Abstract
Social hierarchy is established as an outcome of individual social behaviors, such as dominance behavior during long-term interactions with others. Astrocytes are implicated in optimizing the balance between excitatory and inhibitory (E/I) neuronal activity, which may influence social behavior. However, the contribution of astrocytes in the prefrontal cortex to dominance behavior is unclear. Here we show that dorsomedial prefrontal cortical (dmPFC) astrocytes modulate E/I balance and dominance behavior in adult male mice using in vivo fiber photometry and two-photon microscopy. Optogenetic and chemogenetic activation or inhibition of dmPFC astrocytes show that astrocytes bidirectionally control male mouse dominance behavior, affecting social rank. Dominant and subordinate male mice present distinct prefrontal synaptic E/I balance, regulated by astrocyte activity. Mechanistically, we show that dmPFC astrocytes control cortical E/I balance by simultaneously enhancing presynaptic-excitatory and reducing postsynaptic-inhibitory transmission via astrocyte-derived glutamate and ATP release, respectively. Our findings show how dmPFC astrocyte–neuron communication can be involved in the establishment of social hierarchy in adult male mice.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
All data generated in this study are provided in the Source Data file. The raw data images supporting the current study’s findings are available from the corresponding author upon reasonable request. Source data are provided with this paper.
Change history
26 September 2023
A Correction to this paper has been published: https://doi.org/10.1038/s41593-023-01470-w
References
Dallerac, G., Zapata, J. & Rouach, N. Versatile control of synaptic circuits by astrocytes: where, when and how? Nat. Rev. Neurosci. 19, 729–743 (2018).
Perea, G. et al. Activity-dependent switch of GABAergic inhibition into glutamatergic excitation in astrocyte–neuron networks. eLife 5, e20362 (2016).
Lee, J. H. et al. Astrocytes phagocytose adult hippocampal synapses for circuit homeostasis. Nature 590, 612–617 (2021).
Volterra, A. & Meldolesi, J. Astrocytes, from brain glue to communication elements: the revolution continues. Nat. Rev. Neurosci. 6, 626–640 (2005).
Sohal, V. S. & Rubenstein, J. L. R. Excitation–inhibition balance as a framework for investigating mechanisms in neuropsychiatric disorders. Mol. Psychiatry 24, 1248–1257 (2019).
Yizhar, O. et al. Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature 477, 171–178 (2011).
Scheggia, D. et al. Somatostatin interneurons in the prefrontal cortex control affective state discrimination in mice. Nat. Neurosci. 23, 47–60 (2020).
Desmichel, P. & Rucker, D. D. Social-rank cues: decoding rank from physical characteristics, behaviors, and possessions. Curr. Opin. Psychol. 43, 79–84 (2022).
Sauder, M., Lynn, F. & Podolny, J. M. Status: insights from organizational sociology. Annu. Rev. Sociol. 38, 267–283 (2012).
Komori, T., Makinodan, M. & Kishimoto, T. Social status and modern-type depression: a review. Brain Behav. 9, e01464 (2019).
Sapolsky, R. M. The influence of social hierarchy on primate health. Science 308, 648–652 (2005).
Rivenbark, J. et al. Adolescents' perceptions of family social status correlate with health and life chances: a twin difference longitudinal cohort study. Proc. Natl Acad. Sci. USA 117, 23323–23328 (2020).
Wang, F., Kessels, H. W. & Hu, H. L. The mouse that roared: neural mechanisms of social hierarchy. Trends Neurosci. 37, 674–682 (2014).
Zhou, T. et al. History of winning remodels thalamo-PFC circuit to reinforce social dominance. Science 357, 162–168 (2017).
Wang, F. et al. Bidirectional control of social hierarchy by synaptic efficacy in medial prefrontal cortex. Science 334, 693–697 (2011).
Tan, S. et al. Postnatal TrkB ablation in corticolimbic interneurons induces social dominance in male mice. Proc. Natl Acad. Sci. USA 115, E9909–E9915 (2018).
Zhang, C. et al. Dynamics of a disinhibitory prefrontal microcircuit in controlling social competition. Neuron 110, 516–531 e516 (2022).
Fan, Z. X. et al. Using the tube test to measure social hierarchy in mice. Nat. Protoc. 14, 819–831 (2019).
Lindzey, G., Winston, H. & Manosevitz, M. Social dominance in inbred mouse strains. Nature 191, 474–476 (1961).
Cui, G. et al. Deep brain optical measurements of cell type-specific neural activity in behaving mice. Nat. Protoc. 9, 1213–1228 (2014).
Zhao, J., Wang, D. & Wang, J. H. Barrel cortical neurons and astrocytes coordinately respond to an increased whisker stimulus frequency. Mol. Brain 5, 12 (2012).
Yu, X. Z. et al. Reducing astrocyte calcium signaling in vivo alters striatal microcircuits and causes repetitive behavior. Neuron 99, 1170–1187 (2018).
Harada, K., Kamiya, T. & Tsuboi, T. Gliotransmitter release from astrocytes: functional, developmental, and pathological implications in the brain. Front. Neurosci. 9, 499 (2015).
Jourdain, P. et al. Glutamate exocytosis from astrocytes controls synaptic strength. Nat. Neurosci. 10, 331–339 (2007).
Rose, C. R. et al. Astroglial glutamate signaling and uptake in the hippocampus. Front. Mol. Neurosci. 10, 451 (2017).
Flanagan, B., McDaid, L., Wade, J., Wong-Lin, K. & Harkin, J. A computational study of astrocytic glutamate influence on post-synaptic neuronal excitability. PLoS Comput. Biol. 14, e1006040 (2018).
Lalo, U. et al. Exocytosis of ATP from astrocytes modulates phasic and tonic inhibition in the neocortex. PLoS Biol. 12, e1001747 (2014).
Won, J. et al. Opto-vTrap, an optogenetic trap for reversible inhibition of vesicular release, synaptic transmission, and behavior. Neuron 110, 423–435 (2022).
Tada, H. et al. Neonatal isolation augments social dominance by altering actin dynamics in the medial prefrontal cortex. Proc. Natl Acad. Sci. USA 113, E7097–E7105 (2016).
Ma, M. et al. A novel pathway regulates social hierarchy via lncRNA AtLAS and postsynaptic synapsin IIb. Cell Res. 30, 105–118 (2020).
Escartin, C. et al. Reactive astrocyte nomenclature, definitions, and future directions. Nat. Neurosci. 24, 312–325 (2021).
Ben Haim, L. & Rowitch, D. H. Functional diversity of astrocytes in neural circuit regulation. Nat. Rev. Neurosci. 18, 31–41 (2017).
Batiuk, M. Y. et al. Identification of region-specific astrocyte subtypes at single cell resolution. Nat. Commun. 11, 1220 (2020).
Matos, M. et al. Astrocytes detect and upregulate transmission at inhibitory synapses of somatostatin interneurons onto pyramidal cells. Nat. Commun. 9, 4254 (2018).
Kingsbury, L. et al. Correlated neural activity and encoding of behavior across brains of socially interacting animals. Cell 178, 429–446 (2019).
Endo, F. et al. Molecular basis of astrocyte diversity and morphology across the CNS in health and disease. Science 378, eadc9020 (2022).
Padilla-Coreano, N. et al. Cortical ensembles orchestrate social competition through hypothalamic outputs. Nature 603, 667–671 (2022).
Adamsky, A. et al. Astrocytic activation generates de novo neuronal potentiation and memory enhancement. Cell 174, 59–71 e14 (2018).
Kol, A. et al. Astrocytes contribute to remote memory formation by modulating hippocampal–cortical communication during learning. Nat. Neurosci. 23, 1229–1239 (2020).
van der Kooij, M. A. & Sandi, C. Social memories in rodents: methods, mechanisms and modulation by stress. Neurosci. Biobehav. Rev. 36, 1763–1772 (2012).
Fan, Z. et al. Neural mechanism underlying depressive-like state associated with social status loss. Cell 186, 560–576 (2023).
Low, R. J., Gu, Y. & Tank, D. W. Cellular resolution optical access to brain regions in fissures: imaging medial prefrontal cortex and grid cells in entorhinal cortex. Proc. Natl Acad. Sci. USA 111, 18739–18744 (2014).
Pnevmatikakis, E. A. & Giovannucci, A. NoRMCorre: an online algorithm for piecewise rigid motion correction of calcium imaging data. J. Neurosci. Meth. 291, 83–94 (2017).
Giovannucci, A. et al. CaImAn an open source tool for scalable calcium imaging data analysis. eLife 8, e38173 (2019).
Pnevmatikakis, E. A. et al. Simultaneous denoising, deconvolution, and demixing of calcium imaging data. Neuron 89, 285–299 (2016).
Fan, Z. X. et al. Using the tube test to measure social hierarchy in mice. Nat. Protoc. 14, 2595–2595 (2019).
Remedios, R. et al. Social behaviour shapes hypothalamic neural ensemble representations of conspecific sex. Nature 550, 388–392 (2017).
Deacon, R. M. J. Measuring the strength of mice. J. Vis. Exp. https://doi.org/10.3791/2610 (2013).
Lee, S. J., Zhou, T., Choi, C. H., Wang, Z. & Benveniste, E. N. Differential regulation and function of Fas expression on glial cells. J. Immunol. 164, 1277–1285 (2000).
Cho, W. H. et al. Hippocampal astrocytes modulate anxiety-like behavior. Nat. Commun. 13, 6536 (2022).
Woo, J. et al. Control of motor coordination by astrocytic tonic GABA release through modulation of excitation/inhibition balance in cerebellum. Proc. Natl Acad. Sci. USA 115, 5004–5009 (2018).
Acknowledgements
This study was supported by the Samsung Science & Technology Foundation (SSTF-BA1502-13) and the National Research Foundation of Korea (NRF-2021R1A4A1021594 and NRF-2021R1A6A3A01088164).
Author information
Authors and Affiliations
Contributions
K.N. and S.J.L. designed the research. K.N., B.H.L. and D.W.K. performed in vivo two-photon imaging and analysis. K.N., Y.K.C. and M.H. performed TCSPC. K.N. performed immunohistochemistry, stereotactic surgery and electrophysiology. K.N., K.P. and M.H. performed all behavioral tests. K.N., W.-H.C. and E.B. performed glutamate and ATP assays, and W.-H.C. performed cell culture experiments and in vitro Ca2+ assay. K.N., M.H., E.B. and Y.S.K. performed microdialysis. H.Y.P. and S.B.J. supervised the in vivo two-photon imaging and TCSPC experiments. S.-Y.C. supervised the electrophysiology experiment. C.J.L. and B.-E.Y. supervised the microdialysis experiments. K.N., K.P. and S.J.L. wrote the manuscript, and all authors commented on the manuscript. S.J.L. supervised the project.
Corresponding author
Ethics declarations
Competing interests
All authors declare that they have no competing interests.
Peer review
Peer review information
Nature Neuroscience thanks Weizhe Hong, Thomas Papouin and Gertrudis Perea for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Ca2+ responses of astrocytes in dmPFC and formation of social hierarchy in group-housed mice.
(a) Top: Generation of astrocyte-specific GCaMP6s transgenic mice (GFAP-GCaMP6s); Bottom: experimental scheme. (b and c) Astrocyte (S100β-positive)-specific GCaMP6s expression in tamoxifen-injected GFAP-GCaMP6s mice (n = 3 in each group). Scale bars, 100 μm. (d) Using two-photon imaging, astrocyte-specific GCaMP6s-expressing mice (GFAP-GCaMP6s) show astrocytic Ca2+ elevations when mice are gently pocked with a stick. (e) Region of interests (ROIs) for analysis of dmPFC astrocyte Ca2+ activities. Scale bars: 200 μm. The experiment was repeated 15 times independently. (f) (left) Representative heat map image shows an increased Ca2+ response in most GCaMP6s-expressing astrocytes of the dmPFC (35 cells from a mouse). Ca2+ traces of five representative astrocytes are shown (right). (g) Percent of responsive cells and normalized ΔF/F before and after nose poking (p = 4.0056 × 10-14; n = 35 cells). Two-sided Paired t-test. (h and i) Experimental scheme used to measure basal social hierarchy in group-housed mice (4 mice per cage) and result of the social hierarchy established by daily tube testing of all the mice in a representative cage. We used mouse cages showing a stable hierarchy among cage mates for further experiments. (j) The numbers of pushing and resistance behaviors per tube test trial for winner mice compared to losers (p = 8.69 × 10-5 (Push) and p = 2.59 × 10-8 (Resistance)) (n = 22 in each group). Two-tailed Wilcoxon rank-sum test. The winner mice show more pushing and resistance behavior compared to loser mice. (k and l) Hierarchy formation according to tube test schedules (experiments conducted during daytime, night, and random phases) and mouse ages (8-, 12-, and 16-week-old male mice). The percentage of cages in which a hierarchy was established was consistent regardless of the time of day for tube test (62%–68%) and age of the mice tested (61%–68%), validating the test consistency. (m) GCaMP6s expression among hierarchical cage mates (n = 3 in each group). Scale bars: 100 μm. One-way ANOVA with Bonferroni post-hoc analysis. ***p < 0.005. n.s., not statistically significant. Data are presented as the mean ± s.e.m.
Extended Data Fig. 2 Astrocyte Ca2+ activity changes and mouse dominance behavioral stances by astrocyte activity modulations.
(a) Astrocyte Ca2+ activities upon chemogenetic activation of hM3Dq or inhibition by hPMCA2w/b. (b) Quantification of astrocytic Ca2+ events (p = 0.001 (mCherry vs. hM3Dq), p = 0.034 (mCherry vs. hPMCA2w/b), and p < 0.001 (hM3Dq vs. hPMCA2w/b); n = 4 mice in each group). One-way ANOVA with Bonferroni post-hoc analysis. (c and d) Quantification of four dominance behavioral stances (push, resistance, retreat, and approach) during tube test upon dmPFC astrocyte activation (c) and inhibition (d) (n = 20 mice for hM3Dq-injected rank-4; n = 8 mice for hPMCA2w/b-injected rank-1). *p < 0.05; **p < 0.01; ***p < 0.005; n.s., not statistically significant. Data are presented as the mean ± s.e.m.
Extended Data Fig. 3 Mouse muscle strength, aggression, locomotion, and emotion by dmPFC astrocyte Ca2+ activation or inhibition.
(a and b) Hierarchical rank change by chemogenetic dmPFC astrocyte activation in rank-1 mice and their behavioral stances (n = 6 for mCherry, 8 for hM3Dq-injected rank-1). Two-sided Wilcoxon signed-rank test. (c) Schematic illustration of the inverted screen test. The behavioral test was performed 2 h after CNO injection (3 mg/kg, i.p.). Adult male mice were hanged onto a grid mesh and the time to fall was measured. When comparing the time to fall and body weight of mCherry- and hM3Dq-expressing mice, no substantial difference was observed between the groups (n = 9 for mCherry, 8 for hM3Dq). Two-tailed Wilcoxon rank-sum test. (d) There is no correlation between the time to fall and mouse body weight indicating that latency to fall does not depend on mouse weight (n = 17; Pearson’s correlation) (e) Time to fall in rank-1, rank-4, and CNO-injected rank-4 mice from the inverted screen test (n = 6 in each group). One-way ANOVA with Bonferroni post-hoc analysis. (f) Schematic illustration of the resident-intruder test. Male and female mice were co-housed for 3 weeks. On the day of the test, the female mouse was replaced by a new male mouse as an intruder. The behavioral test was conducted 2 h after CNO injection for 10 min during an experimental period extending over 3 days. The aggressive behaviors of resident male mice were measured by counting their (i) chasing duration, (ii) attack duration, and (iii) attack frequency on three consecutive days. (g) Diagram of the resident-intruder test results divided by aggressive (67.6%) and non-aggressive resident mice (32.4%). (h and i) Mean results of chasing duration, attack duration, and attack frequency of (h) aggressive resident mice (n = 10 for mCherry and n = 13 for hM3Dq) and (i) non-aggressive resident mice (n = 5 for mCherry and n = 6 for hM3Dq). Two-tailed Wilcoxon rank-sum test. Both attack duration and frequency were increased daily in aggressive resident mice upon repeated exposure to an intruder; however, the differences between the two groups (mCherry vs. hM3Dq) were not significant. (j) Mouse locomotive activity measuring the distance moved in the OFT 2 h after CNO injection during a 10 min experimental period. (left) Representative traces of mouse locomotion for 10 min, (middle) total distance traveled, and (right) time in center between mCherry- and hM3Dq-expressing mice (n = 8 in each group). Two-tailed Wilcoxon rank-sum test. (k) Percent of freezing time during open field test between mCherry- and hM3Dq-expressing mice (n = 8 in each group). Chemogenetic dmPFC astrocyte stimulation did not induce freezing behavior. (l) Depressive-like behavior measured by immobility time in the FST and TST (n = 8 in each group). Two-tailed Wilcoxon rank-sum test. The tests were performed 2 h after CNO injection. (m and n) Hierarchical rank change by dmPFC astrocyte inhibition upon hPMCA2w/b injection in rank-4 mice and their behavioral stances (n = 5 in mCherry and n = 6 in hPMCA2w/b). Wilcoxon signed-rank test. (o, p) Mouse muscle strength upon hPMCA2w/b injection in wild-type mice (n = 7 in each group). Pearson’s correlation and one-way ANOVA with Bonferroni post-hoc analysis. (q–s) Aggressive behaviors upon hPMCA2w/b injection in wild-type mice (in aggressive mice: n = 6 for mCherry and n = 6 for hPMCA2w/b; in non-aggressive mice: n = 5 for mCherry and n = 6 for hPMCA2w/b). Two-tailed Wilcoxon rank-sum test. (t–u) Locomotion upon hPMCA2w/b injection in wild-type mice (p = 0.0119 for Time in center) (n = 7 in each group). Two-tailed Wilcoxon rank-sum test. (v) Depressive-like behaviors upon hPMCA2w/b injection (p = 0.0274 for TST) (n = 7 in each group). Two-tailed Wilcoxon rank-sum test. *p < 0.05. n.s., not statistically significant. Data are presented as mean ± s.e.m.
Extended Data Fig. 4 Optogenetic astrocyte stimulation increases intracellular Ca2+ activity and mouse social rank.
(a) Development of astrocytic ChR2-expressing transgenic mice. (b) Fluorescent images of primary cultured ChR2-expressing astrocytes from GFAP-ChR2 mice showing ChR2 expression detected by eYFP signal in GFAP-positive cells. Scale bar, 100 μm. The experiment was repeated three times independently. (c) Ca2+ response in primary astrocytes in vitro. Rhod-2 fluorescence from individual (gray) and averaged trace (red) from ChR2-positive astrocytes (n = 7 cells). Rhod-2 fluorescence intensity was substantially increased after blue light stimulation of ChR2-expressing astrocytes (n = 7). Scale bar: 1 min. (d) Comparison of Rhod-2 fluorescence intensity before (Pre) and after (Post) the light stimulation (p = 1.23 × 10−5). Two-sided Paired t-test. (e) Experimental scheme to test the effects of optogenetic astrocytes stimulation on mouse social rank. (f and g) AAVDJ-GFAP-ChR2-mCherry was injected into the PL subregion of the dmPFC. Scale bar: 1 mm. ChR2 (mCherry) was expressed in astrocytes (GFAP-positive cells) with more than 97% specificity. Negligible co-localization of ChR2 (mCherry) was detected with the neuronal marker NeuN (0.8%) and the microglial marker Iba1 (1.6%). Scale bar: 100 μm. The experiment was repeated five times independently. (h) Mean rank change of light-stimulated rank-4 mice injected with AAVDJ-GFAP-ChR2-mCherry virus compared with the control group (p = 0.041 (0 d), p = 0.038 (1 d), and p = 0.034 (2 d)) (GFAP-mCherry; n = 10 for GFAP-mCherry and n = 16 for GFAP-ChR2). Two-sided Wilcoxon signed-rank test. (i) Rank elevation of light-stimulated rank-4 mice showed (left) first day or (right) delayed rank change. (j) Ratio between First day (62.5%) and Delayed rank elevation (37.5%) of light-stimulated rank-4 mice. (k) Experimental scheme to perform tube test 30 sec after optogenetic dmPFC astrocyte stimulation. (l) (left) Hierarchical rank change observed upon optogenetic dmPFC astrocyte stimulation of rank-4 mice. Light stimulation was delivered 30 sec prior to tube test and continued during the test (Prior stimulation). Other mice received light immediately after entering the tube (Immediate stimulation; n = 5 for Immediate, n = 7 for Prior) (p = 0.046 (0 d), p = 0.034 (1 d), and p = 0.038 (2 d)). Two-sided Wilcoxon signed-rank test. (right) Percentage of rank elevation between the two groups. (m) Comparison of behavioral performances (pushing and resistance) of rank-1, rank-4, and optogenetic astrocyte-stimulated rank-4 mice during tube test [Push: p = 3.35 × 10−4 (Rank 1 vs. Rank 4), p = 0.0027 (Rank 1 vs. Rank 4 + CNO); Resistance: p = 2.11 × 10−4 (Rank 1 vs. Rank 4), p = 6.70 × 10−6 (Rank 1 vs. Rank 4 + CNO)] (14 mice in each group). One-way ANOVA, Bonferroni post-hoc analysis. *p < 0.05; ***p < 0.005; n.s., not statistically significant. Data are presented as mean ± s.e.m.
Extended Data Fig. 5 Optogenetic activation of dmPFC astrocytes increases the excitatory synaptic input without affecting neuronal excitability.
(a) (left) Representative traces of the excitability of dmPFC pyramidal neurons of GFAP-mCherry and GFAP-ChR2 mice. Number of action potentials at different current steps of (middle) GFAP-mCherry (9 cells from 3 mice) and (right) GFAP-ChR2 (17 cells from 4 mice) mice. Two-sided Paired t-test. (b) The minimal injected current to induce an action potential from resting and light-stimulated pyramidal neurons of GFAP-mCherry and GFAP-ChR2 mice (9 cells in mCherry and 17 cells in ChR2). Two-sided Paired t-test. (c) (left) Representative traces of sEPSC events, and (middle and right) the number of mESPC and sEPSC events per 10 sec time bin, during and after astrocyte stimulation (1 min at 20-Hz) [mEPSC: p = 0.022 (at 120 sec), p = 0.028 (at 140 sec), p = 0.012 (at 150 sec), p = 0.025 (at 160 sec), p = 0.044 (at 170 sec); sEPSC: p = 0.045 (at 90 sec), p = 0.031 (at 100 sec), p = 0.002 (from 110 to 190 sec), p = 0.014 (at 200 sec), p = 0.011 (at 210 sec), p = 0.019 (at 220 sec), p = 0.028 (at 230 sec)] (mEPSC: 10 cells from 3 GFAP-mCherry mice and 11 cells from 3 GFAP-ChR2 mice; sEPSC: 23 cells from 5 GFAP-mCherry mice and 17 cells from 4 GFAP-ChR2 mice). Two-tailed Student’s t-test. (d and e) Cumulative probabilities and mean results of both amplitude and frequency of (d) mEPSC (p = 0.034) and (e) sEPSC (p < 0.001) (mEPSC: 10 cells from 3 GFAP-mCherry mice and 11 from 3 GFAP-ChR2 mice; sEPSC: 23 cells from 5 GFAP-mCherry mice and 17 from 4 GFAP-ChR2 mice). Two-tailed Student’s t-test. *p < 0.05; ***p < 0.005; n.s., not statistically significant. Data are presented as the mean ± s.e.m.
Extended Data Fig. 6 Optogenetic dmPFC astrocyte activation does not alter locomotion, anxiety, depression, or aggressive behaviors.
(a) Mouse locomotor activity was assessed by measuring the distance moved in the open field during intermittent light stimulation over a 10-min experimental period (n = 6 in each group). Two-tailed Wilcoxon rank-sum test. (b) (left) Representative traces of locomotion over 10 min and (middle and right) total distance traveled per minute with the light on or off (n = 6 in each group). Two-sided paired t-test. (c) Distance traveled in the center area during intermittent light stimulation over a 10-min period (n = 6 in each group). Two-tailed Wilcoxon rank-sum test. (d) Distance traveled in the center per minute with the light on or off during the test session (n = 6 in each group). Two-sided paired t-test. The results indicate that optogenetic dmPFC astrocyte activation does not affect mouse locomotion. (e) Difference in locomotion speed before and after light stimulation (n = 6 in each group). Two-sided paired t-test. Mice with optogenetic dmPFC astrocyte stimulation did not alter their locomotive speed, indicating that dmPFC astrocyte-activated resistance behavior is not due to reduced locomotive activity. (f) Schematic illustration of the EPM test during light stimulation. Light was delivered to the mice during the entire experimental session (5 min). (g) Anxiety-like behaviors assessed by the time spent in the open and closed arms. Mice with optogenetic dmPFC astrocyte stimulation did not show any anxiety-like behavior (n = 6 in each group). Two-tailed Wilcoxon rank-sum test. (h) Depressive-like behavior measured by the immobility time in the FST (n = 6 in each group). Light was delivered to the mice during the entire experimental session (5 min). Optogenetic dmPFC astrocyte stimulation did not increase immobility time in the FST. Two-tailed Wilcoxon rank-sum test. (i) Schematic illustration of the resident-intruder test. Male and female mice were housed together for 3 weeks. On the day of the test, the female mouse was removed and replaced by a new male mouse introduced as an intruder. The behavioral test was conducted for 10 min with light stimulation (473 nm, 20 Hz) during the entire experimental period (3 days). The animals were videotaped, and the aggressive behaviors of resident mice were measured by counting their (i) chasing duration, (ii) attack duration, and (iii) attack frequency during three consecutive days. (j) Diagram of the resident-intruder test results divided by aggressive (62.1%) and non-aggressive resident mice (37.9%). (k and l) Mean results of chasing duration, attack duration, and attack frequency of (top row) non-aggressive resident mice and (bottom row) aggressive resident mice during optogenetic light stimulation. Both attack duration and frequency were increased daily in aggressive resident mice with repeated exposure to an intruder. However, the differences between the two groups (GFAP-mCherry vs. GFAP-ChR2) were not statistically significant (n = 5 for mCherry and n = 6 for ChR2 for non-aggressive mice; n = 8 for mCherry and n = 10 for ChR2 for aggressive mice). Two-tailed Wilcoxon rank-sum test. These results indicate that optogenetic dmPFC astrocyte stimulation does not affect mouse aggressive behavior. n.s., not statistically significant. Data are presented as mean ± s.e.m.
Extended Data Fig. 7 Electrophysiological changes upon CNO treatment and hPMCA2w/b injection in wild type mice.
(a) mEPSC (12 cells from 3 mice; Paired t-test) and mIPSC (14 cells from 3 mice; Two-sided paired t-test), (b) excitatory and inhibitory PPRs (15 cells from 4 mice; Two-sided paired t-test), (c) EPSC and IPSC input-output ratio (7 cells from 3 mice; Two-sided paired t-test), (d) neuronal excitability (12 cells from 3 mice; Two-sided paired t-test) before and after CNO bath application. (e) mEPSC (11 cells from three mCherry-injected mice and 13 cells from three hPMCA2w/b-injected mice; Two-tailed Student’s t-test.) and mIPSC (16 cells from three mCherry-injected mice and 17 cells from three hPMCA2w/b-injected mice; Two-tailed Student’s t-test.), (f) excitatory and inhibitory PPRs [Excitatory PPR: p = 0.0437 (at 100 ms)] (12 cells from three mCherry-injected mice and 13 cells from three hPMCA2w/b-injected mice; Two-tailed Student’s t-test.), (g) EPSC and IPSC input-output ratio [IPSC IO: p = 0.0486 (at 0.1); E/I ratio: p = 0.0453 (at 0.2), p = 0.0285 (at 0.3), and p = 0.0395 (at 0.4)] (8 cells from three mCherry- or hPMCA2w/b-injected; Two-tailed Student’s t-test.), (h) neuronal excitability (12 cells from three mCherry-injected mice and 14 cells from three hPMCA2w/b-injected mice; Two-tailed Student’s t-test.) between mCherry and hPMCA2w/b-injected mice. Left dashed squares indicate schematic illustration of experiments. *p < 0.05; n.s., not statistically significant. Data are presented as the mean ± s.e.m.
Extended Data Fig. 8 Dominant and subordinate mice show distinct prefrontal E/I balance.
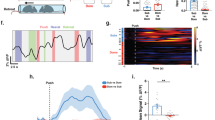
(a) Illustration of AAV5-hSyn1-jRGECO1a-mCherry (hSyn1-jRGECO1a) injection into the dmPFC and expression of jRGECO1a in the PL cortex of dmPFC. Scale bars: 1 mm and (inset) 100 μm. The experiment was repeated three times independently. (b) Experimental setting for two-photon imaging while performing tube test in a head-fixed manner. (c) ROIs for analysis of neuronal Ca2+ activities using in vivo two-photon imaging. Scale bars: 100 μm. The experiment was repeated three times independently. (d) Heat map visualization of normalized neuronal Ca2+ activities in dominant (left) and subordinate (right) mice (62 cells from one dominant mouse and 74 cells from one subordinate mouse). (e) Representative neuronal Ca2+ traces show activation or suppression upon interacting with an opponent mouse in a tube. (f) Percentages of neurons activated, suppressed, or not changed upon interaction with an opponent mouse in dominant and subordinate mice.
Extended Data Fig. 9 Changes in gliotransmitters after astrocyte inhibition and their effects on mouse behaviors.
(a) Glutamate and ATP assays from acute mPFC tissues (n = 8 in each group) and by in vivo microdialysis (n = 8 in each group). Two-tailed Wilcoxon rank-sum test. (b) Mouse locomotion, anxiety and depressive-like behaviors upon Opto-vTrap injection in dmPFC with blue light stimulation during whole experimental sessions (1 Hz, 0.5 sec duration) (n = 8 in each group). Two-tailed Wilcoxon rank-sum test. Scale bar, 500 μm. n.s.: not significant. Data are presented as mean ± s.e.m.
Extended Data Fig. 10 Working hypothesis for the astrocyte-regulated dominance behaviors in dmPFC.
Astrocyte activities in dmPFC enhance the prefrontal E/I balance by upregulating presynaptic-dependent excitatory synaptic transmission and down-regulating postsynaptic-independent inhibitory synaptic transmission by releasing glutamate and ATP, respectively. The increased dmPFC E/I balance induces dominance winning through an enhancement of defensive resistance behavior, which is sufficient to win against opponents. These behavioral adaptations would enable mice to obtain high social status in the social hierarchy.
Supplementary information
Supplementary Video 1
Real-time dmPFC astrocyte activities during tube test
Supplementary Video 2
Behavior of rank-4 mice during tube test with or without chemogenetic dmPFC astrocyte stimulation
Supplementary Video 3
Push training
Supplementary Video 4
Measure of mouse pushing and resisting force
Supplementary Table
Statistical information
Source data
Source Data for all Figures
Source Data file for all main and Extended Data figures
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Noh, K., Cho, WH., Lee, B.H. et al. Cortical astrocytes modulate dominance behavior in male mice by regulating synaptic excitatory and inhibitory balance. Nat Neurosci 26, 1541–1554 (2023). https://doi.org/10.1038/s41593-023-01406-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41593-023-01406-4
This article is cited by
-
Acetylcholine muscarinic M1 receptors in the rodent prefrontal cortex modulate cognitive abilities to establish social hierarchy
Neuropsychopharmacology (2024)
-
Microbiota–gut–brain axis and its therapeutic applications in neurodegenerative diseases
Signal Transduction and Targeted Therapy (2024)
-
Prefrontocortical Astrocytes Regulate Dominance Hierarchy in Male Mice
Neuroscience Bulletin (2024)
-
Control of social hierarchy beyond neurons
Nature Neuroscience (2023)