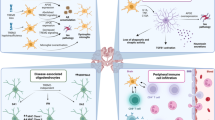
Abstract
Microglial cells are the major immune cells of the central nervous system (CNS), and directly react to neurodegeneration, but other immune cell types are also able to react to pathology and can modify the course of neurodegenerative processes. These mainly include monocytes/macrophages and lymphocytes. While these peripheral immune cells were initially considered to act only after infiltrating the CNS, recent evidence suggests that some of them can also act directly from the periphery. We will review the existing and emerging evidence for a role of peripheral immune cells in neurodegenerative diseases, both with and without CNS infiltration. Our focus will be on amyotrophic lateral sclerosis, but we will also compare to Alzheimer’s disease and Parkinson’s disease to highlight similarities or differences. Peripheral immune cells are easily accessible, and therefore may be an attractive therapeutic target for neurodegenerative diseases. Thus, understanding how these peripheral immune cells communicate with the CNS deserves deeper investigation.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Michaud, J. P., Bellavance, M. A., Prefontaine, P. & Rivest, S. Real-time in vivo imaging reveals the ability of monocytes to clear vascular amyloid beta. Cell Rep. 5, 646–653 (2013). Seminal study using in vivo imaging to show the clearance of vascular amyloid beta by monocytes.
Mildner, A. et al. Distinct and non-redundant roles of microglia and myeloid subsets in mouse models of Alzheimer’s disease. J. Neurosci. 31, 11159–11171 (2011).
Peter, I. et al. Anti-tumor necrosis factor therapy and incidence of Parkinson disease among patients with inflammatory bowel disease. JAMA Neurol. 75, 939–946 (2018).
Villumsen, M., Aznar, S., Pakkenberg, B., Jess, T. & Brudek, T. Inflammatory bowel disease increases the risk of Parkinson’s disease: a Danish nationwide cohort study 1977–2014. Gut 68, 18–24 (2019).
Weimers, P. et al. Inflammatory bowel disease and Parkinson’s disease: a nationwide Swedish cohort study. Inflamm. Bowel Dis. 25, 111–123 (2019).
Beers, D. R. et al. Wild-type microglia extend survival in PU.1 knockout mice with familial amyotrophic lateral sclerosis. Proc. Natl Acad. Sci. USA 103, 16021–16026 (2006). Seminal study in mice showing the contribution of microglial cells to ALS progression, but also that they do not initiate motor neuron degeneration.
Boillee, S. et al. Onset and progression in inherited ALS determined by motor neurons and microglia. Science 312, 1389–1392 (2006). Study showing that motor neurons drive initiation of ALS and microglial cells/peripheral macrophages influence disease progression.
Wang, L., Sharma, K., Grisotti, G. & Roos, R. P. The effect of mutant SOD1 dismutase activity on non-cell autonomous degeneration in familial amyotrophic lateral sclerosis. Neurobiol. Dis. 35, 234–240 (2009).
Yamanaka, K. et al. Astrocytes as determinants of disease progression in inherited amyotrophic lateral sclerosis. Nat. Neurosci. 11, 251–253 (2008).
Chen, H. et al. Nonsteroidal antiinflammatory drug use and the risk for Parkinson’s disease. Ann. Neurol. 58, 963–967 (2005).
Chen, H. et al. Nonsteroidal anti-inflammatory drugs and the risk of Parkinson disease. Arch. Neurol. 60, 1059–1064 (2003).
Gao, X., Chen, H., Schwarzschild, M. A. & Ascherio, A. Use of ibuprofen and risk of Parkinson disease. Neurology 76, 863–869 (2011).
Schrag, A., Horsfall, L., Walters, K., Noyce, A. & Petersen, I. Prediagnostic presentations of Parkinson’s disease in primary care: a case–control study. Lancet Neurol. 14, 57–64 (2015).
Devos, D. et al. Colonic inflammation in Parkinson’s disease. Neurobiol. Dis. 50, 42–48 (2013).
Pan, W. & Kastin, A. J. TNF transport across the blood–brain barrier is abolished in receptor knockout mice. Exp. Neurol. 174, 193–200 (2002).
Qin, L. et al. Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia 55, 453–462 (2007).
Holmes, C. et al. Systemic inflammation and disease progression in Alzheimer disease. Neurology 73, 768–774 (2009).
Dunn, N., Mullee, M., Perry, V. H. & Holmes, C. Association between dementia and infectious disease: evidence from a case–control study. Alzheimer Dis. Assoc. Disord. 19, 91–94 (2005).
Cui, C. et al. Associations between autoimmune diseases and amyotrophic lateral sclerosis: a register-based study. Amyotroph. Lateral Scler. Frontotemporal Degener. 22, 211–219 (2021).
Miller, Z. A. et al. Increased prevalence of autoimmune disease within C9 and FTD/MND cohorts: completing the picture. Neurol. Neuroimmunol. Neuroinflamm 3, e301 (2016).
DeJesus-Hernandez, M. et al. Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-linked FTD and ALS. Neuron 72, 245–256 (2011).
Atanasio, A. et al. C9orf72 ablation causes immune dysregulation characterized by leukocyte expansion, autoantibody production and glomerulonephropathy in mice. Sci. Rep. 6, 23204 (2016).
Burberry, A. et al. Loss-of-function mutations in the C9ORF72 mouse ortholog cause fatal autoimmune disease. Sci. Transl. Med. 8, 347ra393 (2016).
O’Rourke, J. G. et al. C9orf72 is required for proper macrophage and microglial function in mice. Science 351, 1324–1329 (2016).
Sudria-Lopez, E. et al. Full ablation of C9orf72 in mice causes immune system-related pathology and neoplastic events but no motor neuron defects. Acta Neuropathol. 132, 145–147 (2016).
Ahmad, L., Zhang, S. Y., Casanova, J. L. & Sancho-Shimizu, V. Human TBK1: a gatekeeper of neuroinflammation. Trends Mol. Med. 22, 511–527 (2016).
Freischmidt, A. et al. Haploinsufficiency of TBK1 causes familial ALS and fronto-temporal dementia. Nat. Neurosci. 18, 631–636 (2015).
Griciuc, A. & Tanzi, R. E. The role of innate immune genes in Alzheimer’s disease. Curr. Opin. Neurol. 34, 228–236 (2021).
Jonsson, T. et al. Variant of TREM2 associated with the risk of Alzheimer’s disease. N. Engl. J. Med. 368, 107–116 (2013).
Naj, A. C. et al. Common variants at MS4A4/MS4A6E, CD2AP, CD33 and EPHA1 are associated with late-onset Alzheimer’s disease. Nat. Genet. 43, 436–441 (2011).
Alam, M. M. et al. Alpha synuclein, the culprit in Parkinson disease, is required for normal immune function. Cell Rep. 38, 110090 (2022).
Gardet, A. et al. LRRK2 is involved in the IFN-gamma response and host response to pathogens. J. Immunol. 185, 5577–5585 (2010).
Lee, H., James, W. S. & Cowley, S. A. LRRK2 in peripheral and central nervous system innate immunity: its link to Parkinson’s disease. Biochem. Soc. Trans. 45, 131–139 (2017).
Gillardon, F., Schmid, R. & Draheim, H. Parkinson’s disease-linked leucine-rich repeat kinase 2R1441G mutation increases proinflammatory cytokine release from activated primary microglial cells and resultant neurotoxicity. Neuroscience 208, 41–48 (2012).
Lall, D. et al. C9orf72 deficiency promotes microglial-mediated synaptic loss in aging and amyloid accumulation. Neuron 109, 2275–2291 e2278 (2021).
McCauley, M. E. et al. C9orf72 in myeloid cells suppresses STING-induced inflammation. Nature 585, 96–101 (2020).
Taft, J. et al. Human TBK1 deficiency leads to autoinflammation driven by TNF-induced cell death. Cell 184, 4447–4463 (2021). Study describing rare patients with systemic autoimmune disease linked to full deletion of TBK1, a gene for which haploinsufficiency is linked to ALS.
Marchlik, E. et al. Mice lacking Tbk1 activity exhibit immune cell infiltrates in multiple tissues and increased susceptibility to LPS-induced lethality. J. Leukoc. Biol. 88, 1171–1180 (2010).
Kim, G., Gautier, O., Tassoni-Tsuchida, E., Ma, X. R. & Gitler, A. D. ALS genetics: gains, losses, and implications for future therapies. Neuron 108, 822–842 (2020).
Ajami, B., Bennett, J. L., Krieger, C., Tetzlaff, W. & Rossi, F. M. Local self-renewal can sustain CNS microglia maintenance and function throughout adult life. Nat. Neurosci. 10, 1538–1543 (2007). Influential study showing self-renewal of microglia and that the CNS infiltration of myeloid cells from the periphery, measured in studies using irradiation, is an artifact of accidental BBB opening due to irradiation.
Butovsky, O. et al. Modulating inflammatory monocytes with a unique microRNA gene signature ameliorates murine ALS. J. Clin. Invest. 122, 3063–3087 (2012).
Chiot, A. et al. Modifying macrophages at the periphery has the capacity to change microglial reactivity and to extend ALS survival. Nat. Neurosci. 23, 1339–1351 (2020). Study showing that peripheral macrophages can influence disease progression, in ALS without infiltrating the CNS and that peripheral nerve macrophages and microglia react differently to the same motor neuron degenerating.
Chiu, I. M. et al. A neurodegeneration-specific gene-expression signature of acutely isolated microglia from an amyotrophic lateral sclerosis mouse model. Cell Rep. 4, 385–401 (2013). Study showing the microglial transcriptome modifications over the disease course in ALS mice, but also indicating that monocytes do not infiltrate the CNS.
Zondler, L. et al. Peripheral monocytes are functionally altered and invade the CNS in ALS patients. Acta Neuropathol. 132, 391–411 (2016). Study showing differences in ALS patient monocytes compared to controls, and that these modifications happen early on, even before disease onset in asymptomatic mutation carriers.
Parillaud, V. R. et al. Analysis of monocyte infiltration in MPTP mice reveals that microglial CX3CR1 protects against neurotoxic over-induction of monocyte-attracting CCL2 by astrocytes. J. Neuroinflammation 14, 60 (2017).
Gao, L. et al. Infiltration of circulating myeloid cells through CD95L contributes to neurodegeneration in mice. J. Exp. Med. 212, 469–480 (2015).
Martin, E., Boucher, C., Fontaine, B. & Delarasse, C. Distinct inflammatory phenotypes of microglia and monocyte-derived macrophages in Alzheimer’s disease models: effects of aging and amyloid pathology. Aging Cell 16, 27–38 (2017). Study showing that CNS infiltration of monocytes is weak and linked to aging in AD mice.
Reed-Geaghan, E. G., Croxford, A. L., Becher, B. & Landreth, G. E. Plaque-associated myeloid cells derive from resident microglia in an Alzheimer’s disease model. J. Exp. Med. 217, e20191374 (2020). Study showing in AD mice that infiltrating monocytes do not contribute to the parenchymal myeloid pool and microglial population.
Yan, P. et al. Peripheral monocyte-derived cells counter amyloid plaque pathogenesis in a mouse model of Alzheimer’s disease. J. Clin. Invest. 132, e152565 (2022). Study showing that only 6% of the parenchymal myeloid cells derive from infiltrating monocytes in AD mice.
Chiu, I. M. et al. Activation of innate and humoral immunity in the peripheral nervous system of ALS transgenic mice. Proc. Natl Acad. Sci. USA 106, 20960–20965 (2009). Data showing progressive peripheral macrophage activation in peripheral nerves of ALS mice.
Ben-Yaakov, K. et al. Axonal transcription factors signal retrogradely in lesioned peripheral nerve. EMBO J. 31, 1350–1363 (2012).
Baleriola, J. et al. Axonally synthesized ATF4 transmits a neurodegenerative signal across brain regions. Cell 158, 1159–1172 (2014).
El Khoury, J. et al. Ccr2 deficiency impairs microglial accumulation and accelerates progression of Alzheimer-like disease. Nat. Med. 13, 432–438 (2007). Study showing the importance of peripheral myeloid cells to amyloid beta clearance.
Naert, G. & Rivest, S. CC chemokine receptor 2 deficiency aggravates cognitive impairments and amyloid pathology in a transgenic mouse model of Alzheimer’s disease. J. Neurosci. 31, 6208–6220 (2011). Study showing the impact of peripheral myeloid cells in improving AD mouse cognitive decline.
Simard, A. R., Soulet, D., Gowing, G., Julien, J. P. & Rivest, S. Bone marrow-derived microglia play a critical role in restricting senile plaque formation in Alzheimer’s disease. Neuron 49, 489–502 (2006). Study showing the importance of peripheral myeloid cells to amyloid beta clearance.
Zhao, W. et al. Characterization of gene expression phenotype in amyotrophic lateral sclerosis monocytes. JAMA Neurol. 74, 677–685 (2017).
Du, Y. et al. Increased activation ability of monocytes from ALS patients. Exp. Neurol. 328, 113259 (2020).
Navarro, E. et al. Dysregulation of mitochondrial and proteolysosomal genes in Parkinson’s disease myeloid cells. Nat. Aging 1, 850–863 (2021).
Shigemizu, D. et al. Identification of potential blood biomarkers for early diagnosis of Alzheimer’s disease through RNA sequencing analysis. Alzheimers Res Ther. 12, 87 (2020).
Bradshaw, E. M. et al. CD33 Alzheimer’s disease locus: altered monocyte function and amyloid biology. Nat. Neurosci. 16, 848–850 (2013).
Kleinberger, G. et al. TREM2 mutations implicated in neurodegeneration impair cell surface transport and phagocytosis. Sci. Transl. Med. 6, 243ra286 (2014).
Tian, L. et al. Decreased expression of cathepsin D in monocytes is related to the defective degradation of amyloid-beta in Alzheimer’s disease. J. Alzheimers Dis. 42, 511–520 (2014).
Zaghi, J. et al. Alzheimer disease macrophages shuttle amyloid-beta from neurons to vessels, contributing to amyloid angiopathy. Acta Neuropathol. 117, 111–124 (2009).
Beers, D. R., Henkel, J. S., Zhao, W., Wang, J. & Appel, S. H. CD4+ T cells support glial neuroprotection, slow disease progression, and modify glial morphology in an animal model of inherited ALS. Proc. Natl Acad. Sci. USA 105, 15558–15563 (2008). Original study showing that CD4+ T cells are protective in ALS mice.
Chiu, I. M. et al. T lymphocytes potentiate endogenous neuroprotective inflammation in a mouse model of ALS. Proc. Natl Acad. Sci. USA 105, 17913–17918 (2008).
Engelhardt, J. I., Tajti, J. & Appel, S. H. Lymphocytic infiltrates in the spinal cord in amyotrophic lateral sclerosis. Arch. Neurol. 50, 30–36 (1993).
Evans, F. L., Dittmer, M., de la Fuente, A. G. & Fitzgerald, D. C. Protective and regenerative roles of T cells in central nervous system disorders. Front. Immunol. 10, 2171 (2019).
Kawamata, T., Akiyama, H., Yamada, T. & McGeer, P. L. Immunologic reactions in amyotrophic lateral sclerosis brain and spinal cord tissue. Am. J. Pathol. 140, 691–707 (1992).
Lampson, L. A., Kushner, P. D. & Sobel, R. A. Major histocompatibility complex antigen expression in the affected tissues in amyotrophic lateral sclerosis. Ann. Neurol. 28, 365–372 (1990).
Troost, D., Van den Oord, J. J. & Vianney de Jong, J. M. Immunohistochemical characterization of the inflammatory infiltrate in amyotrophic lateral sclerosis. Neuropathol. Appl. Neurobiol. 16, 401–410 (1990).
Weiss, F., Labrador-Garrido, A., Dzamko, N. & Halliday, G. Immune responses in the Parkinson’s disease brain. Neurobiol. Dis. 168, 105700 (2022).
Naor, S. et al. Development of ALS-like disease in SOD-1 mice deficient of B lymphocytes. J. Neurol. 256, 1228–1235 (2009).
Beers, D. R. et al. Endogenous regulatory T lymphocytes ameliorate amyotrophic lateral sclerosis in mice and correlate with disease progression in patients with amyotrophic lateral sclerosis. Brain 134, 1293–1314 (2011). Original study showing hints that the Treg cell proportion correlates with disease progression in ALS patients.
Banerjee, R. et al. Adaptive immune neuroprotection in G93A-SOD1 amyotrophic lateral sclerosis mice. PLoS ONE 3, e2740 (2008).
Sheean, R. K. et al. Association of regulatory T cell expansion with progression of amyotrophic lateral Sclerosis: a study of humans and a transgenic mouse model. JAMA Neurol. 75, 681–689 (2018). Extensive study in ALS mice and human patients showing the potential of Treg cells in improving disease progression.
Dansokho, C. et al. Regulatory T cells delay disease progression in Alzheimer-like pathology. Brain 139, 1237–1251 (2016).
Reynolds, A. D. et al. Regulatory T cells attenuate TH17 cell-mediated nigrostriatal dopaminergic neurodegeneration in a model of Parkinson’s disease. J. Immunol. 184, 2261–2271 (2010).
Brochard, V. et al. Infiltration of CD4+ lymphocytes into the brain contributes to neurodegeneration in a mouse model of Parkinson disease. J. Clin. Invest. 119, 182–192 (2009). Study showing evidence for T cell infiltration in postmortem brains of PD patients, and that such infiltration is neurotoxic in a PD mouse model.
Williams, G. P. et al. CD4+T cells mediate brain inflammation and neurodegeneration in a mouse model of Parkinson’s disease. Brain 144, 2047–2059 (2021).
Baruch, K. et al. Breaking immune tolerance by targeting Foxp3+ regulatory T cells mitigates Alzheimer’s disease pathology. Nat. Commun. 6, 7967 (2015).
Ito, M. et al. Brain regulatory T cells suppress astrogliosis and potentiate neurological recovery. Nature 565, 246–250 (2019).
Pasciuto, E. et al. Microglia Require CD4+ T cells to complete the fetal-to-adult transition. Cell 182, 625–640 (2020).
Coque, E. et al. Cytotoxic CD8+ T lymphocytes expressing ALS-causing SOD1 mutant selectively trigger death of spinal motoneurons. Proc. Natl Acad. Sci. USA 116, 2312–2317 (2019). Study showing the negative impact of CD8+ T cells on motor neuron survival in ALS mice.
Komine, O. et al. Innate immune adaptor TRIF deficiency accelerates disease progression of ALS mice with accumulation of aberrantly activated astrocytes. Cell Death Differ. 25, 2130–2146 (2018). Study showing that blocking CD8+ T cells or NK cells does not influence disease progression in ALS mice.
Cui, C. et al. Correlation between leukocyte phenotypes and prognosis of amyotrophic lateral sclerosis. Elife 11, e74065 (2022).
Nardo, G. et al. Counteracting roles of MHCI and CD8+ T cells in the peripheral and central nervous system of ALS SOD1G93A mice. Mol. Neurodegener. 13, 42 (2018). Study suggesting a possible role of CD8+ T cells, directly at the periphery, in peripheral nerves of ALS mice.
Beers, D. R. et al. ALS patients’ regulatory T lymphocytes are dysfunctional, and correlate with disease progression rate and severity. JCI Insight 2, e89530 (2017).
Henkel, J. S. et al. Regulatory T lymphocytes mediate amyotrophic lateral sclerosis progression and survival. EMBO Mol. Med. 5, 64–79 (2013). Study showing the potential of using Treg cell markers to identify patients with fast-progressing ALS.
Jin, M., Gunther, R., Akgun, K., Hermann, A. & Ziemssen, T. Peripheral proinflammatory TH1/TH17 immune cell shift is linked to disease severity in amyotrophic lateral sclerosis. Sci. Rep. 10, 5941 (2020).
Saresella, M. et al. T helper-17 activation dominates the immunologic milieu of both amyotrophic lateral sclerosis and progressive multiple sclerosis. Clin. Immunol. 148, 79–88 (2013).
Beers, D. R. et al. Tregs attenuate peripheral oxidative stress and acute phase proteins in ALS. Ann. Neurol. 92, 195–200 (2022).
Camu, W. et al. Repeated 5-day cycles of low dose aldesleukin in amyotrophic lateral sclerosis (IMODALS): a phase 2a randomised, double-blind, placebo-controlled trial. EBioMedicine 59, 102844 (2020). Phase 2a trial showing safety of using IL-2 to increase Treg cells in ALS patients.
Garofalo, S. et al. Natural killer cells modulate motor neuron–immune cell crosstalk in models of amyotrophic lateral sclerosis. Nat. Commun. 11, 1773 (2020).
Murdock, B. J. et al. NK cells associate with ALS in a sex- and age-dependent manner. JCI Insight 6, e147129 (2021).
Zhang, Y. et al. Depletion of NK cells improves cognitive function in the Alzheimer disease mouse model. J. Immunol. 205, 502–510 (2020).
Earls, R. H. et al. NK cells clear alpha-synuclein and the depletion of NK cells exacerbates synuclein pathology in a mouse model of alpha-synucleinopathy. Proc. Natl Acad. Sci. USA 117, 1762–1771 (2020).
Graves, M. C. et al. Inflammation in amyotrophic lateral sclerosis spinal cord and brain is mediated by activated macrophages, mast cells and T cells. Amyotroph. Lateral Scler. Other Motor Neuron Disord. 5, 213–219 (2004).
Trias, E. et al. Mast cells and neutrophils mediate peripheral motor pathway degeneration in ALS. JCI Insight 3, e123249 (2018).
Trias, E. et al. Schwann cells orchestrate peripheral nerve inflammation through the expression of CSF1, IL-34, and SCF in amyotrophic lateral sclerosis. Glia 68, 1165–1181 (2020).
Trias, E. et al. Post-paralysis tyrosine kinase inhibition with masitinib abrogates neuroinflammation and slows disease progression in inherited amyotrophic lateral sclerosis. J. Neuroinflammation 13, 177 (2016).
Trias, E. et al. Evidence for mast cells contributing to neuromuscular pathology in an inherited model of ALS. JCI Insight 2, e95934 (2017).
Kovacs, M. et al. The pathogenic role of c-Kit+ mast cells in the spinal motor neuron-vascular niche in ALS. Acta Neuropathol. Commun. 9, 136 (2021).
Li, T. et al. Effects of chronic masitinib treatment in APPswe/PSEN1dE9 transgenic mice modeling Alzheimer’s disease. J. Alzheimers Dis. 76, 1339–1345 (2020).
Mora, J. S. et al. Long-term survival analysis of masitinib in amyotrophic lateral sclerosis. Ther. Adv. Neurol. Disord. 14, 17562864211030365 (2021).
Ginhoux, F. et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 330, 841–845 (2010).
Goldmann, T. et al. Origin, fate and dynamics of macrophages at central nervous system interfaces. Nat. Immunol. 17, 797–805 (2016).
Van Hove, H. et al. A single-cell atlas of mouse brain macrophages reveals unique transcriptional identities shaped by ontogeny and tissue environment. Nat. Neurosci. 22, 1021–1035 (2019).
Bennett, F. C. et al. A combination of ontogeny and CNS environment establishes microglial identity. Neuron 98, 1170–1183 (2018).
Bennett, M. L. et al. New tools for studying microglia in the mouse and human CNS. Proc. Natl Acad. Sci. USA 113, E1738–E1746 (2016).
Gosselin, D. et al. An environment-dependent transcriptional network specifies human microglia identity. Science 356, eaal3222 (2017).
Lund, H. et al. Competitive repopulation of an empty microglial niche yields functionally distinct subsets of microglia-like cells. Nat. Commun. 9, 4845 (2018).
Bruttger, J. et al. Genetic cell ablation reveals clusters of local self-renewing microglia in the mammalian central nervous system. Immunity 43, 92–106 (2015).
Staats, K. A., Borchelt, D. R., Tansey, M. G. & Wymer, J. Blood-based biomarkers of inflammation in amyotrophic lateral sclerosis. Mol. Neurodegener. 17, 11 (2022).
Moreno-Martinez, L., Calvo, A. C., Munoz, M. J. & Osta, R. Are circulating cytokines reliable biomarkers for amyotrophic lateral sclerosis? Int. J. Mol. Sci. 20, 2759 (2019).
Hu, Y. et al. Increased peripheral blood inflammatory cytokine levels in amyotrophic lateral sclerosis: a meta-analysis study. Sci. Rep. 7, 9094 (2017).
Buttini, M., Limonta, S. & Boddeke, H. W. Peripheral administration of lipopolysaccharide induces activation of microglial cells in rat brain. Neurochem. Int. 29, 25–35 (1996).
Quan, N. & Banks, W. A. Brain-immune communication pathways. Brain Behav. Immun. 21, 727–735 (2007).
Liu, X. et al. Cell-type-specific interleukin-1 receptor-1 signaling in the brain regulates distinct neuroimmune activities. Immunity 50, 317–333 (2019).
Moreno-Martinez, L. et al. Circulating cytokines could not be good prognostic biomarkers in a mouse model of amyotrophic lateral sclerosis. Front Immunol. 10, 801 (2019).
Gowing, G., Dequen, F., Soucy, G. & Julien, J. P. Absence of tumor necrosis factor-alpha does not affect motor neuron disease caused by superoxide dismutase-1 mutations. J. Neurosci. 26, 11397–11402 (2006).
Han, Y., Ripley, B., Serada, S., Naka, T. & Fujimoto, M. Interleukin-6 deficiency does not affect motor neuron disease caused by superoxide dismutase-1 mutation. PLoS ONE 11, e0153399 (2016).
Nguyen, M. D., Julien, J. P. & Rivest, S. Induction of proinflammatory molecules in mice with amyotrophic lateral sclerosis: no requirement for proapoptotic interleukin-1β in neurodegeneration. Ann. Neurol. 50, 630–639 (2001).
Guttenplan, K. A. et al. Knockout of reactive astrocyte activating factors slows disease progression in an ALS mouse model. Nat. Commun. 11, 3753 (2020).
Fulling, C., Dinan, T. G. & Cryan, J. F. Gut microbe to brain signaling: what happens in vagus. Neuron 101, 998–1002 (2019).
Zheng, D., Liwinski, T. & Elinav, E. Interaction between microbiota and immunity in health and disease. Cell Res. 30, 492–506 (2020).
Bercik, P. et al. The intestinal microbiota affect central levels of brain-derived neurotropic factor and behavior in mice. Gastroenterology 141, 599–609 (2011).
Wang, X. et al. Evidences for vagus nerve in maintenance of immune balance and transmission of immune information from gut to brain in STM-infected rats. World J. Gastroenterol. 8, 540–545 (2002).
Erny, D. et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat. Neurosci. 18, 965–977 (2015).
Sampson, T. R. et al. Gut microbiota regulate motor deficits and neuroinflammation in a model of Parkinson’s disease. Cell 167, 1469–1480 (2016).
Blacher, E. et al. Potential roles of gut microbiome and metabolites in modulating ALS in mice. Nature 572, 474–480 (2019).
Burberry, A. et al. C9orf72 suppresses systemic and neural inflammation induced by gut bacteria. Nature 582, 89–94 (2020).
Acknowledgements
The author’s research is supported by grants from the Fondation pour la Recherche Médicale (FRM, Equipe FRM EQU202103012581), the ALS association (ALSA, 20-IIA-530), NRJ-Institut de France, Fondation Thierry Latran, l’ARMC, S.L.A.F.R., ‘La longue route des malades de la SLA’, ‘Un pied devant l’autre’, Ferblanc and private donors to ALS at the ICM. F.B. was supported by a fellowship from the French ministry of research. We thank M. Mallat for helpful comments. Figures were partly drawn with free-to-use Servier Medical Art images available at https://smart.servier.com/.
Author information
Authors and Affiliations
Contributions
F.B., C.S.L. and S.B. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Neuroscience thanks Serge Rivest and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Berriat, F., Lobsiger, C.S. & Boillée, S. The contribution of the peripheral immune system to neurodegeneration. Nat Neurosci 26, 942–954 (2023). https://doi.org/10.1038/s41593-023-01323-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41593-023-01323-6
This article is cited by
-
Microbiota–gut–brain axis and its therapeutic applications in neurodegenerative diseases
Signal Transduction and Targeted Therapy (2024)
-
Myasthenia gravis concurrent with Parkinson’s disease in a Spanish cohort. Causation or correlation?
Neurological Sciences (2024)
-
Neural precursor cell delivery induces acute post-ischemic cerebroprotection, but fails to promote long-term stroke recovery in hyperlipidemic mice due to mechanisms that include pro-inflammatory responses associated with brain hemorrhages
Journal of Neuroinflammation (2023)