner and outer body for self-consciousness. Trends Cogn. Sci. 23, 377–388 (2019).
Azzalini, D., Rebollo, I. & Tallon-Baudry, C. Visceral signals shape brain dynamics and cognition. Trends Cogn. Sci. 23, 488–509 (2019).
Koch, E. Die irradiation der pressoreceptorischen kreislaufreflexe. Klinische Wochenschr. 11, 225–227 (1932).
Bonvallet, M., Dell, P. & Hiebel, G. Tonus sympathique et activité électrique corticale. Electroencephalogr. Clin. Neurophysiol. 6, 119–144 (1954).
Persson, B. & Svensson, T. H. Control of behaviour and brain noradrenaline neurons by peripheral blood volume receptors. J. Neural Transm. 52, 73–82 (1981).
Dworkin, B. R. et al. Central effects of baroreceptor activation in humans: attenuation of skeletal reflexes and pain perception. Proc. Natl Acad. Sci. USA 91, 6329–6333 (1994).
Wilkinson, M., McIntyre, D. & Edwards, L. Electrocutaneous pain thresholds are higher during systole than diastole. Biol. Psychol. 94, 71–73 (2013).
Edwards, L., Ring, C., McIntyre, D. & Carroll, D. Modulation of the human nociceptive flexion reflex across the cardiac cycle. Psychophysiology 38, 712–718 (2001).
Edwards, L., McIntyre, D., Carroll, D., Ring, C. & Martin, U. The human nociceptive flexion reflex threshold is higher during systole than diastole. Psychophysiology 39, 678–681 (2002).
Skora, L. I., Livermore, J. J. A. & Roelofs, K. The functional role of cardiac activity in perception and action. Neurosci. Biobehav. Rev. 137, 104655 (2022).
Al, E. et al. Heart-brain interactions shape somatosensory perception and evoked potentials. Proc. Natl Acad. Sci. USA 117, 10575–10584 (2020).
Al, E., Iliopoulos, F., Nikulin, V. V. & Villringer, A. Heartbeat and somatosensory perception. Neuroimage 238, 118247 (2021).
Saxon, S. A. Detection of near threshold signals during four phases of cardiac cycle. Ala. J. Med. Sci. 7, 427–430 (1970).
Schulz, A. et al. Cardiac modulation of startle: effects on eye blink and higher cognitive processing. Brain Cogn. 71, 265–271 (2009).
Schulz, A. et al. Cardiac cycle phases affect auditory-evoked potentials, startle eye blink and pre-motor reaction times in response to acoustic startle stimuli. Int. J. Psychophysiol. 157, 70–81 (2020).
Cohen, R., Lieb, H. & Rist, F. Loudness judgments, evoked-potentials, and reaction-time to acoustic stimuli early and late in the cardiac cycle in chronic-schizophrenics. Psychiatry Res. 3, 23–29 (1980).
Delfini, L. F. & Campos, J. J. Signal detection and the ‘cardiac arousal cycle’. Psychophysiology 9, 484–491 (1972).
Velden, M. & Juris, M. Perceptual performance as a function of intra-cycle cardiac activity. Psychophysiology 12, 685–692 (1975).
Elliott, R. & Graf, V. Visual sensitivity as a function of phase of cardiac cycle. Psychophysiology 9, 357–361 (1972).
Sandman, C. A., Mccanne, T. R., Kaiser, D. N. & Diamond, B. Heart-rate and cardiac phase influences on visual-perception. J. Comp. Physiol. Psych. 91, 189–202 (1977).
Ohl, S., Wohltat, C., Kliegl, R., Pollatos, O. & Engbert, R. Microsaccades are coupled to heartbeat. J. Neurosci. 36, 1237–1241 (2016).
Galvez-Pol, A., McConnell, R. & Kilner, J. M. Active sampling in visual search is coupled to the cardiac cycle. Cognition 196, 104149 (2020).
Kunzendorf, S. et al. Active information sampling varies across the cardiac cycle. Psychophysiology https://doi.org/10.1111/psyp.13322 (2019).
Palser, E. R., Glass, J., Fotopoulou, A. & Kilner, J. M. Relationship between cardiac cycle and the timing of actions during action execution and observation. Cognition 217, 104907 (2021).
Konttinen, N., Mets, T., Lyytinen, H. & Paananen, M. Timing of triggering in relation to the cardiac cycle in nonelite rifle shooters. Res. Q. Exerc. Sport 74, 395–400 (2003).
Helin, P., Sihvonen, T. & Hänninen, O. Timing of triggering action of shooting in relation to the cardiac cycle. Br. J. Sports Med. 21, 33–36 (1987).
Herman, A. M. & Tsakiris, M. Feeling in control: the role of cardiac timing in the sense of agency. Affect. Sci. 1, 155–171 (2020).
Park, H. D. et al. Breathing is coupled with voluntary action and the cortical readiness potential. Nat. Commun. 11, 289 (2020).
Gray, M. A., Minati, L., Paoletti, G. & Critchley, H. D. Baroreceptor activation attenuates attentional effects on pain-evoked potentials. Pain 151, 853–861 (2010).
Schandry, R. & Montoya, P. Event-related brain potentials and the processing of cardiac activity. Biol. Psychol. 42, 75–85 (1996).
Gray, M. A. et al. A cortical potential reflecting cardiac function. Proc. Natl Acad. Sci. USA 104, 6818–6823 (2007).
Park, H. D. & Blanke, O. Heartbeat-evoked cortical responses: underlying mechanisms, functional roles, and methodological considerations. Neuroimage 197, 502–511 (2019).
Buot, A., Azzalini, D., Chaumon, M. & Tallon-Baudry, C. Does stroke volume influence heartbeat-evoked responses. Biol. Psychol. 165, 108165 (2021).
Coll, M. P., Hobson, H., Bird, G. & Murphy, J. Systematic review and meta-analysis of the relationship between the heartbeat-evoked potential and interoception. Neurosci. Biobehav. Rev. 122, 190–200 (2021).
Kern, M., Aertsen, A., Schulze-Bonhage, A. & Ball, T. Heart cycle-related effects on event-related potentials, spectral power changes, and connectivity patterns in the human ECoG. Neuroimage 81, 178–190 (2013).
Azzalini, D., Buot, A., Palminteri, S. & Tallon-Baudry, C. Responses to heartbeats in ventromedial prefrontal cortex contribute to subjective preference-based decisions. J. Neurosci. 41, 5102–5114 (2021).
Babo-Rebelo, M., Richter, C. G. & Tallon-Baudry, C. Neural responses to heartbeats in the default network encode the self in spontaneous thoughts. J. Neurosci. 36, 7829–7840 (2016).
Babo-Rebelo, M., Wolpert, N., Adam, C., Hasboun, D. & Tallon-Baudry, C. Is the cardiac monitoring function related to the self in both the default network and right anterior insula? Philos. Trans. R Soc. Lond. B Biol. Sci. https://doi.org/10.1098/rstb.2016.0004 (2016).
Park, H. D. et al. Transient modulations of neural responses to heartbeats covary with bodily self-consciousness. J. Neurosci. 36, 8453–8460 (2016).
Babo-Rebelo, M., Buot, A. & Tallon-Baudry, C. Neural responses to heartbeats distinguish self from other during imagination. Neuroimage 191, 10–20 (2019).
Couto, B. et al. Heart evoked potential triggers brain responses to natural affective scenes: a preliminary study. Auton. Neurosci. 193, 132–137 (2015).
Park, H. D. et al. Neural sources and underlying mechanisms of neural responses to heartbeats, and their role in bodily self-consciousness: an intracranial EEG study. Cereb. Cortex 28, 2351–2364 (2018).
Kim, J. et al. Sad faces increase the heartbeat-associated interoceptive information flow within the salience network: a MEG study. Sci. Rep. 9, 430 (2019).
Engelen, T., Buot, A., Grezes, J. & Tallon-Baudry, C. Whose emotion is it? Perspective matters to understand brain–body interactions in emotions. Neuroimage 268, 119867 (2023).
Schandry, R., Sparrer, B. & Weitkunat, R. From the heart to the brain: a study of heartbeat contingent scalp potentials. Int. J. Neurosci. 30, 261–275 (1986).
Montoya, P., Schandry, R. & Muller, A. Heartbeat evoked potentials (HEP): topography and influence of cardiac awareness and focus of attention. Electroencephalogr. Clin. Neurophysiol. 88, 163–172 (1993).
Pollatos, O. & Schandry, R. Accuracy of heartbeat perception is reflected in the amplitude of the heartbeat-evoked brain potential. Psychophysiology 41, 476–482 (2004).
Pollatos, O., Kirsch, W. & Schandry, R. Brain structures involved in interoceptive awareness and cardioafferent signal processing: a dipole source localization study. Hum. Brain Mapp. 26, 54–64 (2005).
Villena-Gonzalez, M. et al. Attending to the heart is associated with posterior alpha band increase and a reduction in sensitivity to concurrent visual stimuli. Psychophysiology 54, 1483–1497 (2017).
García-Cordero, I. et al. Feeling, learning from and being aware of inner states: interoceptive dimensions in neurodegeneration and stroke. Philos. Trans. R. Soc. B Biol. Sci. 371, 20160006 (2016).
Petzschner, F. H. et al. Focus of attention modulates the heartbeat evoked potential. Neuroimage 180, 595–606 (2019).
Katkin, E. S., Cestaro, V. L. & Weitkunat, R. Individual differences in cortical evoked potentials as a function of heartbeat detection ability. Int. J. Neurosci. 61, 269–276 (1991).
Fittipaldi, S. et al. A multidimensional and multi-feature framework for cardiac interoception. Neuroimage 212, 116677 (2020).
Brener, J. & Ring, C. Towards a psychophysics of interoceptive processes: the measurement of heartbeat detection. Philos. Trans. R. Soc. Lond. B Biol. Sci. 371, 20160015 (2016).
Desmedt, O., Luminet, O. & Corneille, O. The heartbeat counting task largely involves non-interoceptive processes: evidence from both the original and an adapted counting task. Biol. Psychol. 138, 185–188 (2018).
Damasio, A. R. The somatic marker hypothesis and the possible functions of the prefrontal cortex. Philos. Trans. R. Soc. Lond. B Biol. Sci. 351, 1413–1420 (1996).
Fukushima, H., Terasawa, Y. & Umeda, S. Association between interoception and empathy: evidence from heart-beat evoked brain potential. Int. J. Psychophysiol. 79, 259–265 (2011).
Gentsch, A., Sel, A., Marshall, A. C. & Schutz-Bosbach, S. Affective interoceptive inference: evidence from heart-beat evoked brain potentials. Hum. Brain Mapp. 40, 20–33 (2019).
MacKinnon, S., Gevirtz, R., McCraty, R. & Brown, M. Utilizing heartbeat evoked potentials to identify cardiac regulation of vagal afferents during emotion and resonant breathing. Appl. Psychophysiol. Biofeedback 38, 241–255 (2013).
Luft, C. D. B. & Bhattacharya, J. Aroused with heart: modulation of heartbeat evoked potential by arousal induction and its oscillatory correlates. Sci. Rep. 5, 15717 (2015).
Bradley, M. M. Natural selective attention: orienting and emotion. Psychophysiology 46, 1–11 (2009).
Marshall, A. C., Gentsch, A., Blum, A.-L., Broering, C. & Schütz-Bosbach, S. I feel what I do: relating interoceptive processes and reward-related behavior. Neuroimage 191, 315–324 (2019).
Damasio, A. Self Comes to Mind: Constructing the Conscious Brain (Pantheon Books, 2010).
Blanke, O. & Metzinger, T. Full-body illusions and minimal phenomenal selfhood. Trends Cogn. Sci. 13, 7–13 (2009).
Shao, S., Shen, K., Wilder-Smith, E. P. V. & Li, X. Effect of pain perception on the heartbeat evoked potential. Clin. Neurophysiol. 122, 1838–1845 (2011).
Candia-Rivera, D. et al. Neural responses to heartbeats detect residual signs of consciousness during resting state in postcomatose patients. J. Neurosci. 41, 5251–5262 (2021).
Schulz, A. et al. Altered patterns of heartbeat-evoked potentials in depersonalization/derealization disorder: neurophysiological evidence for impaired cortical representation of bodily signals. Psychosom. Med. 77, 506–516 (2015).
Zhang, Y. S. & Ghazanfar, A. A. A hierarchy of autonomous systems for vocal production. Trends Neurosci. 43, 115–126 (2020).
Smith, J. C., Ellenberger, H. H., Ballanyi, K., Richter, D. W. & Feldman, J. L. Pre-Botzinger complex: a brainstem region that may generate respiratory rhythm in mammals. Science 254, 726–729 (1991).
Del Negro, C. A., Funk, G. D. & Feldman, J. L. Breathing matters. Nat. Rev. Neurosci. 19, 351–367 (2018).
Ito, J. et al. Whisker barrel cortex delta oscillations and gamma power in the awake mouse are linked to respiration. Nat. Commun. 5, 3572 (2014).
Yanovsky, Y., Ciatipis, M., Draguhn, A., Tort, A. B. & Brankack, J. Slow oscillations in the mouse hippocampus entrained by nasal respiration. J. Neurosci. 34, 5949–5964 (2014).
Zelano, C. et al. Nasal respiration entrains human limbic oscillations and modulates cognitive function. J. Neurosci. 36, 12448–12467 (2016).
Grosmaitre, X., Santarelli, L. C., Tan, J., Luo, M. & Ma, M. Dual functions of mammalian olfactory sensory neurons as odor detectors and mechanical sensors. Nat. Neurosci. 10, 348–354 (2007).
Davenport, P. W., Reep, R. L. & Thompson, F. J. Phrenic nerve afferent activation of neurons in the cat SI cerebral cortex. J. Physiol. 588, 873–886 (2010).
Nair, J. et al. Anatomy and physiology of phrenic afferent neurons. J. Neurophysiol. 118, 2975–2990 (2017).
Kim, S. H. et al. Mapping of the sensory innervation of the mouse lung by specific vagal and dorsal root ganglion neuronal subsets. eNeuro https://doi.org/10.1523/ENEURO.0026-22.2022 (2022).
Radna, R. J. & MacLean, P. D. Vagal elicitation of respiratory-type and other unit responses in basal limbic structures of squirrel monkeys. Brain Res. 213, 45–61 (1981).
Yang, C. F. & Feldman, J. L. Efferent projections of excitatory and inhibitory pre-Botzinger Complex neurons. J. Comp. Neurol. 526, 1389–1402 (2018).
Yackle, K. et al. Breathing control center neurons that promote arousal in mice. Science 355, 1411–1415 (2017).
Karalis, N. & Sirota, A. Breathing coordinates cortico-hippocampal dynamics in mice during offline states. Nat. Commun. 13, 467 (2022).
Tort, A. B. L., Brankack, J. & Draguhn, A. Respiration-entrained brain rhythms are global but often overlooked. Trends Neurosci. 41, 186–197 (2018).
Zhong, W. et al. Selective entrainment of gamma subbands by different slow network oscillations. Proc. Natl Acad. Sci. USA 114, 4519–4524 (2017).
Lockmann, A. L., Laplagne, D. A., Leao, R. N. & Tort, A. B. A respiration-coupled rhythm in the rat hippocampus independent of theta and slow oscillations. J. Neurosci. 36, 5338–5352 (2016).
Nguyen Chi, V. et al. Hippocampal respiration-driven rhythm distinct from theta oscillations in awake mice. J. Neurosci. 36, 162–177 (2016).
Biskamp, J., Bartos, M. & Sauer, J. F. Organization of prefrontal network activity by respiration-related oscillations. Sci. Rep. https://doi.org/10.1038/srep45508 (2017).
Bagur, S. et al. Breathing-driven prefrontal oscillations regulate maintenance of conditioned-fear evoked freezing independently of initiation. Nat. Commun. 12, 2605 (2021).
Moberly, A. H. et al. Olfactory inputs modulate respiration-related rhythmic activity in the prefrontal cortex and freezing behavior. Nat. Commun. 9, 1528 (2018).
Frysinger, R. C. & Harper, R. M. Cardiac and respiratory correlations with unit discharge in epileptic human temporal lobe. Epilepsia 31, 162–171 (1990).
Herrero, J. L., Khuvis, S., Yeagle, E., Cerf, M. & Mehta, A. D. Breathing above the brain stem: volitional control and attentional modulation in humans. J. Neurophysiol. 119, 145–159 (2018).
Arshamian, A., Iravani, B., Majid, A. & Lundstrom, J. N. Respiration modulates olfactory memory consolidation in Humans. J. Neurosci. 38, 10286–10294 (2018).
Nakamura, N. H., Fukunaga, M. & Oku, Y. Respiratory modulation of cognitive performance during the retrieval process. PLoS ONE 13, e0204021 (2018).
Perl, O. et al. Human non-olfactory cognition phase-locked with inhalation. Nat. Hum. Behav. 3, 501–512 (2019).
Rojas-Libano, D., Wimmer Del Solar, J., Aguilar-Rivera, M., Montefusco-Siegmund, R. & Maldonado, P. E. Local cortical activity of distant brain areas can phase-lock to the olfactory bulb’s respiratory rhythm in the freely behaving rat. J. Neurophysiol. 120, 960–972 (2018).
Maier, E., Lauer, S. & Brecht, M. Layer 4 organization and respiration locking in the rodent nose somatosensory cortex. J. Neurophysiol. 124, 822–832 (2020).
Kluger, D. S. & Gross, J. Depth and phase of respiration modulate cortico-muscular communication. Neuroimage 222, 117272 (2020).
Schulz, A., Schilling, T. M., Vogele, C., Larra, M. F. & Schachinger, H. Respiratory modulation of startle eye blink: a new approach to assess afferent signals from the respiratory system. Philos. Trans. R Soc. Lond. B Biol. Sci. https://doi.org/10.1098/rstb.2016.0019 (2016).
Johannknecht, M. & Kayser, C. The influence of the respiratory cycle on reaction times in sensory-cognitive paradigms. Sci. Rep. 12, 2586 (2022).
Kluger, D. S., Balestrieri, E., Busch, N. A. & Gross, J. Respiration aligns perception with neural excitability. Elife https://doi.org/10.7554/eLife.70907.sa2 (2021).
Flexman, J. E. & Demaree, R. G. & Simpson, D. D Respiratory phase and visual signal detection. Percept. Psychophys. 16, 337–339 (1974).
Kluger, D. & Gross, J. Respiration modulates oscillatory neural network activity at rest. PLoS Biol. https://doi.org/10.1371/journal.pbio.3001457 (2021).
Monti, A., Porciello, G., Tieri, G. & Aglioti, S. M. The ‘embreathment’ illusion highlights the role of breathing in corporeal awareness. J. Neurophysiol. 123, 420–427 (2020).
Adler, D., Herbelin, B., Similowski, T. & Blanke, O. Breathing and sense of self: visuo-respiratory conflicts alter body self-consciousness. Respir. Physiol. Neurobiol. 203, 68–74 (2014).
Tort, A. B. L., Hammer, M., Zhang, J., Brankack, J. & Draguhn, A. Temporal relations between cortical network oscillations and breathing frequency during REM Sleep. J. Neurosci. 41, 5229–5242 (2021).
Gandevia, S. C. & Rothwell, J. C. Activation of the human diaphragm from the motor cortex. J. Physiol. 384, 109–118 (1987).
Maskill, D., Murphy, K., Mier, A., Owen, M. & Guz, A. Motor cortical representation of the diaphragm in man. J. Physiol. 443, 105–121 (1991).
Poe, G. R., Kristensen, M. P., Rector, D. M. & Harper, R. M. Hippocampal activity during transient respiratory events in the freely behaving cat. Neuroscience 72, 39–48 (1996).
Kaelberer, M. M. et al. A gut-brain neural circuit for nutrient sensory transduction. Science https://doi.org/10.1126/science.aat5236 (2018).
Han, W. et al. A neural circuit for gut-induced reward. Cell 175, 887–888 (2018).
Ichiki, T. et al. Sensory representation and detection mechanisms of gut osmolality change. Nature 602, 468–474 (2022).
Mercado-Perez, A. & Beyder, A. Gut feelings: mechanosensing in the gastrointestinal tract. Nat. Rev. Gastroenterol. Hepatol. 19, 283–296 (2022).
Kim, M., Heo, G. & Kim, S.-Y. Neural signalling of gut mechanosensation in ingestive and digestive processes. Nat. Rev. Neurosci. 23, 135–156 (2022).
Bozler, E. The action potentials of the stomach. Am. J. Physiol. 144, 693–700 (1945).
Suzuki, N., Prosser, C. L. & Dahms, V. Boundary cells between longitudinal and circular layers—essential for electrical slow waves in cat intestine. Am. J. Physiol. 250, G287–G294 (1986).
Sanders, K. M., Koh, S. D. & Ward, S. M. Interstitial cells of Cajal as pacemakers in the gastrointestinal tract. Annu. Rev. Physiol. 68, 307–343 (2006).
Huizinga, J. D. & Chen, J. H. Interstitial cells of Cajal: update on basic and clinical science. Curr. Gastroenterol. Rep. 16, 363 (2014).
Hong, G.-S. et al. Effect of transcutaneous vagus nerve stimulation on muscle activity in the gastrointestinal tract (transVaGa): a prospective clinical trial. Int. J. Colorectal Dis. 34, 417–422 (2019).
Teckentrup, V. et al. Non-invasive stimulation of vagal afferents reduces gastric frequency. Brain Stimul. 13, 470–473 (2020).
Levinthal, D. J. & Strick, P. L. Multiple areas of the cerebral cortex influence the stomach. Proc. Natl Acad. Sci. USA 117, 13078–13083 (2020).
Paintal, A. S. Impulses in vagal afferent fibres from stretch receptors in the stomach and their role in the peripheral mechanism of hunger. Nature 172, 1194–1195 (1953).
Andrews, P. L., Grundy, D. & Scratcherd, T. Vagal afferent discharge from mechanoreceptors in different regions of the ferret stomach. J. Physiol. 298, 513–524 (1980).
Cao, J., Wang, X., Powley, T. L. & Liu, Z. Gastric neurons in the nucleus tractus solitarius are selective to the orientation of gastric electrical stimulation. J. Neural Eng. 18, 056066 (2021).
Traub, R. J., Sengupta, J. N. & Gebhart, G. F. Differential c-Fos expression in the nucleus of the solitary tract and spinal cord following noxious gastric distention in the rat. Neuroscience 74, 873–884 (1996).
Ozaki, N. & Gebhart, G. F. Characterization of mechanosensitive splanchnic nerve afferent fibers innervating the rat stomach. Am. J. Physiol. Gastrointest. Liver Physiol. 281, G1449–G1459 (2001).
Stephan, E. et al. Functional neuroimaging of gastric distention. J. Gastrointest. Surg. 7, 740–749 (2003).
Ladabaum, U. et al. Gastric distention correlates with activation of multiple cortical and subcortical regions. Gastroenterology 120, 369–376 (2001).
Vandenbergh, J. et al. Regional brain activation during proximal stomach distention in humans: a positron emission tomography study. Gastroenterology 128, 564–573 (2005).
van Oudenhove, L. et al. Cortical deactivations during gastric fundus distension in health: visceral pain-specific response or attenuation of ‘default mode’ brain function? A H2 15O-PET study. Neurogastroenterol. Motil. 21, 259–271 (2009).
Spetter, M. S., de Graaf, C., Mars, M., Viergever, M. A. & Smeets, P. A. The sum of its parts–effects of gastric distention, nutrient content and sensory stimulation on brain activation. PLoS ONE 9, e90872 (2014).
Wang, G.-J. et al. Gastric distention activates satiety circuitry in the human brain. Neuroimage 39, 1824–1831 (2008).
Cao, J. et al. Gastric stimulation drives fast BOLD responses of neural origin. Neuroimage 197, 200–211 (2019).
Pigarev, I. N. Neurons of visual cortex respond to visceral stimulation during slow wave sleep. Neuroscience 62, 1237–1243 (1994).
Rebollo, I., Devauchelle, A. D., Beranger, B. & Tallon-Baudry, C. Stomach-brain synchrony reveals a novel, delayed-connectivity resting-state network in humans. Elife 7, e33321 (2018).
Rebollo, I. & Tallon-Baudry, C. The sensory and motor components of the cortical hierarchy are coupled to the rhythm of the stomach during rest. J. Neurosci. 42, 2205–2220 (2022).
Cao, J., Wang, X., Chen, J., Zhang, N. & Liu, Z. The vagus nerve mediates the stomach-brain coherence in rats. Neuroimage 263, 119628 (2022).
Richter, C. G., Babo-Rebelo, M., Schwartz, D. & Tallon-Baudry, C. Phase-amplitude coupling at the organism level: the amplitude of spontaneous alpha rhythm fluctuations varies with the phase of the infra-slow gastric basal rhythm. Neuroimage 146, 951–958 (2017).
Levakov, G. et al. Neural correlates of future weight loss reveal a possible role for brain-gastric interactions. Neuroimage 224, 117403 (2021).
Todd, J., Cardellicchio, P., Swami, V., Cardini, F. & Aspell, J. E. Weaker implicit interoception is associated with more negative body image: evidence from gastric-alpha phase amplitude coupling and the heartbeat evoked potential. Cortex 143, 254–266 (2021).
Laubach, M., Amarante, L. M., Swanson, K. & White, S. R. What, if anything, is rodent prefrontal cortex? eNeuro https://doi.org/10.1523/ENEURO.0315-18.2018 (2018).
Craig, A. D. How do you feel? Interoception: the sense of the physiological condition of the body. Nat. Rev. Neurosci. 3, 655–666 (2002).
Erisir, A., Van Horn, S. C. & Sherman, S. M. Relative numbers of cortical and brainstem inputs to the lateral geniculate nucleus. Proc. Natl Acad. Sci. USA 94, 1517–1520 (1997).
Erisir, A., Van Horn, S. C., Bickford, M. E. & Sherman, S. M. Immunocytochemistry and distribution of parabrachial terminals in the lateral geniculate nucleus of the cat: a comparison with corticogeniculate terminals. J. Comp. Neurol. 377, 535–549 (1997).
Uhlrich, D. J., Tamamaki, N., Murphy, P. C. & Sherman, S. M. Effects of brain stem parabrachial activation on receptive field properties of cells in the cat’s lateral geniculate nucleus. J. Neurophysiol. 73, 2428–2447 (1995).
Farley, G. R., Barlow, S. M. & Netsell, R. Factors influencing neural activity in parabrachial regions during cat vocalizations. Exp. Brain Res. 89, 341–351 (1992).
Smotherman, M., Kobayasi, K., Ma, J., Zhang, S. & Metzner, W. A mechanism for vocal-respiratory coupling in the mammalian parabrachial nucleus. J. Neurosci. 26, 4860–4869 (2006).
Levinthal, D. J. & Strick, P. L. The motor cortex communicates with the kidney. J. Neurosci. 32, 6726–6731 (2012).
van den Heuvel, M. P. & Sporns, O. Network hubs in the human brain. Trends Cogn. Sci. 17, 683–696 (2013).
Criscuolo, A., Schwartze, M. & Kotz, S. A. Cognition through the lens of a body-brain dynamic system. Trends Neurosci. 45, 667–677 (2022).
Singer, W. Synchronization of cortical activity and its putative role in information processing and learning. Annu. Rev. Physiol. 55, 349–374 (1993).
Fries, P. A mechanism for cognitive dynamics: neuronal communication through neuronal coherence. Trends Cogn. Sci. 9, 474–480 (2005).
Buzsaki, G., Logothetis, N. & Singer, W. Scaling brain size, keeping timing: evolutionary preservation of brain rhythms. Neuron 80, 751–764 (2013).
Wolpert, N., Rebollo, I. & Tallon-Baudry, C. Electrogastrography for psychophysiological research: practical considerations, analysis pipeline, and normative data in a large sample. Psychophysiology 57, e13599 (2020).
Shadlen, M. N. & Movshon, J. A. Synchrony unbound: a critical evaluation of the temporal binding hypothesis. Neuron 24, 67–77 (1999).
Behrens, T. E. J. et al. What Is a cognitive map? Organizing knowledge for flexible behavior. Neuron 100, 490–509 (2018).
Busch, N. A. & VanRullen, R. Spontaneous EEG oscillations reveal periodic sampling of visual attention. Proc. Natl Acad. Sci. USA 107, 16048–16053 (2010).
Wachowiak, M. All in a sniff: olfaction as a model for active sensing. Neuron 71, 962–973 (2011).
Lakatos, P., Gross, J. & Thut, G. A new unifying account of the roles of neuronal entrainment. Curr. Biol. 29, R890–R905 (2019).
Allen, M., Levy, A., Parr, T. & Friston, K. J. In the body’s eye: the computational anatomy of interoceptive inference. PLoS Comput. Biol. 18, e1010490 (2022).
Allen, M., Varga, S. & Heck, D. H. Respiratory rhythms of the predictive mind. Psychol. Rev. https://doi.org/10.1037/rev0000391 (2022).
Petzschner, F. H., Garfinkel, S. N., Paulus, M. P., Koch, C. & Khalsa, S. S. Computational models of interoception and body regulation. Trends Neurosci. 44, 63–76 (2021).
Livneh, Y. et al. Estimation of current and future physiological states in insular cortex. Neuron 105, 1094–1111 (2020).
Gu, X., Hof, P. R., Friston, K. J. & Fan, J. Anterior insular cortex and emotional awareness. J. Comp. Neurol. 521, 3371–3388 (2013).
Seth, A. K. Interoceptive inference, emotion, and the embodied self. Trends Cogn. Sci. 17, 565–573 (2013).
Barrett, L. F. & Simmons, W. K. Interoceptive predictions in the brain. Nat. Rev. Neurosci. 16, 419–429 (2015).
Kleckner, I. R. et al. Evidence for a large-scale brain system supporting allostasis and interoception in humans. Nat. Hum. Behav. 1, 0069 (2017).
Bruggemann, J., Shi, T. & Apkarian, A. V. Viscero-somatic neurons in the primary somatosensory cortex (SI) of the squirrel monkey. Brain Res. 756, 297–300 (1997).
Potts, J. T. Neural circuits controlling cardiorespiratory responses: baroreceptor and somatic afferents in the nucleus tractus solitarius. Clin. Exp. Pharm. Physiol. 29, 103–111 (2002).
Stein, B. E. & Stanford, T. R. Multisensory integration: current issues from the perspective of the single neuron. Nat. Rev. Neurosci. 9, 255–266 (2008).
Blanke, O., Slater, M. & Serino, A. Behavioral, neural, and computational principles of bodily self-consciousness. Neuron 88, 145–166 (2015).
Glover, G. H., Li, T. Q. & Ress, D. Image-based method for retrospective correction of physiological motion effects in fMRI: RETROICOR. Magn. Reson. Med. 44, 162–167 (2000).
Murphy, K. & Fox, M. D. Towards a consensus regarding global signal regression for resting state functional connectivity MRI. Neuroimage 154, 169–173 (2017).
Mosher, C. P. et al. Cellular classes in the human brain revealed in vivo by heartbeat-related modulation of the extracellular action potential waveform. Cell Rep. 30, 3536–3551 (2020).
Scholkmann, F. et al. A review on continuous wave functional near-infrared spectroscopy and imaging instrumentation and methodology. Neuroimage 85, 6–27 (2014).
Dirlich, G., Vogl, L., Plaschke, M. & Strian, F. Cardiac field effects on the EEG. Electroencephalogr. Clin. Neurophysiol. 102, 307–315 (1997).
Jousmaki, V. & Hari, R. Cardiac artifacts in magnetoencephalogram. J. Clin. Neurophysiol. 13, 172–176 (1996).
Wolpert, N. & Tallon-Baudry, C. Coupling between the phase of a neural oscillation or bodily rhythm with behavior: evaluation of different statistical procedures. Neuroimage 236, 118050 (2021).
Sherman, M. T., Wang, H. T., Garfinkel, S. N. & Critchley, H. D. The Cardiac Timing Toolbox (CaTT): testing for physiologically plausible effects of cardiac timing on behaviour. Biol. Psychol. 170, 108291 (2022).
Kreibig, S. D. Autonomic nervous system activity in emotion: a review. Biol. Psychol. 84, 394–421 (2010).
Ebert, D., Rassler, B. & Hefter, H. Coordination between breathing and forearm movements during sinusoidal tracking. Eur. J. Appl. Physiol. 81, 288–296 (2000).
Farb, N. A., Segal, Z. V. & Anderson, A. K. Attentional modulation of primary interoceptive and exteroceptive cortices. Cereb. Cortex 23, 114–126 (2013).
Smoller, J. W., Pollack, M. H., Otto, M. W., Rosenbaum, J. F. & Kradin, R. L. Panic anxiety, dyspnea, and respiratory disease. Theoretical and clinical considerations. Am. J. Respir. Crit. Care Med. 154, 6–17 (1996).
Jelincic, V., Van Diest, I., Torta, D. M. & von Leupoldt, A. The breathing brain: The potential of neural oscillations for the understanding of respiratory perception in health and disease. Psychophysiology 59, e13844 (2022).
Harrison, O. K. et al. Interoception of breathing and its relationship with anxiety. Neuron 109, 4080–4093 (2021).
Li, S. & Rymer, W. Z. Voluntary breathing influences corticospinal excitability of nonrespiratory finger muscles. J. Neurophysiol. 105, 512–521 (2011).
Saoji, A. A., Raghavendra, B. R. & Manjunath, N. K. Effects of yogic breath regulation: a narrative review of scientific evidence. J. Ayurveda Integr. Med. 10, 50–58 (2019).
Balasubramaniam, M., Telles, S. & Doraiswamy, P. M. Yoga on our minds: a systematic review of yoga for neuropsychiatric disorders. Front. Psychiatry 3, 117 (2012).
Farb, N. A., Segal, Z. V. & Anderson, A. K. Mindfulness meditation training alters cortical representations of interoceptive attention. Soc. Cogn. Affect Neurosci. 8, 15–26 (2013).
Sevoz-Couche, C. & Laborde, S. Heart rate variability and slow-paced breathing:when coherence meets resonance. Neurosci. Biobehav Rev. 135, 104576 (2022).
Hsu, S. M., Tseng, C. H., Hsieh, C. H. & Hsieh, C. W. Slow-paced inspiration regularizes alpha phase dynamics in the human brain. J. Neurophysiol. 123, 289–299 (2020).
Jafari, H., Courtois, I., Van den Bergh, O., Vlaeyen, J. W. S. & Van Diest, I. Pain and respiration: a systematic review. Pain 158, 995–1006 (2017).
Laborde, S. et al. Psychophysiological effects of slow-paced breathing at six cycles per minute with or without heart rate variability biofeedback. Psychophysiology 59, e13952 (2022).
Lehrer, P. M., Vaschillo, E. G. & Vidali, V. Heart rate and breathing are not always in phase during resonance frequency breathing. Appl. Psychophysiol. Biofeedback 45, 145–152 (2020).
Acknowledgements
The authors thank N. Gerard for help with Fig. 1. This work was supported by the European Research Council under the European Union’s Horizon 2020 research and innovation program (grant agreement no. 670325, advanced grant BRAVIUS) and senior fellowship of the Canadian Institute For Advance Research (CIFAR) program in Brain, Mind and Consciousness to C.T.-B. This research was also funded by Agence Nationale pour la Recherche (ANR-17-EURE-0017, ANR-10- IDEX-0001-02). T.E. is supported by ANR-21-CE37-0031 and M.S. by a fellowship from the Geneva University Hospitals.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Neuroscience thanks Cameron McAlpine, Karin Roelofs, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Engelen, T., Solcà, M. & Tallon-Baudry, C. Interoceptive rhythms in the brain. Nat Neurosci 26, 1670–1684 (2023). https://doi.org/10.1038/s41593-023-01425-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41593-023-01425-1