Abstract
Cortical state, defined by population-level neuronal activity patterns, determines sensory perception. While arousal-associated neuromodulators—including norepinephrine (NE)—reduce cortical synchrony, how the cortex resynchronizes remains unknown. Furthermore, general mechanisms regulating cortical synchrony in the wake state are poorly understood. Using in vivo imaging and electrophysiology in mouse visual cortex, we describe a critical role for cortical astrocytes in circuit resynchronization. We characterize astrocytes’ calcium responses to changes in behavioral arousal and NE, and show that astrocytes signal when arousal-driven neuronal activity is reduced and bi-hemispheric cortical synchrony is increased. Using in vivo pharmacology, we uncover a paradoxical, synchronizing response to Adra1a receptor stimulation. We reconcile these results by demonstrating that astrocyte-specific deletion of Adra1a enhances arousal-driven neuronal activity, while impairing arousal-related cortical synchrony. Our findings demonstrate that astrocytic NE signaling acts as a distinct neuromodulatory pathway, regulating cortical state and linking arousal-associated desynchrony to cortical circuit resynchronization.
Similar content being viewed by others
Main
Patterns of neural activity in the awake cortex are variable1, ranging from states of highly synchronized neuronal activity during periods of low arousal, to states of desynchronized activity during high-arousal periods such as whisking2 and running3. These cortical states can be identified by electrophysiological activity: synchronized cortical states display greater low-frequency (LF) oscillations and reduced high-frequency (HF) oscillations than desynchronized states4. While HF oscillations are important for processing of incoming information5, LF oscillations during the wake state are less well characterized. Generally described within a 2–10 Hz range2,6,7, wakeful LF power differs substantively from cortical activity patterns during sleep2, indicating a unique role for synchronized cortical states in awake animals. One role of waking cortical synchrony may be to modulate sensory responses to external stimuli. Increased LF power is associated with reduced neuronal gain6 and broader tuning8 in sensory cortex, as well as diminished behavioral performance on sensory perception tasks3,7,8.
The mechanisms by which cortical state is regulated, and how the cortex becomes sensitized to external stimuli, are well studied. One central mechanism is signaling by norepinephrine (NE), which regulates a primary hallmark of behavioral arousal—pupil diameter9—and alters the firing properties of cortical neurons leading to desynchronized cortical activity10,11. However, while the desynchronizing effects of NE are well known, mechanisms that increase waking synchrony are less clear. A full understanding of mechanisms that regulate awake cortical synchrony will be necessary to explain fluctuations in perception and behavior.
Neurons are not the only NE-responsive cell types in the cortex12,13. Astrocytes, a non-neuronal cell type abundant throughout the cortex, respond to NE with robust calcium (Ca2+) signaling14,15,16,17,18,19. However, astrocyte Ca2+ has also been linked to increased cortical synchrony when NE signaling is low, including during sleep20 and under anesthesia21. In this Article, we aimed to resolve the disparity between an astrocytic role in sleep generation and their activation by NE and desynchronizing stimuli such as movement. We hypothesized that NE-specific astrocytic signaling might act as a mechanism that regulates NE-driven cortical desynchrony and restores cortical synchrony following changes in arousal.
Here we use in vivo, two-photon (2P) Ca2+ imaging to show that astrocytes in mouse visual cortex respond proportionally and with temporal specificity to changes in arousal and NE, positioning astrocytes as a local feedback mechanism to the effects of arousal. In agreement with this hypothesis, NE-driven astrocyte Ca2+ signaling occurs alongside reductions in arousal-associated neuronal activity. Using in vivo local field potential (LFP) recordings, we further show that arousal-driven astrocyte Ca2+ activity occurs at transitions from cortical desynchrony to synchrony, and that this relationship is dependent on NE signaling. Pharmacological stimulation of Adra1a receptors counterintuitively increases wakeful cortical synchrony, and astrocyte-specific removal of Adra1a enhances total and arousal-driven neuronal activity, while impairing arousal-related cortical synchrony. Our results directly link astrocytic NE receptors to cortical state regulation. We thus identify NE signaling to astrocytes as a new circuit mechanism by which astrocytes act as sensors of NE changes and synchronize the cortex in response to arousal.
Results
Astrocyte Ca2+ correlates with increases in pupil diameter
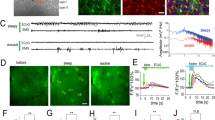
To first determine whether astrocyte Ca2+ activity is dynamically modulated by arousal, we carried out in vivo, 2P Ca2+ imaging in visual cortex of awake, head-fixed mice while simultaneously recording pupil diameter and running speed (Fig. 1a). We expressed the Ca2+ indicator GCaMP in cortical astrocytes under the GfaABC1D promoter and used the Astrocyte Quantitative Analysis (AQuA) toolkit22 to accurately capture dynamic fluorescent astrocyte signals, even from spatially overlapping events (Extended Data Fig. 1a,b).
a, Experimental setup. b, Representative astrocyte Ca2+ (magenta), pupil diameter (gray) and wheel speed (black) show that these measures are closely related (left), even during stationary periods (right, smoothed with a five-frame window). Percent max indicates percent of maximum recorded value. c, Astrocyte Ca2+ correlates better with pupil diameter than speed when comparing across mice (top, two-sided signed-rank test) or using HB of recordings (n = 6 mice). Values of n are applied throughout the figure. d, Left: separating pupil diameter on the basis of size. Right: astrocyte Ca2+ was not different within low or high pupil sizes (n = 3,206 time bins, one-sided Kruskal–Wallis test, P > 0.05 indicated as NS, Supplementary Table 1). Box plot shows median and interquartile range (IQR). Whiskers extend to the most extreme data points. e, Astrocyte Ca2+ and pupil diameter dynamics aligned to mouse movement onset at t = 0 (n = 104 movement onsets). f,g, Linear regression (trend lines) of astrocyte Ca2+ and either wheel speed or pupil diameter, after movement onset (n = 104 movement onsets, two-sided t-test): neither maximum wheel speed (left) nor changes in wheel speed (right) correlate well with astrocyte Ca2+ responses (f); maximum pupil diameter (left) predicts astrocyte Ca2+ less strongly than changes in pupil diameter (right) (g). h, Heat map of r2 values between the variables in f and g. i, Astrocyte Ca2+ response to pupil dilation during stationary periods (n = 188 stationary dilations). j, Changes in stationary pupil diameter correlate with astrocyte Ca2+ (n = 188 stationary dilations, two-sided t-test). k, Astrocyte Ca2+ events (light-pink bars) begin before movement offset, but average astrocyte Ca2+ fluorescence (magenta trace) peaks with pupil diameter at the end of movement (n = 104 movement offsets). l, The latency to maximum astrocyte Ca2+ and pupil diameter after movement onset (n = 104 movement bouts, two-sided t-test) are correlated. m, Astrocyte Ca2+ events (n = 1.17 × 104 astrocyte Ca2+ events) begin (dark pink) with pupil dilation (that is, when pupil derivative is positive) and end (light pink) with constriction (negative pupil derivative). Data are presented as mean ± s.e.m. unless otherwise noted.
We extracted fluorescence traces from all AQuA-detected Ca2+ events and found that movement was accompanied by large increases in astrocyte Ca2+—as well as increases in arousal indicated by pupil dilation—consistent with previous reports6,14,16,23,24 (Fig. 1b, left). Even during stationary periods, astrocyte Ca2+ activity was accompanied by concurrent and proportional fluctuations in arousal (Fig. 1b, right). Population astrocyte Ca2+ activity was better correlated with pupil diameter (0.51 ± 0.09) than running speed (0.21 ± 0.07) when comparing across mice (Fig. 1c, top, n = 6 mice) or using hierarchical bootstrapping (HB, Fig. 1c, bottom). In addition, individual astrocyte Ca2+ events correlated better with arousal compared with either speed- or time-shuffled data (Extended Data Fig. 1c, left). Most Ca2+ events (9,597/11,674; 82.2%) exhibited a maximal cross-correlation with pupil diameter within 10 s and a short (0.32 ± 0.01 s standard error of the mean (s.e.m)) lag, consistent with arousal driving astrocyte activity (Extended Data Fig. 1c, right).
Our analysis indicates that arousal contributes to astrocyte Ca2+ beyond its association with movement. However, cortical activity reflects both absolute pupil diameter23 and relative changes in pupil diameter24. To investigate whether astrocytes sense absolute levels of arousal, we binned the pupil diameter into deciles (Fig. 1d, left), and calculated the average Ca2+ fluorescence in each. Astrocyte Ca2+ dynamically varied with pupil diameter only when the pupil was ~40–80% of maximum diameter (Fig. 1d, right, and Supplementary Table 1). This range matched the overlap in pupil diameter found between stationary and movement periods (Extended Data Fig. 1d), indicating this relationship was a function of movement, and suggesting that absolute pupil diameter did not adequately explain the relationship between arousal and astrocyte Ca2+.
To test whether relative changes in pupil diameter were linked to astrocyte Ca2+ responses, we calculated an event-triggered average relative to the start of movement. As expected, we found that pupil diameter and—with a short delay—astrocyte Ca2+ increased around movement onset (Fig. 1e). We then asked which behavioral variables best explained movement-evoked astrocyte Ca2+ activity (Fig. 1f–h). We found that speed was a poor predictor of the maximal astrocyte Ca2+ fluorescence (Fig. 1f, left) as previously reported16, as was the relative change in speed (Fig. 1f, right). In addition, absolute pupil diameter following movement had a weak correlation with astrocyte Ca2 fluorescence, indicating that, even during behavioral state changes, absolute pupil diameter is a poor predictor of astrocyte responses (Fig. 1g, left). In contrast, the relative change in pupil diameter explained a substantial portion of the astrocyte Ca2+ response to movement, indicating that astrocytes are specifically sensitive to relative changes in arousal (Fig. 1g, right).
We further dissociated the effects of movement and arousal on astrocytes by separating behavioral state periods (Extended Data Fig. 1e). The relationship between astrocyte Ca2+ and increases in pupil diameter persisted during stationary periods (Fig. 1i–j), even though pupil diameter changes were smaller (Extended Data Fig. 1f). These results demonstrate that astrocytes are sensitive to smaller changes in arousal than previously recognized.
We also wondered whether changes in arousal could explain the timing of astrocyte Ca2+ activity. We noticed that astrocyte Ca2+ peaked with pupil diameter around movement offset (Fig. 1k) and the time to maximum pupil diameter was strongly correlated with the time to peak astrocyte activity (Fig. 1l). Both were also dependent on the duration of the movement bout (Extended Data Fig. 1g). When examining all astrocyte Ca2+ events, onsets occurred more often during dilation and offsets during constriction (Fig. 1m). These findings indicate that changes in arousal shape the timing and level of astrocyte Ca2+ activity.
Phasic increases in NE precede astrocyte Ca2+
NE is a key driver of both changes in pupil diameter and brain activity25. While recent work suggests that astrocytes preferentially respond via Ca2+ to “multi-peaked” NE axonal activity15, the impact of behavioral state has not yet been considered. To determine the relationship between NE and astrocyte Ca2+ activity, we simultaneously expressed a fluorescent sensor of NE, GRABNE26, in cortical neurons under the h-syn promoter and the Ca2+ indicator jRGECO1b in cortical astrocytes under the GfaABC1D promoter (Fig. 2a and Supplementary Video 1). Examining the raw GRABNE fluorescence, we saw reductions in fluorescence that matched changes in background vasculature, independent of indicator expression (Extended Data Fig. 2a). Our 2P imaging paradigm was not compatible with techniques to directly measure hemodynamic effects such as reflectance imaging and blind-source separation27. Therefore, we developed a method to approximate and compensate for hemodynamic signals in 2P imaging (Extended Data Fig. 2b and Methods). To further limit hemodynamic contamination, we took the mean of the corrected GRABNE signal, excluding highly contaminated or artifactual pixels (Extended Data Fig. 2c). As our methodology only approximates hemodynamic artifacts and does not account for possible hemodynamics effects from excitation or out-of-plane GRABNE fluorescence, we next sought to confirm the accuracy of our methodology. We found our corrected GRABNE signal showed little correlation with vasculature or hemodynamically contaminated regions (r < 0.2) but correlated well with regions that had active GRABNE signal (Extended Data Fig. 2d). To further validate our methodology in an unbiased manner, we correlated individual pixels with pupil diameter (Extended Data Fig. 2e) and found the corrected GRABNE signal linearly correlated with pupil diameter-related pixels suggesting our method reflects GRABNE signal that occurs from pupil-related increases in NE while reducing hemodynamic effects (Extended Data Fig. 2f).
a, In vivo 2P image showing dual-color expression of neuronal GRABNE and astrocyte jRGECO1b. Scale bar, 50 µm. n = 28 recordings from seven mice throughout figure. b, Representative traces smoothed with a five-frame window. Astrocyte Ca2+ (magenta), GRABNE (green), pupil diameter (gray) and wheel speed (black) show a close relationship over the course of minutes (left), and over the course of seconds during stationary periods (right). c, The power spectrum of GRABNE dynamics shows an inverse relationship with frequency (F−0.95, dotted line) and increased power in slow fluctuations (period >30 s, mean with jackknifed error bars). d, GRABNE positively correlates with pupil diameter (left), but not the derivative of pupil diameter (right). e, GRABNE is more strongly correlated with pupil diameter than pupil derivative across mice (left, two-sided signed-rank test) and across recordings (HB). f, Left: astrocyte Ca2+ activity positively correlates with GRABNE activity. Right: astrocyte Ca2+ activity follows changes in GRABNE. g, No difference was found between the GRABNE correlation with astrocytes compared with pupil diameter across mice (left, P = 0.08, two-sided signed-rank test) or across recordings (HB). h, Left: phasic increases in GRABNE signal, with arrowheads marking a subset of peaks. Right: larger increases in GRABNE had longer durations (P < 0.05 for all bins, one-sided Kruskal–Wallis test, n and P values listed in Supplementary Table 2). Box plot shows median and IQR. i, Example astrocyte Ca2+ traces separated by GRABNE peak amplitude. Left: astrocyte Ca2+ activity showed large and persistent responses to large increases (for example, 3 s.d.) in GRABNE. Right (shaded area, 5 s before and after t = 0): both large and small (for example, 1 s.d.) changes in GRABNE drove small transient increases in astrocyte Ca2+. j, Left: astrocyte Ca2+ persistently increased after large (≥2 s.d.) increases in GRABNE, and scaled with GRABNE amplitude (*P < 0.05, two-sided rank-sum test, n and P values listed in Supplementary Table 3). Right: astrocyte Ca2+ showed proportional, time-locked responses to even small changes (≥1 s.d.) in GRABNE (*P < 0.05, two-sided rank-sum test; for details, see Supplementary Table 3). k, GRABNE activity around astrocyte Ca2+ onsets at t = 0 s (n = 1.2 × 104 events) shows increased extracellular NE before astrocyte Ca2+ events in both movement and stationary periods. Data are presented as mean ± s.e.m.
Using our corrected GRABNE signal, we found that GRABNE fluorescence matched changes in pupil diameter and astrocyte Ca2+ activity, although the GRABNE showed a slow decay, similar to previous reports15 (Fig. 2b, left). This relationship persisted even during stationary periods (Fig. 2b, right). The slow GRABNE decay was reflected in the power spectrum, which showed an inverse relationship with frequency (Fig. 2c). Slow (0.01–0.03 Hz) GRABNE fluctuations had more power than the empirically fit 1/f relationship, suggesting long-lasting fluctuations in “tonic” NE predominate the GRABNE signal.
To confirm the link between GRABNE and arousal, we examined the cross-correlation between pupil diameter and GRABNE. We found a positive correlation between GRABNE and spontaneous changes in pupil diameter (r = 0.13, Fig. 2d), similar to that previously described for cortical NE axons28. However, the maximum cross-correlation was broad and continued for at least 20 s after the pupil diameter (Fig. 2d, left). Further, unlike what has been reported for NE axons28, GRABNE showed poor cross-correlation (r = 0.04) with the derivative of pupil diameter (Fig. 2d right, and 2e). These results suggest that cortical GRABNE dynamics best report arousal level rather than changes in arousal, and may substantively differ from the activity pattern of the underlying NE axons.
We next quantified the relationship between GRABNE and astrocyte Ca2+ activity. We found a positive, broad correlation (r = 0.17) between the two (Fig. 2f, left), with GRABNE preceding astrocyte Ca2+ (−5.7 ± 3.1 s delay, Fig. 2f, right) and GRABNE showing a similar link to astrocyte Ca2+ as arousal (Fig. 2g). Although slow NE fluctuations predominated our GRABNE signal (Fig. 2c), we also noticed phasic increases in NE (Fig. 2h, left, triangles). We segregated phasic NE peaks by amplitude (Fig. 2h, left, triangles) and saw that larger phasic GRABNE activity was also longer lasting (Fig. 2h, right, and Supplementary Table 2). We then examined how astrocyte Ca2+ changed following phasic NE activity. We saw that large (≥2 standard deviations (s.d.)) phasic changes in NE preceded prolonged increases in astrocyte Ca2+ (Fig. 2i–j, left, and Supplementary Table 3, left), while small increases in GRABNE co-occurred with proportional increases in astrocyte Ca2+ (Fig. 2i, right). These increases were observed even with smaller (1 s.d.) changes in NE (Fig. 2j, right, and Supplementary Table 3, right). These results suggest astrocytes dynamically respond to phasic changes in NE, in both the duration and amplitude of their Ca2+ signaling. We also calculated the event-triggered average GRABNE signal relative to astrocyte Ca2+ events and confirmed that GRABNE increased before astrocyte Ca2+, and peaked shortly after astrocyte Ca2+ signaling had begun (Fig. 2k). This relationship was true for both astrocyte Ca2+ events during movement (Fig. 2k, left) and stationary periods (Fig. 2k, right). In sum, these results indicate that astrocytes are sensitive to changes in NE across behavioral states.
To corroborate these findings, we performed freely moving, dual-color fiber photometry recordings of GRABNE and astrocyte Ca2+ (Extended Data Fig. 3a) and saw similar GRABNE dynamics (Extended Data Fig. 3b,c). To confirm the relationship between NE and astrocyte Ca2+, we used tail lifts to evoke startle responses14, and saw expected increases in both GRABNE and astrocyte Ca2+ (Extended Data Fig. 3d). The GRABNE signal began increasing before astrocyte Ca2+ and persisted after the startle response (Extended Data Fig. 3e). These results further support the hypothesis that astrocytes respond to phasic increases in NE.
Astrocyte Ca2+ may reduce arousal-driven neuronal activity
We next wondered how arousal-mediated changes in nearby neurons relate to astrocyte Ca2+ activity, as arousal also strongly modulates neuronal activity6,24,29. To answer this, we expressed hSyn-GCaMP6f in neurons and GfaABC1D-jRGECO1b in astrocytes to record the Ca2+ activity of both cellular populations simultaneously (Fig. 3a,b and Supplementary Video 2).
a, Experimental paradigm for dual-color Ca2+ imaging of neurons and astrocytes. n = 33 recordings from eight mice throughout figure. b, 2P images from one recording of in vivo neuronal GCaMP6f (gray) and astrocyte jRGECO1b (magenta). Yellow arrows indicate bleed-through in the red channel which was accounted for (Methods), while white arrows show a neuron with no bleed through. Scale bar, 50 µm. c, Example of neuronal (dark gray) and astrocyte (magenta) Ca2+ activity, with pupil diameter (light gray) and wheel speed (black). d, Astrocyte and neuronal Ca2+ were positively correlated, and increased their correlation during movement compared with stationary periods (two-sided rank sum test). e, Top: an example of pupil diameter (light gray) fluctuations alongside changes in the neuronal mean fluorescence (dark gray) and arousal-associated PC of neuronal activity (red). Bottom: an example heat map of single-cell neuronal activity sorted by arousal PC weight shows that the arousal PC captured heterogeneous neuronal responses to arousal, including around movement events (red dashed lines). f, The component number of the arousal PC. g, Both neurons (gray) and astrocytes (pink) showed positive correlations between mean Ca2+ fluorescence and pupil diameter (left). Using PC analysis, we identified neuronal activity that showed strong correlation with pupil diameter, whereas astrocyte Ca2+ activity was not amenable to this analysis. h, Scatter plot of the correlation between either arousal PC (y axis) or mean fluorescence (x axis) for each recording. PC analysis identified arousal-associated neuronal activity for neurons (gray dots), but not astrocytes (magenta dots). i, Astrocyte Ca2 signaling (magenta) occurred alongside increases in arousal-associated neuronal activity (gray), and peaked with reductions in neuronal activity, for both movement (top, n = 134 movement bouts) and stationary increases in arousal (bottom, n = 772 pupil dilations). j, Top: arousal-associated neuronal activity tends to peak and then decrease around astrocyte Ca2+ activity both overall (left, n = 8.9e4 events) and during stationary periods (right, n = 3.2 × 104 events). Bottom: the derivative of arousal-associated neuronal activity demonstrates that arousal-associated neuronal activity decreases directly following astrocyte Ca2+ events both overall (left) and during stationary periods (right). All data are presented as mean ± s.e.m. unless otherwise noted.
We found that astrocyte and neuronal Ca2+ fluctuated together along with movement and pupil diameter (Fig. 3c). The average fluorescence of neurons and astrocytes were maximally correlated with no time shift, a relationship enhanced during movement (Fig. 3d, black, r = 0.46 ± 0.06) compared with stationary periods (Fig. 3d, gray, r = 0.34 ± 0.04). We next investigated how astrocyte Ca2+ relates to arousal-driven neuronal activity specifically. To do this, we used principal component (PC) analysis to identify the PC of neuronal activity most correlated with arousal (arousal PC)29. We found that the arousal PC of neuronal activity (Fig. 3e, top, red line) often better matched the pupil diameter (Fig. 3e, top, light gray) than the mean neuronal activity (Fig. 3e, top, dark gray), probably due to the variability in neuronal responses to arousal (Fig. 3e, bottom).
In agreement with previous work29, we found that the arousal PC was usually PC1 (Fig. 3f, gray, 28/35 recordings). Furthermore, we found that average astrocyte Ca2+ activity (Fig. 3g, left, magenta) was better correlated with pupil diameter than average neuronal Ca2+ activity (Fig. 3f, left, gray). This relationship was reversed when using the arousal PC of astrocyte (Fig. 3g, right, magenta) and neuronal (Fig. 3g, right, gray) activity instead. The arousal PC consistently reflected arousal-associated activity better than using mean fluorescence for neurons (Fig. 3h, gray dots) but not for astrocytes (Fig. 3h, magenta dots), suggesting that astrocytic responses to arousal are best characterized by a consistent increase reflected in the mean fluorescence while neuronal responses are best captured by describing the variability in neuronal activity using PC analysis.
We next looked at changes in astrocyte Ca2+ and arousal-driven neuronal activity with arousal. We found that, with both movement (Fig. 3i, top) and stationary increases in pupil diameter (Fig. 3i, bottom), arousal-associated neuronal activity (Fig. 3i, gray) increased alongside astrocyte Ca2+ (Fig. 3i, magenta) and astrocyte Ca2+ appeared to peak when neuronal activity began to diminish (Fig. 3i). To investigate this further, we looked at the average arousal PC around the onset of all astrocyte Ca2+ events. We similarly found that astrocyte Ca2+ events occurred at the peak of arousal-associated neuronal activity (Fig. 3j, top left), and using the first derivative of the neuronal PC (Fig. 3j, bottom left), we saw astrocyte Ca2+ occurred at a transition point between increasing and decreasing arousal-associated neuronal activity. This was also true for astrocyte Ca2+ events during stationary periods (Fig. 3j, right). These results indicate that astrocyte activity is positioned to counteract the effects of arousal on the local neuronal population.
These results raised the possibility that astrocyte Ca2+ may respond to changes in local arousal-associated neuronal activity, rather than arousal or NE per se. To dissect the effects of neuronal activity and arousal on astrocyte Ca2+ we used Random Forest Regression to predict astrocyte Ca2+ (Extended Data Fig. 4). Using this strategy, we could explain ~85% of the variance in astrocyte Ca2+ fluorescence (Extended Data Fig. 4a, left). Permutation-based feature importance analysis indicated that pupil diameter was the most important predictor of astrocyte Ca2+, and a substantially better predictor than the next most important factor, local neuronal Ca2+ fluorescence (Extended Data Fig. 4a, right). These results remained consistent when using the neuronal arousal PC data (Extended Data Fig. 4b). We interpret these results as evidence that astrocytes, while responsive to local neuronal activity, are sensitive to arousal beyond the influence of nearby neurons.
Arousal-driven astrocyte Ca2+ is not local neuron-dependent
To further investigate how arousal and local neuronal activity drive arousal-associated astrocyte Ca2+, we expressed hM4Di, an inhibitory Gi-coupled Designer Receptor Exclusively Activated by Designer Drugs (DREADD)30 in neurons, as well as GCaMP6f in astrocytes to image Ca2+ (Fig. 4a). We found no difference in the average frequency of astrocyte Ca2+ events from baseline following administration of either the hM4Di agonist CNO or saline, although the overall variability increased (Fig. 4b, resampled distributions from each condition). We also found no difference in astrocyte Ca2+ responses to movement with either saline or CNO administration (Fig. 4c,d and Supplementary Table 4). There was no difference in astrocyte Ca2+ responses to arousal during stationary periods, except at a high CNO concentration (5 mg kg−1), where we recorded a potential enhancement of arousal-associated Ca2+ (Fig. 4e,f and Supplementary Table 5). These results indicate that local neuronal activity is not necessary for astrocyte Ca2+ responses to arousal, and that astrocytes may monitor the state of local circuit activity when responding to arousal.
a, Confocal image showing representative cortical expression from one mouse of both astrocyte GCaMP6f (magenta) and neuronal inhibitory DREADD hM4Di (gray). Scale bar, 500 µm. b, Bootstrapped change in astrocyte event rate (n = 1 × 105 resampled events from five mice) after saline (gray) or CNO (purple) administration. No significant change in astrocyte Ca2+ event rate was found (P < 0.05, One-sided test of the proportion of bootstrapped change in event rates below saline). c, Average change in astrocyte Ca2+ response to movement after saline (left) or CNO (right, 5 mg kg−1) compared with the baseline in each animal (black lines). d, No significant (P < 0.05) difference in astrocyte Ca2+ responses (% change in astrocyte Ca2+ relative to t = 0; box-and-whisker plot with outliers as open circles), to movement after saline or either 1 mg kg−1 or 5 mg kg−1 of CNO was found (one-sided Kruskal–Wallis test, n and P values listed in Supplementary Table 4). e, Average change in astrocyte Ca2+ response to stationary pupil dilation after saline (left) or CNO (right, 5 mg kg−1). f, No significant difference (P < 0.05) was found within saline or 1 mg kg−1 CNO conditions. 5 mg kg−1 CNO caused an enhancement in astrocyte responses to arousal (Kruskal–Wallis test, n and P values listed in Supplementary Table 5). Box plots show median and IQR, with whiskers extended to 1.5× IQR. Line plots are presented as mean ± s.e.m.
NE ties astrocyte Ca2+ to changes in cortical state
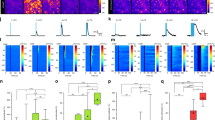
In Fig. 3, we showed that astrocyte Ca2+ events occur before reductions in arousal-associated neuronal activity. We wondered whether astrocyte Ca2+ was similarly related to activity changes in the broader cortical circuit. To answer this question, in a subset of mice expressing Ca2+ indicators in neurons (hysn-GCaMP6f) and astrocytes (GFAP-jRGECO1b), we implanted low-impedance electrodes bilaterally into the visual cortex (Fig. 5a). Using this methodology, we recorded the LFP (Fig. 5b, top), which unlike our spatially restricted imaging window, reflects the integrated electrical inputs and activity of the visual cortex and surrounding brain regions31. In these LFP recordings, we observed spontaneous transitions between cortical states dominated by LF power (2–7 Hz; running-evoked 7–10 Hz was excluded) and those dominated by HF (70–100 Hz) power, indicative of synchronous and desynchronized cortical states respectively (Fig. 5b)1. We noticed that, during transitions to a low-arousal state following movement, astrocyte Ca2+ peaked as LF power increased (Fig. 5c, left) and HF power decreased (Fig. 5c, right).
a, Experimental setup. b, Example LFP data (black) with calculated LF (2–7 Hz, blue) and HF (70–100 Hz, orange) power. c, Average LFP LF (left) and HF (right) power with astrocyte Ca2+ activity (magenta) around movement offset at t = 0 s (n = 52 offsets from four mice). d, Top: cross-correlation between astrocyte (magenta) or neuronal Ca2+ (gray) activity and LF power (left), or the derivative of LF power (right). Bottom: significance (1/P, two-sided signed-rank test) for astrocytes and neurons. Dashed line is the threshold of correction for multiple comparison. e, HF power and its derivative are cross-correlated to neuronal and astrocyte Ca2+, as for LF power in d. Neuronal Ca2+ correlated with LFP power, while astrocyte Ca2+ correlated with the derivative of LFP power. f,g, LFP power dynamics in ipsilateral (f) and contralateral (g) cortex, centered around astrocyte Ca2+ onsets at t = 0 s. LFP power in a 5 s window around astrocyte Ca2+ events was computed, and each frequency was normalized by its median power. (All p-values from one-sided HB). Left: astrocyte Ca2+ events occur at the crux of ipsilateral (f, n = 4 mice) and contralateral (g, n = 5 mice) LFP state transitions from HF- to LF-dominated states. Comparisons are made using HB between empirical data (colored bars) and shuffled distributions (light gray). Right: HF power decreased (orange) and LF power (blue) increased after astrocyte Ca2+ events. h,i, This relationship was abolished in both ipsilateral (h) and contralateral (i) cortical LFP recordings after administration of the Adra1 receptor antagonist Prazosin (5 mg kg−1, i.p., n = 4 mice), for both LF and HF power (comparisons are made using HB between state changes without Prazosin (colored bars) and with the addition of Prazosin (dark gray). Line plots are presented as mean ± s.e.m.
To investigate this relationship, we cross-correlated astrocyte and neuronal Ca2+ with LFP power, and with the derivative of LFP power. We found that astrocyte cross-correlations with LFP power were right-shifted from the equivalent neuronal cross-correlations (Fig. 5d,e). When looking at LF power specifically (Fig. 5d, left, magenta), we found that astrocyte Ca2+ preceded increases in LF power (Fig. 5d, left, magenta). In contrast, neuronal Ca2+ was only significantly positively correlated with LF power with zero lag (Fig. 5d, left, gray), indicating that LF power and neuronal population activity are coordinated. These findings were confirmed when examining the derivative of LF power. We found that changes in LF power were maximally correlated with astrocyte activity without any lag (Fig. 5d, right, magenta), while LF power changes preceded neuronal activity (Fig. 5d, right, gray). Our results demonstrate that, while neuronal Ca2+ is correlated with LF power, astrocyte Ca2+ is correlated with changes in LF power.
We found a similar relationship when using HF power, although inverted about the y axis. HF power preceded both neuronal and astrocyte Ca2+, with neurons maximally negatively correlated with zero time lag (Fig. 5e, left). In addition, both astrocyte and neuronal Ca2+ were negatively correlated with the derivative of HF power, but for astrocytes this relationship was true both when HF power preceded or had no lag with astrocyte Ca2+ (Fig. 5e, right). These results indicate that, while HF power precedes both neuronal and astrocyte Ca2+, neuronal Ca2+ is inversely related to absolute HF power while astrocyte Ca2+ is inversely related to changes in HF power. Overall, this analysis demonstrates that neuronal Ca2+ activity reflects absolute LFP power while astrocyte Ca2+ is instead tied to changes in LFP power.
We next asked whether arousal-driven astrocyte Ca2+ was generally tied to changes in LFP power by computing an astrocyte Ca2+ event-triggered spectrogram. We found that astrocyte Ca2+ signaling occurred at the crux of a transition from a HF-dominated cortical state to one dominated by LF power (Fig. 5f). This relationship was also seen in the LFP power of the contralateral cortex (Fig. 5g), indicating that astrocyte Ca2+ is tied to the transition of neuronal activity from a high- to low-arousal state not just at a local level (Fig. 3j), and at the level of the nearby cortex (Fig. 5f), but also to bi-hemispheric changes in cortical state.
On the basis of our identified changes in arousal and NE as major drivers of spontaneous changes in astrocyte Ca2+ (Figs. 1 and 2), we hypothesized that NE was necessary for the link between astrocyte Ca2+ and cortical state transitions. In particular, we hypothesized that Adra1 receptors might underlie this relationship due to their importance for astrocyte physiology14,16,19,32. We pharmacologically blocked Adra1 receptors using Prazosin (5 mg kg−1, intraperitoneal (i.p.)) and found that the relationship between astrocytes and cortical state was reduced (Fig. 5h,i), confirming that NE signaling links astrocyte Ca2+ to cortical state changes.
Enrichment of Adra1a mRNA in visual cortex astrocytes
We next sought to determine which Adra1 receptors were most likely to underlie the connection between astrocytes and cortical state. We analyzed a previously published dataset that profiled the ribosome-associated messenger RNA expression of astrocytes in the mouse visual cortex33. We looked at adrenergic receptor expression in the adult (P120) dataset and confirmed astrocytic expression of most adrenergic receptors, although astrocytes showed little Adra2b, Adrab2 and Adrab3 in this dataset (Extended Data Fig. 5a, left). When analyzing the astrocytic expression of adrenergic receptors relative to an input control, the relative expression of Adra1a mRNA was highest and greater than the input control, indicating that Adra1a mRNA may be preferentially enriched in visual cortex astrocytes (Extended Data Fig. 5a, right).
We also used spatial transcriptomics to assess the mRNA expression of Adra1a, Adra1b and Adra2a (Extended Data Fig. 5b). Using LaST map single-molecule in situ hybridization (smFISH)34, we saw striking heterogeneity throughout the brain (Extended Data Fig. 5b, left). All three receptors showed cortical expression in astrocytes, as delineated by expression of the astrocyte-specific mRNA SlcA3 (GLAST), and in non-astrocytic cell types (Extended Data Fig. 5b, right). We quantified mRNA spots per astrocyte in the visual cortex for each of these receptors and found higher expression at deep layers of cortex (Extended Data Fig. 5c). This was particularly true for Adra2a and Adra1b receptors, while Adra1a receptors also showed higher expression in intermediate layers of cortex (Extended Data Fig. 5c), where the effects of cortical synchrony are particularly important for perception35.
Astrocyte Adra1a receptor signaling shapes neuronal activity
On the basis of pharmacological and transcriptomic data, as well as its activation of astrocytes in other contexts15,16,17,36, we identified Adra1a receptors as a likely connector between astrocytes and cortical state. To test how Adra1a receptors modulated cortical activity, we injected the Adra1a receptor agonist A61603 (10 μg kg−1, i.p.)—which stimulates astrocytes in acute cortical slices32—while recording cortical astrocyte and neuron Ca2+ activity (Fig. 6a). We saw robust responses within minutes of A61603 administration (Fig. 6b). Astrocyte Ca2+ was more homogeneous (Fig. 6c) and elevated (Fig. 6d, left), while neuronal Ca2+ showed a general reduction in baseline Ca2+ (Fig. 6d, right), while exhibiting similar oscillatory activity as astrocytes. To better quantify these neuronal changes, we took the power spectrum of each neuron and separated the activity into slower (<0.05 Hz, Fig. 6e, left) and faster fluctuations (>0.05 Hz, Fig. 6f, left). We found that after A61603 administration there was more power in <0.05 Hz fluctuations (Fig. 6e, right) and less power in >0.05 Hz fluctuations (Fig. 6f, right). Our results indicate that A61603 administration, while increasing the coordination and level of astrocyte Ca2+ activity, broadly inhibits and alters the pattern of neuronal activity.
a, Experimental schematic for dual-color imaging with in vivo pharmacology. b, A striking example of population astrocyte (magenta) and neuron (gray) Ca2+ activity in response to A61603 (10 µg kg−1, i.p.). c–f, Histograms show differences between metrics at baseline (gray) or after A61603 (red) using HB (n = 4 mice in c–g): astrocyte Ca2+ activity became more homogeneous as measured by autocorrelation (c); astrocytes (left) showed increased population Ca2+ fluorescence while neurons (right) showed decreased population Ca2+ fluorescence after A61603 administration (d); neuronal activity power spectra <0.05 Hz (left) showed more power after A61603 injection (right, HB) (e); same as in e for power >0.05 Hz (f). g, Schematic for neuronal Ca2+ imaging in homozygous Adra1afl/fl mice and wild-type littermate controls; all mice were injected with GFAP-Cre (n = 4 Adra1afl/fl and four wild-type littermate controls in h–l). h, Overall, neuronal activity was increased in Adra1afl/fl mice (green) relative to wild type (gray). i,j, Neuronal activity <0.05 Hz was decreased and neuronal activity >0.05 Hz was increased in Adra1afl/fl mice. k, Arousal-associated neuronal activity was increased following movement in Adra1afl/fl mice (green, n = 177 movement bouts) compared with wild type (gray, n = 400). l, Adra1afl/fl mice (green, n = 923 pupil dilations) showed increased arousal-associated neuronal activity compared with wild type (gray, n = 1,396 pupil dilations) following stationary increases in pupil diameter. m, Arousal-associated neuronal activity was less correlated with the pupil diameter overall in Adra1afl/fl (green, n = 23 recordings) compared with wild-type mice (gray, n = 14 recordings). For all panels, histograms show HB distributions (n = 4 Adra1afl/fl and 4 wild-type littermate controls). All line plots are presented as mean ± s.e.m. and P values from histograms show one-sided HB tests.
To determine how astrocytic Adra1a receptors specifically affect cortical neuronal activity, we generated a mouse line with LoxP sites flanking Adra1a, allowing for Cre-specific deletion of the receptor (Extended Data Fig. 6). We injected an astrocyte-specific Cre-GFP virus into the cortex of Adra1afl/fl mice (or wild-type littermate controls), and performed neuronal Ca2+ imaging while recording movement and pupillometry (Fig. 6g). In agreement with previous work37, we saw astrocyte-specific Cre expression which colocalized with the astrocytic marker S100β, but not the neuronal marker NeuN (Extended Data Fig. 7). Overall activity in these mice showed more neuronal Ca2+ events (Fig. 6h), as well as less power in <0.05 Hz Ca2+ fluctuations (Fig. 6i) and more power in >0.05 Hz Ca2+ fluctuations (Fig. 6j), the inverse of stimulating Adra1a receptors using A61603 (Fig. 6d–f). These results suggest NE signaling through astrocyte Adra1a receptors is a major pathway for modulating the level and pattern of neuronal activity. To ensure these results were not an artifact of imaging rate, we recorded neuronal activity using resonant galvanometers and saw the same results (Extended Data Fig. 8).
We next determined whether astrocyte Adra1a receptors impacted arousal-related neuronal activity. As before (Fig. 3i), we assessed how arousal-related neuronal activity changed either with movement (Fig. 6k) or stationary pupil dilation (Fig. 6l). In this dataset, the distribution of durations during movement bouts (Extended Data Fig. 9a) and stationary pupil dilations (Extended Data Fig. 9b) was wide, and differed between Adra1afl/fl mice and wild-type controls. To account for this, we analyzed only arousal-related neuronal activity during the movement (Extended Data Fig. 9b) or pupil dilation event (Extended Data Fig. 9c), including a ~1 s offset to account for a delayed response in neuronal activity. In both cases, Adra1afl/fl mice displayed enhanced arousal-related neuronal activity (Fig. 6k,l). This increase could not be explained by an overall stronger neuronal connection to arousal; Adra1afl/fl neuronal activity was less correlated with pupil diameter than that in wild-type littermates (Fig. 6m). These results suggest that NE signaling through astrocyte Adra1a receptors regulates both the magnitude and shape of neuronal responses to arousal.
NE signaling via astrocyte Adra1a modulates cortical state
On the basis of the Adra1a-dependent relationship between astrocytes and cortical state, we hypothesized that, in contrast to the desynchronizing effect of NE on the cortex generally11, signaling through astrocytic Adra1a receptors would lead to cortical synchrony. We again injected the Adra1a receptor agonist A61603 (1 μg kg−1, i.p.) while recording cortical LFP (Fig. 7a). Compared with saline control, A61603 treatment resulted in high-amplitude LFP fluctuations (Fig. 7b) reflected by increased LF power (Fig. 7c,d). In contrast, we saw no significant difference in HF power (70–100 Hz, Fig. 7e). These effects show that, contrary to the role of NE more broadly10,11,25, stimulation of Adra1a receptors increases cortical synchrony.
a, Experimental setup for LFP recording with in vivo pharmacology. b, Example cortical LFP data following i.p. injections of either saline (black) or the Adra1a-specific agonist A61603 (1 µg kg−1, red). c, Representative spectrograms from saline-injected (top) and A61603 -injected (bottom) mice. Arrows and vertical lines indicate time of injection. d, Left: example of 2–7 Hz band power change with saline or A61603 injection, smoothed with a 2 min moving average. Right: A61603 increased LF power compared with saline (two-sided signed-rank test, n = 10 mice). e, Left: representative trace of 70–100 Hz band power with saline or A61603 injection. Right: A61603 did not affect HF power compared with saline (two-sided signed-rank test, n = 10 mice). f, LFP recordings were performed in homozygous Adra1afl/fl mice and wild-type littermate controls; all mice were injected with GFAP-Cre. g, Representative 30 s of LFP data from Adra1afl/fl (green) and wild-type (black) mice. h, Average LFP spectra from Adra1afl/fl (n = 5) and wild-type (n = 9) mice, with total LFP power higher in Adra1afl/fl mice (two-sided t-test, shaded region shows theoretical error bars with P = 0.05). i, Average LFP spectrograms around movement offset (t = 0 s) for wild-type (left) and Adra1afl/fl (right) mice. Data are normalized by the median power at each frequency to show state-related changes in LFP. Top: entire spectrograms from 0–100 Hz. Middle: 70–100 Hz range showing increased power in Adra1afl/fl mice compared with wild type during movement. Bottom: 2–7 Hz range showing reduced power after movement offset in Adra1afl/fl mice. j, There was increased 70–100 Hz power in Adra1afl/fl mice during movement (left, n = 303 wild-type and 194 Adra1afl/fl movement offsets) between cohorts (right, n = 5 Adra1afl/fl and 9 wild-type mice, two-sided t-test). k, Same as in j for 2–7 Hz power after mice stopped moving. For all scatter plots, individual data are plotted as mean ± s.e.m.
However, the manipulation using A61603 was body-wide due to i.p. injection and affected all Adra1a-expressing cells. On the basis of our Ca2+ imaging results (Fig. 6g–m) in Adra1afl/fl mice, we wondered whether astrocytic Adra1a receptor signaling might modulate cortical state. To test this, we used the same receptor knockout strategy and recorded cortical LFP (Fig. 7f). In Adra1afl/fl mice, the cortical LFP was generally higher amplitude than in controls (Fig. 7g), with higher power across all frequency bands (Fig. 7h, left), and higher total LFP power (Fig. 7h, right). These results further support the idea that NE signaling to astrocytes modulates overall cortical neuronal activity.
We also hypothesized that removing astrocytic Adra1a receptors would effect NE-related cortical state transitions. We focused on times when mice stopped moving (Fig. 7i, top row), and found Adra1afl/fl mice had increased HF power during movement (Fig. 7i, middle row; Fig. 7j) and less LF power in the Adra1afl/fl mice compared with wild-type mice (Fig. 7i, bottom row; Fig. 7k). Taken together, these data show that selectively removing a NE receptor from astrocytes alters arousal-related cortical state changes, enhancing the desynchronizing effects of arousal and reducing resynchronization afterwards.
Discussion
Understanding awake cortical state regulation is crucial for understanding perception, attention and behavior1. Most research examining cortical states has focused on arousal mechanisms—such as release of NE—that desynchronize the cortex and increase sensitivity to external stimuli6,7,24. However, we found that NE (Fig. 8, green) not only desynchronizes cortical neuronal activity, but also leads to cortical synchrony by activating astrocytic Adra1a receptors. This model identifies astrocytes as key regulators in the awake cortex, acting as a feedback mechanism for arousal-associated desynchrony (Fig. 8).
NE (green arrows) drives changes from states of low arousal with synchronized cortical activity (left) to states of high arousal with desynchronized cortical activity (right). Simultaneous activation of astrocytes through the Adra1a receptor leads to Ca2+ signaling (magenta arrow) that drives the cortex back to a synchronized state following increases in arousal. Scale bar, 10 µm. Mouse images from SciDraw.io under a Creative Commons licence CC BY 4.0.
Astrocytes regulate arousal-associated cortical circuits
We found that astrocytes responded more sensitively to arousal (Fig. 1) and NE (Fig. 2) than previously recognized14,15,16,17. Arousal-associated astrocyte Ca2+ activity occurred at the crux of reductions in arousal-associated neuronal activity (Fig. 3) and bi-hemispheric cortical state changes (Fig. 5), and was not dependent on local neuronal activity (Fig. 4). These results suggest direct NE signaling to astrocytes acts as a separate neuromodulatory pathway. To support this, we found that NE signaling through astrocyte Adra1a receptors regulates overall neuronal activity (Fig. 6h–j), the response of neurons specifically to arousal (Fig. 6k–m), and arousal-associated cortical states (Fig. 7f–k). Notably, we find that astrocytes detect relative changes in arousal, suggesting they act as a feedback mechanism without working against the arousal system more generally. This role may be particularly useful in the context of heterogeneous cortical NE dynamics. We observed extracellular fluctuations in NE26 on a timescale of seconds, similar to the reported activity patterns of the underlying locus coeruleus axons28, as well as much slower signaling on the order of tens of seconds to minutes (Fig. 2c). Thus, change detection may allow astrocytes to ignore the tonic fluctuations of cortical NE and sensitively respond to phasic increases in NE.
Astrocyte Ca2+ is poised to regulate neuronal activity
Of course, astrocytes are not the sole detectors of cortical NE. Neurons, among other cell types12,13, also show strong responses to arousal2,3,7,24 and NE10. Understanding the relative effects of arousal on neuronal and astrocytic activity is crucial to understanding how these cellular populations contribute to arousal-mediated cortical state changes. Our results are notable in the context of previous research placing astrocyte activity downstream of neuronal activity, or to driving changes in neuronal activity on long timescales32,38,39. In contrast, our data show that arousal-associated astrocyte Ca2+ signaling occurs at the crux of the neuronal response to arousal, co-occurring with transitions in arousal-associated neuronal activity (Fig. 3). This relationship was also seen more broadly, with astrocyte Ca2+ occurring when bi-hemispheric arousal-associated desynchronized electrical activity decreased and cortical synchrony increased (Fig. 5). Furthermore, we found that NE-specific astrocyte Ca2+ signaling, and its relationship to arousal, was not dependent on the activity of nearby neurons, suggesting that astrocytes act directly downstream of neuromodulatory signaling to modulate neuronal activity (Fig. 4). These findings support a model in which astrocytes dynamically respond to NE to modulate neuronal activity on the physiological timescales of arousal.
Receptor-specific relationship between NE and cortical state
Our work positions cortical astrocytes as drivers of neuronal synchrony on multiple scales in the wake state, and suggests new roles for NE in cortical state regulation. In contrast to the generally desynchronizing role of NE11, we found that specific pharmacological stimulation of the Adra1a receptor reduced cortical neuronal activity (Fig. 6d, right) and altered its pattern (Fig. 6e, f). Adra1a receptor stimulation also led to cortical synchrony increases without altering HF power (Fig. 7a–d). This relationship was initially hypothesized by early pharmacological work40, but to our knowledge has not been validated nor widely accepted. Our findings suggest that even within the same family, activation of different NE-receptor subtypes may have profoundly different effects on cortical state and by extension arousal, attention and behavior, which may help explain disparate effects of adrenergic signaling on perception and cognition41,42.
What is arousal?
Here we used pupil diameter as an external read-out for a complicated set of biological processes that influence neural activity brain-wide23,25,43,44. While we focused on NE, many neuromodulators including acetylcholine28,45 and serotonin46 vary with arousal and modulate cortical state. Fully describing how neuromodulatory inputs affect astrocyte and neuronal activity will be vital for a complete description of cortical state regulation. Other internal states, such as motivation, interact with arousal and may involve neuromodulatory systems, but are not fully captured with the present arousal metrics. Here we were interested in pupil diameter and movement as external readouts of NE-related states, but future work should investigate the contributions of other internal states, using tools such as facial motion energy29, to further dissect astrocyte function in the context of internal states.
While our work establishes a role for astrocytes in mediating arousal-associated changes in neuronal activity and cortical state, we did not directly link these changes to behavioral outcomes. Future studies should focus on how arousal-associated astrocyte Ca2+ influences the general perceptual effects of arousal7 and extend these findings to the specific effects of attention1,35,43,47. It will also be important to examine how sensory stimuli-driven48,49,50 and arousal-driven astrocyte Ca2+ signaling leads to changes in behaviorally relevant neuronal population activity and cortical synchrony.
How does arousal modulate different cellular subpopulations?
To expand the impact of our findings, the experiments described here could be extended to dissect the role of specific subpopulations of neurons and astrocytes, or types of Ca2+ activity in these populations. In this work, we analyzed Ca2+ imaging data as homogeneous cellular populations, in part due to an acquisition rate (~2 Hz) necessary for imaging astrocyte populations on a wider scale (0.5 mm2). However, there may be differences between the population-level Ca2+ activity we identified and fast Ca2+ responses to arousal.
Both astrocytes34,51 and neurons52 can be split into multiple subpopulations, and neuronal subtypes—in particular inhibitory interneuron subtypes—have unique relationships to arousal and cortical state53. In our work, we used PC analysis to account for heterogeneous neuronal responses to arousal, and we found Adra1a-mediated astrocytic modulation of neuronal responses to arousal. However, we did not identify any specific effects on neuronal subtypes. On the basis of the overall reduction of neuronal activity with A61603 injection (Fig. 6d, right), and the overall increase in neuronal activity (Fig. 6h) and LFP power with knockout of astrocytic-Adra1a receptors (Fig. 7h), our work suggests astrocytic modulation of cortical inhibition is a likely candidate for the downstream circuit mechanisms controlling cortical state, as has been proposed in other contexts36,54. Cortical interneurons have been shown both to play a critical role in the control of cortical state45,55,56, and to be particularly sensitive to their electrostatic extracellular environment57. Neuromodulator-driven astrocytic modulation of the extracellular environment58 and inhibitory regulation of nearby neurons18 may act as prime loci of control for cortical circuit activity and state.
Within our smFISH dataset (Extended Data Fig. 5) and in published single-cell data51, cortical astrocytes show variable expression of neuromodulatory receptors, and some astrocytes lack any transcripts for Adra1a. This suggests molecularly distinct, and potentially functionally distinct, subpopulations of cortical astrocytes with differential sensitivity to arousal-related neuromodulators. It also suggests that gap junctions, ATP signaling and other mechanisms59,60 that create networks within the astrocyte syncytium may be important for arousal-associated astrocyte activity and modulation of cortical circuits. Identifying the specific relationships among subpopulations of astrocytes and neurons, and determining the manner and magnitude of their modulation by arousal, will be important for understanding what constitutes a cortical circuit and how cortical circuit activity is regulated. Furthermore, much like the effects of arousal, these relationships may be layer35 or cortical area44 specific. Future study of cellular subpopulation-specific effects on cortical state, particularly in additional cortical regions and in combination with behavioral assessments, will provide a richer appreciation of the cellular mechanisms that regulate arousal-associated cortical state.
Methods
Animals
All procedures were carried out in accordance with protocols approved by the University of California, San Francisco Institutional Animal Care and Use Committee. Animals were housed and maintained in a temperature-controlled environment on a 12 h light–dark cycle, with ad libitum food and water. Male and female mice were used whenever available. All imaging/electrophysiology experiments were performed at the same time each day. Adult C57BL/6 mice, Adra1afl/fl mice or Adra1a wild-type mice aged 1–6 months at time of surgery were used. For experiments involving Adra1a fl/fl mice, the experimenter was blind to animal genotype before surgery, recording and analysis.
Generation of Adra1afl/fl mice
The Mouse Biology Program at the University of California, Davis, constructed the mouse. A floxed FLAG-α1A knock-in vector was made using standard methods as follows: The targeting vector contained a 5′ arm of 5.4 kb and a 3′ arm of 5.5 kb. Lox P sites were placed upstream and downstream of the α1A-AR gene first coding exon. A Kozak sequence and single FLAG tag were upstream, and the neomycin-resistance gene was downstream.
The vector was electroporated into the C57BL/6N ES cell line JM8.F6. The resulting ES cell clones were screened by long-range PCR for loss of the native allele and homologous recombination, and containing a single copy of the plasmid by LoxP PCR. Two clones passed these screens and had normal chromosome counts. Both ES clones were microinjected into blastocysts, and transferred to embryonic day 2.5 stage pseudo-pregnant recipients. The resulting chimeras were screened for percent ES cell derived coat color, and those greater than 50% were mated to C57BL/6N females. Germline heterozygous mice were produced, and mated to MMRRC strain C57BL/6-Tg(CAG-Flpo)1Afst/Mmucd, RRID: MMRRC:036512-UCD, for excision of the Neo selection cassette. Neo-excised mice were identified via PCR, and mated further to C57BL/6J mice to remove the FLP transgene. Mice were continued in C57BL/6J.
Routine PCR genotyping used 5′-gcttcctcaggctcacgtttcc and 3′-gccttagaaatgttcacctgtgc primers upstream and downstream of the LoxP site (Extended Data Fig. 6). These mice are available upon request from the corresponding author.
Surgical procedures and viral infection
Mice were administered dexamethasone (5 mg kg−1, subcutaneous) at least 1 h before surgery, and anesthetized using 1.5% isoflurane (Patterson Veterinary Supply, 78908115). After hair removal and three alternating swabs of 70% ethanol (Thermo Fisher Scientific, 04-355-720) and Betadine (Thermo Fisher Scientific, NC9850318), a custom-made titanium headplate was attached to the skull using cyanoacrylate glue and C&B Metabond (Parkell, S380). If recording LFP, 0.5 mm burr holes were made bilaterally over the visual cortex, and bilaterally over the cerebellum for reference, and a ~200-µm-diameter perfluoroalkoxy-coated tungsten wire (A-M Systems, 796500) was implanted -0.2 mm into each hole and secured with Metabond. For imaging and LFP experiments, a 3 mm craniotomy was made over the right visual cortex and the right visual cortex burr hole was made lateral to the craniotomy.
For viral infection, 400–800 nl total volume of the following viruses were injected alone, or in combination by premixing the solutions before aspiration: AAV5.GfaABC1D.GCaMP6f.SV40 (Addgene, 52925-AAV5), AAV9.hSyn.NE2h (WZ Biosciences, YL003011-AV9), AAV9.GfaABC1D.jRGECO1b (Vigene, custom-ordered), AAV9.Syn.GCaMP6f.WPRE.SV40 (Penn Vector Core, AV-8-PV2822), AAV2.hSYN.hM4D(Gi).mCherry (Addgene, 50475-AAV2) and AAV5.GFAP(0.7).EGFP.T2A.iCre (Vector Biolabs, VB1131).
Injections were made through a glass pipette and UMP3 microsyringe pump (World Precision Instruments) into one or two locations in the right visual cortex at coordinates centered on +2.5 mm medial/lateral, +0.5 mm anterior/posterior and −0.3 dorsal/ventral from lambda. After allowing at least 10 min for viral diffusion, the pipette was slowly withdrawn and a glass cranial window implanted using a standard protocol61.
For in vivo fiber photometry recordings, GRABNE2h (AAV9.hSyn.NE2h) and astrocytic jRGECO1b (AAV9.GfaABC1D.jRGECO1b) were expressed via viral vectors in C57Bl/6 mice. A 1-mm-diameter craniotomy was made over the PFC (+1.7–1.8 mm rostral, +0.5 mm lateral from bregma), and viral vectors were delivered to −2.3 to 2.4 mm ventral. A fiber optic cannula (Mono Fiberoptic Cannula, 400 µm core, 0.66 NA, 2.8 mm length, Doric Lenses) was then lowered to −2.3 mm ventral and secured in place using dental cement.
Immunohistochemistry and in situ hybridization
Immunohistochemistry
Following in vivo experiments, mice were overdosed on isoflurane and then perfused transcardially with phosphate-buffered saline (PBS, Sigma-Aldrich P3813) followed by 4% paraformaldehyde in PBS (Santa Cruz Biotechnology, CAS 30525-89-4). Brains were removed and postfixed overnight in 4% paraformaldehyde, followed by cryoprotection in 30% sucrose in PBS for 2 days at 4 °C. Brains were snap frozen in dry ice and stored at −80 °C until adhesion onto a sectioning block with Optimal Cutting Temperature Compound (Thermo Fisher Scientific, 23-730-571). Forty-micrometer coronal sections were taken on a cryostat and stored in cryoprotectant at −20 °C until immunohistochemistry was performed. For immunohistochemistry, free-floating sections spanning the rostral–caudal axis were selected and washed with 1× PBS for 5 min three times on an orbital shaker, followed by permeabilization with 0.01% PBS-Triton X for 30 min. Sections were then blocked with 10% NGS (Sigma-Aldrich, S26-100ML) for 1 h. Immediately after, sections were incubated with chicken α-GFP (1:3,000, Abcam, ab13970) and either rabbit α-NeuN (1:1,000, EMD Millipore, ABN78) or mouse α-NeuN (1:1,000, Millipore Sigma, MAB377) and rabbit α-S100B (1:500, Millipore Sigma, SAB5500172). Sections were then washed with 1x PBS for five minutes three times on a shaker, followed by secondary incubation with Thermo Fisher Scientific goat α-chicken Alexa Fluor 488 and either goat α-rabbit Alexa Fluor 405 or goat α-mouse Alexa Fluor 405; goat α-rabbit Alexa Fluor 555 for 2 h at 20 °C on a shaker. Sections were washed again with 1× PBS for 5 min three times, then mounted and cover slipped with Fluoromount-G. For whole section examples, images were taken using a Keyence BZ-X800 fluorescence microscope to assess viral spread. Then, 2× images were acquired and the images were computationally stitched with Keyence Analysis Software. For cell counting 60× z-stacks were captured on a spinning-disk confocal (Zeiss). Slides were oil-immersed. The Fiji plugin Cell Counter was used to quantify the number of GFP+, NeuN+ and GFP+/NeuN+ cells to determine colocalization. Each animal had two sections, with each section having six distinct field of views containing 20 z-planes. A cell was considered GFP+ when signal was confined to soma and processes, and GFP+/NeuN+ when cells exhibited merged signals.
Single-molecule fluorescent in situ hybridization
Single-molecule, fluorescent in situ hybridization data collection was performed using LaST map smFISH as previously described34. Astrocyte cell boundaries were segmented using an ilastik pixel classifier and a customized watershed segmentation. To preserve processes of astrocyte and not cut them off from the DAPI signals, a pixel classifier was trained by using only large Gaussian filters (5/10 pixels) during the feature extraction step. As a result, astrocyte processes were detected with fewer splits. Pixel classification carves out only non-background pixels from the image and does not identify the astrocyte boundaries separating neighboring cells, that is, instance segmentation. To address this problem, we first used CellPose to identify all nuclei from the image. Subsequently, the centroids of all nuclei were extracted and used as the seeds to generate astrocyte boundaries between adjacent cells using a watershed segmentation. As a result, astrocytes with touching processes were separated. Finally, as non-cell debris might remain in the segmentation image, we further used an object classification workflow in ilastik to remove them.
All mRNA signals were detected using a Python package called TrackPy using five pixels as diameter and 96 as percentile threshold. These detected spots were then assigned to each astrocyte that they sat within by using a Python package called shapely. Due to the tissue damage that occurred during the sample preparation step, only seven cortex surface areas from all sections were eligible for downstream processing to ensure the comparability between tissue sections. For all these regions, both white matter boundaries and the superficial cortex area were manually annotated. For each cell, the distance to both the white matter boundary (d_WM) and distance to the cortex surface (d_ep) were calculated. The relative cortical depth (D_relaCortex) was thus defined as:
Recording setup
Animals were given at least 1 week after surgery for recovery and viral expression. They were then habituated on a custom-made circular running wheel over at least 2 days, and for a cumulative time of at least 2.5 h, before experimental recordings began. After habituation, mice were head-fixed on the wheel and movements were recorded by monitoring deflections of colored tabs on the edge of the wheel using an optoswitch (Newark, HOA1877-003).
Pupillometry
Pupil recordings were made using a Genie near-infrared camera (1stVision, M640) and a telescopic lens (Thorlabs, MVL50TM23), and acquired at 30 Hz using the MATLAB Image Acquisition toolbox. A small monitor (Amazon, B06XKLNMW3) showing a consistent teal background color (RGB: 0,1,1) was placed by the mouse to allow for observation of the full range of pupil dynamics in an otherwise dark room. For experiments without 2P illumination, a near-infrared light (Amazon, B00NFNJ7FS) was used to visualize the pupil.
2P imaging
2P imaging was performed on a microscope (Bruker) with two tunable Ti:sapphire lasers (MaiTai, SpectraPhysics) and a Nikon 16×, 0.8 numerical aperture water-dipping objective with a 2× optical zoom (frame rate 1.7 Hz, field of view 412 µm2, resolution 512 × 512 pixels). A 950 nm excitation light with a 515/530 emission filter was used to image green-emitting fluorophores, and 1,040 nm light with a 605/615 emission filter was used to image red-emitting fluorophores. Recordings lasted from 10 min to 1 h.
Electrophysiology
Visual cortex LFP was recorded at 1 kHz and subtracted from the ipsilateral cerebellar LFP before 1 kHz amplification (Warner Instruments, DP-304A) and acquired using PrairieView (Bruker) or PackIO62.
In vivo pharmacology
Recordings were taken before and after saline, Prazosin–HCl (5 mg kg−1 Sigma-Aldrich, P7791-50MG), A61603(1 µg kg−1 or 10 µg kg−1, Tocris, 1052), or clozapine N-oxide (CNO, 1 mg kg−1 or 5 mg kg−1, Tocris, 4936) were injected intraperitonially while animals remained head-fixed on the wheel, to ensure post-treatment recordings were comparable with baseline measures.
Fiber photometry
Dual-color fiber photometry recordings were performed on a Tucker-Davis Technologies RZ10X processor with 405, 465 and 560 nm LEDs. LED drivers were modulated such that light power was approximately 15 μW for 405 nm and 20 µW for 465 nm and 560 nm wavelengths at the tip of the light path. Animals were recorded in a freely moving arena in which the mouse was able to move in all directions, after coupling to low-autofluorescence fiberoptic patchcords connected to photosensors through a rotary joint (Doric Lenses). Fluorescence signals were recorded for 10 min, during which tail lifts were performed every 2 min. For a tail lift stimulation, the experimenter held and lifted the tail of the animal until its hind paws disconnected from the ground; after that the tail was released. With this experimental paradigm, no pain or harm is caused to the animal.
Statistics and reproducibility
All data analysis was done in MATLAB unless otherwise indicated. No statistical methods were used to predetermine sample sizes, but they are similar to previous reports14,28. Box plots are shown with the central mark indicating the median and the bottom and top edges of the box indicating the 25th and 75th percentiles, respectively. Whiskers extend to the most extreme data point or within 1.5 times the interquartile range (IQR) from the bottom or top of the box, and all other data are plotted as individual points, as listed in the figure legends. For statistical comparisons, nonparametric tests were used, or where indicated, normality was assumed but not formally tested, and t-tests were used. HB was performed on the basis of a MATLAB implementation (https://github.com/jenwallace/Hierarchical_bootstrap_Matlab) of the methodology, and used to reduce the statistical error rate of comparisons while retaining statistical power63. All multiway comparisons were adjusted for using Tukey–Kramer correction. No data were excluded from analyses except for the following (not predetermined): In hSYN-hM4Di experiments, outliers were excluded across all conditions from small stationary responses to avoid confounding effects from other influences on astrocyte Ca2+, as described in methods. For in vivo pharmacology experiments, electrical artifacts in band power were excluded before analysis, as described in methods. Samples were allocated into experimental groups by cell-type expression of each individual fluorescent sensor. Only adult animals (1–6 months of age) were used in experiments, and both males and females were used and randomly selected. For imaging and electrical recordings of spontaneous activity, blinding was not relevant because cell-type viral expression is evident from expression pattern. For in vivo pharmacology, blinding was not possible because control recordings were taken before treatment recordings to avoid confounding the treatment effects. For Adra1afl/fl mice, the experimenter was blinded to genotype before data collection and analysis.
Speed calculations
To compute wheel speed, a detected break in the optoswitch circuit was determined when the absolute value of the derivative of the raw voltage trace was at least 2 s.d. above the mean. For recordings with very little movement (s.d. <0.1), this threshold generated false positives so a set threshold of 0.1 was used. The number of breaks in the optoswitch circuit per second was then calculated, and using the circumference and number of evenly spaced colored tabs at the edge of the wheel, the wheel speed was determined and used for all subsequent analyses using speed. Movement periods were defined by wheel speed ≥10 cm s−1, and movement bouts that were separated by ≤2 s were considered one event. To ensure that movement-related dynamics were not included in stationary analysis, data were excluded from at least 10 s around identified movement periods.
Pupillometry
Following acquisition, pupil data were processed through a Python function that used contrast detection to identify the edges of the backlit pupil from the sclera and fit an ellipse whose major radius was taken as the pupil diameter. The diameter was then low-pass filtered to 0.5 Hz and normalized to a range between 0 and 1 to give pupil diameter as percent maximum. The pupil derivate was normalized to the acquisition rate (30 Hz) to compute pupil phase and to determine the phase of astrocyte Ca2+ events and the cross-correlations with GRABNE. Stationary arousal dilations and constrictions were identified by the sign of the calculated pupil derivative, and only changes in pupil diameter >10% were used for subsequent analyses.
LFP
All spectral analysis was done using Chronux64. Raw LFP data were visually inspected to confirm useable signal was present, and then 60 Hz noise was filtered out and drifting baselines were compensated for using linear fitting. LFP power for frequency bands was computed using built-in Chronux functions with a time bandwidth of 2.5 and two tapers, no frequency padding and 5 s moving windows. For changes in LFP band power around arousal or astrocyte Ca2+ events, the median band-limited power was obtained and then normalized to the median band-limited power in the event-triggered time window to get relative band power. The median power before an event onset versus after was combined for each recording, and for Adra1afl/fl mice, the ratio between the two was computed and compared with Adra1a wild-type mice. For changes in LFP power after A61603 administration, the band-limited power for saline and drug data was calculated, outliers were removed to avoid contamination by recording artifacts, and then this power was normalized to the band-limited power for the respective baseline recording.
For total power in Adra1afl/fl and control mice, no baseline correction was done to avoid skewing the analysis. Spectrum power was calculated by concatenating recordings from all mice of each genotype and computing average or individual spectra with a time bandwidth of six and eight tapers to increase accuracy. The total power was then computed by summing across all frequency bands. Relative power was computing by dividing the spectrum from each mouse by its total power, and relative band power was computed by summing the power from each frequency band and dividing by the total power.
Ca2+ imaging
Astrocyte Ca2+ events and fluorescence was extracted using the AQuA software analysis package22. For dual-color imaging with neuronal Ca2+ indicators, particular care was taken to avoid AQuA detection of neuronal activity; the s.d. of the neuronal channel was taken in FIJI and a mask was created in AQuA to exclude areas of high neuronal activity and soma from analysis. Astrocyte events were included only if they had an area greater than 10 µm, lasted for at least two frames and had an AQuA P value <0.05. To obtain the average astrocyte Ca2+ fluorescence, the compensated fluorescence traces that account for spatially overlapping events were taken, normalized to their maximum value and then averaged together.
Neuronal Ca2+ events and fluorescence were extracted from neuropil background semi-automatically using Suite2P65. We identified Ca2+ events by taking identified spikes in the Ca2+ fluorescence data and thresholding them for only the largest (>3 s.d. over the mean) events. To calculate the average neuronal Ca2+ fluorescence, the trace from each neuron was normalized to its maximum value and averaged together.
Machine-learning based analysis of input contribution to astrocyte Ca2+
As an alternative to assess the contribution of biological inputs on astrocyte Ca2+, data from dual-color Ca2+ imaging were used to train a machine-learning model to predict average Ca2+ activity. To include LFP data, the spectrogram data from each LFP recording were decomposed using PC analysis based on the eigenvectors from the ipsilateral recording that accounted for the largest proportion of the variance. The PC1 in this data corresponded to cortical synchrony, with positive weights for HF and negative weights for low frequencies, matching a previous report but with inverse sign66. LFP PC1 for ipsilateral and contralateral recordings, as well as speed, pupil diameter and average Ca2+ fluorescence for neurons and astrocytes, was then z-scored and resampled to 10 Hz before being concatenated. This dataset was then imported into Python for machine learning analysis using the SciKit-learn toolbox. For analysis, randomly generated data were added for comparison, and rows without both ipsilateral and contralateral LFP recordings were excluded from subsequent analysis. Average astrocyte Ca2+ data were used as the target dataset and data were split into training (80%) and testing (20%) before classification using a random forest regression model. The model was validated using the R2 between the predicted average astrocyte Ca2+ fluorescence from the model and the actual average astrocyte Ca2+ data of the test set. Permutation testing of the predictors was then used to determine their relative contributions to model prediction.
GRABNE analysis
In GRABNE imaging data, we observed background fluorescence fluctuations that we thought might arise from hemodynamic artifacts. To ensure the data reflected the NE signal, hemodynamic artifacts in the data were removed by a custom-designed, data processing pipeline. The predominant hemodynamic artifact in the data was assumed to reflect fluctuating hemoglobin levels altering brain absorptivity causing an attenuation of light. As such, the signal from each pixel could be modeled as
where k is index of pixel, Yk(t) and Fk(t) are respectively the observed curve and the real fluorescence of kth pixel, μk(t) is the absorption coefficient for kth pixel, X is the path distance, the term \(e^{h_k\left( t \right)}\) represents the intensity attenuation, and N(t) is the noise. In our data, the identified hemodynamic signal across pixels was approximately synchronous but varied in magnitude; thus, the attenuation of one pixel can be represented by another, that is \(e^{h_j\left( t \right)} = e^{ah_k\left( t \right) + b}\left( \,{j \ne k} \right)\). On the basis the findings above, we designed the following pipeline:
-
1.
We selected one connected vascular region with minimal fluorescence in the average projection of the data and calculated an initial vascular reference curve. This region was assumed to have the lowest possibility to contain any true GRABNE signal.
-
2.
To avoid compensating for slow changes in the true GRABNE signal, we subtracted the curve of each pixel by a 100-frame moving average.
-
3.
We applied linear regression and fit the logarithm of the processed curve to the initial vascular reference curve. The exponential of the fit data was then taken to represent the hemodynamic effect for each pixel. To account for cases where the initial reference curve was contaminated, we iteratively refined the reference curve before fitting, that is, we calculated the weighted average of the original curve for all pixels (fitting parameter a in \(e^{ah_k\left( t \right) + b}\) for each pixel is considered as the weight) and subtracted its moving average.
-
4.
We removed the hemodynamic artifact (if any) by dividing the raw pixel curve by the exponential of the fitting data, \(F_k\left( t \right) \approx \frac{{Y_k\left( t \right)}}{{e^{h_k(t)}}}\).
Next, only the least (1–25%) hemodynamically affected pixels with the lowest a were taken, excluding the bottom 1%, which often contained artifacts, and these were averaged together and used as the final GRABNE signal. For spectral analysis, each recording was concatenated together in 10 min segments, padding with its median value if necessary, and then run in Chronux with no frequency padding, a time bandwidth of 3, five tapers, and passing frequencies above half the window size (3 × 10−3 Hz) and less than the Nyquist frequency (0.9 Hz). To identify phasic increases in the GRABNE signal, built-in MATLAB functions were used to determine local peaks in the signal and a prominence threshold was used to determine the different magnitudes of GRABNE increases.
Event-triggered averages
All averages were computed by identifying events (for example, movement offset, pupil dilation and so on) and taking data in a symmetric time window around the events. The data are subsequently plotted as the mean and standard error across all events, except for spectrograms where the median was used.
Correlations
For comparisons of maximum and change in astrocyte Ca2+/pupil/speed after arousal, values were computed for each trace separately and then linearly correlated with P values describing the probability of a true R2 relationship between each two metrics. For correlations between astrocyte and neuronal Ca2+ activity, the average population fluorescence was taken for each and z-scored before cross-correlation. For behavioral state-separated cross-correlations, the same procedure applied, but only z-scored data from either moving periods or stationary periods were used. For correlations within neuronal and astrocyte populations, the pairwise correlation between each cell (neurons) or event (astrocytes) was computed, the symmetric and autocorrelations were excluded, and the overall mean was taken to obtain a single value indicating the synchrony of Ca2+ dynamics within each cellular population. For cross-correlations between pupil and imaging data (GRABNE and astrocyte Ca2+), all data were z-scored, resampled to either 10 or 30 Hz, padded with nan values if unequal in length, and then cross-correlated. For cross-correlations between LFP band power and Ca2+ imaging data, all data were z-scored, averaged and then resampled to 2 Hz to match the LFP resolution before cross-correlation.
hSYN-hM4Di
To estimate the effect of CNO on astrocyte Ca2+, the average Ca2+ event properties and overall event rate for each recording were randomly sampled 104 times and CNO data were subtracted from corresponding baseline data. This procedure generated the range of treatment effects possible from the sampled data, and a P value was calculated as the proportion of CNO difference from baseline that was less than the maximum, or greater than the minimum, difference found in saline conditions. For calculating the modulation of astrocyte Ca2+ responses to arousal, the absolute change in average astrocyte Ca2+ fluorescence after either movement onset or pupil dilation was determined. Outliers during small stationary responses, which might reflect the influence of other variables on astrocyte Ca2+, were excluded. The magnitude of astrocyte Ca2+ responses to arousal after treatment was then compared with baseline responses.
Neuronal arousal PC analysis
PCA was done using the built-in MATLAB function on z-scored neuronal Ca2+ data. The pupil diameter and PC data were then resampled to an effective rate of 10 Hz, and the Pearson’s correlation between the pupil diameter and each PC was used to identify the arousal PC for each recording This PC was then normalized to the maximum value before subsequent analysis.
For comparisons between wild-type and Adra1afl/fl mice, the response to each movement onset or stationary pupil dilation was normalized to the median value in the window, and then the average arousal PC value during the event period was taken with a two-frame offset to account for a slight lag in the arousal-associated neuronal response.
Fiber photometry
Photometry data were detrended using linear regression to correct bleaching and normalized by z-scoring. For startle experiments, recordings were denoised using a FIR filter (cutoff at 2 Hz, transition width 0.5 Hz). Startle responses were aligned to the onset of the jRGECO signal by fitting a sigmoid to the evoked jRGECO and taking the fourth derivative to identify the onset inflection point.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The data presented in this study are publicly available on Dryad (https://doi.org/10.7272/Q6XK8CS6).
Code availability
The code used to generate the findings of this study is publicly available on Zenodo (https://doi.org/10.5281/zenodo.7098082).
Change history
03 May 2023
In the version of this article initially published, the legends for Supplementary Videos 3 and 4 were interchanged and have now been updated in the HTML version of the article.
References
Harris, K. D. & Thiele, A. Cortical state and attention. Nat. Rev. Neurosci. 12, 509–523 (2011).
Crochet, S. & Petersen, C. C. H. Correlating whisker behavior with membrane potential in barrel cortex of awake mice. Nat. Neurosci. 9, 608–610 (2006).
Niell, C. M. & Stryker, M. P. Modulation of visual responses by behavioral state in mouse visual cortex. Neuron 65, 472–479 (2010).
Steriade, M., McCormick, D. A. & Sejnowski, T. J. Thalamocortical oscillations in the sleeping and aroused brain. Science 262, 679–685 (1993).
Fries, P. Neuronal gamma-band synchronization as a fundamental process in cortical computation. Annu. Rev. Neurosci. 32, 209–224 (2009).
Vinck, M., Batista-Brito, R., Knoblich, U. & Cardin, J. A. Arousal and locomotion make distinct contributions to cortical activity patterns and visual encoding. Neuron 86, 740–754 (2015).
McGinley, M. J. et al. Waking state: rapid variations modulate neural and behavioral responses. Neuron 87, 1143–1161 (2015).
Einstein, M. C., Polack, P.-O., Tran, D. T. & Golshani, P. Visually evoked 3–5 Hz membrane potential oscillations reduce the responsiveness of visual cortex neurons in awake behaving mice. J. Neurosci. 37, 5084–5098 (2017).
Liu, Y., Rodenkirch, C., Moskowitz, N., Schriver, B. & Wang, Q. Dynamic lateralization of pupil dilation evoked by locus coeruleus activation results from sympathetic, not parasympathetic, contributions. Cell Rep. 20, 3099–3112 (2017).
Polack, P.-O., Friedman, J. & Golshani, P. Cellular mechanisms of brain state-dependent gain modulation in visual cortex. Nat. Neurosci. 16, 1331–1339 (2013).
Constantinople, C. M. & Bruno, R. M. Effects and mechanisms of wakefulness on local cortical networks. Neuron 69, 1061–1068 (2011).
Nakadate, K., Imamura, K. & Watanabe, Y. Cellular and subcellular localization of alpha-1 adrenoceptors in the rat visual cortex. Neuroscience 141, 1783–1792 (2006).
Liu, Y. U. et al. Neuronal network activity controls microglial process surveillance in awake mice via norepinephrine signaling. Nat. Neurosci. 22, 1771–1781 (2019).
Ding, F. et al. α1-Adrenergic receptors mediate coordinated Ca2+ signaling of cortical astrocytes in awake, behaving mice. Cell Calcium 54, 387–394 (2013).
Oe, Y. et al. Distinct temporal integration of noradrenaline signaling by astrocytic second messengers during vigilance. Nat. Commun. 11, 471 (2020).
Paukert, M. et al. Norepinephrine controls astroglial responsiveness to local circuit activity. Neuron 82, 1263–1270 (2014).
Ye, L. et al. Ethanol abolishes vigilance-dependent astroglia network activation in mice by inhibiting norepinephrine release. Nat. Commun. 11, 6157 (2020).
Ma, Z., Stork, T., Bergles, D. E. & Freeman, M. R. Neuromodulators signal through astrocytes to alter neural circuit activity and behaviour. Nature 539, 428–432 (2016).
Mu, Y. et al. Glia accumulate evidence that actions are futile and suppress unsuccessful behavior. Cell 178, 27–43.e19 (2019).
Bojarskaite, L. et al. Astrocytic Ca2+ signaling is reduced during sleep and is involved in the regulation of slow wave sleep. Nat. Commun. 11, 3240 (2020).
Poskanzer, K. E. & Yuste, R. Astrocytes regulate cortical state switching in vivo. Proc. Natl Acad. Sci. USA 113, E2675–E2684 (2016).
Wang, Y. et al. Accurate quantification of astrocyte and neurotransmitter fluorescence dynamics for single-cell and population-level physiology. Nat. Neurosci. 22, 1936–1944 (2019).
McGinley, M. J., David, S. V. & McCormick, D. A. Cortical membrane potential signature of optimal states for sensory signal detection. Neuron 87, 179–192 (2015).
Reimer, J. et al. Pupil fluctuations track fast switching of cortical states during quiet wakefulness. Neuron 84, 355–362 (2014).
Aston-Jones, G. & Cohen, J. D. Adaptive gain and the role of the locus coeruleus-norepinephrine system in optimal performance. J. Comp. Neurol. 493, 99–110 (2005).
Feng, J. et al. A genetically encoded fluorescent sensor for rapid and specific in vivo detection of norepinephrine. Neuron 102, 745–761.e8 (2019).
Ma, Y. et al. Wide-field optical mapping of neural activity and brain haemodynamics: considerations and novel approaches. Philos. Trans. R. Soc. Lond. B 371, 20150360 (2016).
Reimer, J. et al. Pupil fluctuations track rapid changes in adrenergic and cholinergic activity in cortex. Nat. Commun. 7, 13289 (2016).
Stringer, C. et al. Spontaneous behaviors drive multidimensional, brainwide activity. Science 364, 255 (2019).
Armbruster, B. N., Li, X., Pausch, M. H., Herlitze, S. & Roth, B. L. Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proc. Natl Acad. Sci. USA 104, 5163–5168 (2007).
Herreras, O. Local field potentials: myths and misunderstandings. Front. Neural Circuits 10, 101 (2016).
Pankratov, Y. & Lalo, U. Role for astroglial α1-adrenoreceptors in gliotransmission and control of synaptic plasticity in the neocortex. Front. Cell Neurosci. 9, 230 (2015).
Farhy-Tselnicker, I. et al. Activity-dependent modulation of synapse-regulating genes in astrocytes. eLife 10, e70514 (2021).
Bayraktar, O. A. et al. Astrocyte layers in the mammalian cerebral cortex revealed by a single-cell in situ transcriptomic map. Nat. Neurosci. 23, 500–509 (2020).
Speed, A., Del Rosario, J., Burgess, C. P. & Haider, B. Cortical state fluctuations across layers of V1 during visual spatial perception. Cell Rep. 26, 2868–2874.e3 (2019).
Wahis, J. & Holt, M. G. Astrocytes, Noradrenaline, α1-adrenoreceptors, and neuromodulation: evidence and unanswered questions. Front. Cell Neurosci. 15, 645691 (2021).
Papouin, T., Dunphy, J. M., Tolman, M., Dineley, K. T. & Haydon, P. G. Septal cholinergic neuromodulation tunes the astrocyte-dependent gating of hippocampal NMDA receptors to wakefulness. Neuron 94, 840–854.e7 (2017).
Di Castro, M. A. et al. Local Ca2+ detection and modulation of synaptic release by astrocytes. Nat. Neurosci. 14, 1276–1284 (2011).
Bernardinelli, Y. et al. Activity-dependent structural plasticity of perisynaptic astrocytic domains promotes excitatory synapse stability. Curr. Biol. 24, 1679–1688 (2014).
Stumpf, C. & Pichler, L. Synchronizing effect of the alpha 1-adrenoceptor agonist 2-(2-chloro-5-trifluoromethylphenylimino)imidazolidine on rabbit electroencephalogram. Arzneimittelforschung 38, 770–774 (1988).
Berridge, C. W. & Spencer, R. C. Differential cognitive actions of norepinephrine a2 and a1 receptor signaling in the prefrontal cortex. Brain Res. 1641, 189–196 (2016).
Perez, D. M. α1-Adrenergic receptors in neurotransmission, synaptic plasticity, and cognition. Front. Pharmacol. 11, 581098 (2020).
Thiele, A. & Bellgrove, M. A. Neuromodulation of attention. Neuron 97, 769–785 (2018).
Shimaoka, D., Harris, K. D. & Carandini, M. Effects of arousal on mouse sensory cortex depend on modality. Cell Rep. 22, 3160–3167 (2018).
Lee, A. M. et al. Identification of a brainstem circuit regulating visual cortical state in parallel with locomotion. Neuron 83, 455–466 (2014).
Celada, P., Puig, M. V. & Artigas, F. Serotonin modulation of cortical neurons and networks. Front Integr. Neurosci. 7, 25 (2013).
Speed, A., Del Rosario, J., Mikail, N. & Haider, B. Spatial attention enhances network, cellular and subthreshold responses in mouse visual cortex. Nat. Commun. 11, 505 (2020).
Slezak, M. et al. Distinct mechanisms for visual and motor-related astrocyte responses in mouse visual cortex. Curr. Biol. 29, 3120–3127.e5 (2019).
Stobart, J. L. et al. Cortical circuit activity evokes rapid astrocyte calcium signals on a similar timescale to neurons. Neuron 98, 726–735.e4 (2018).
Lines, J., Martin, E. D., Kofuji, P., Aguilar, J. & Araque, A. Astrocytes modulate sensory-evoked neuronal network activity. Nat. Commun. 11, 3689 (2020).
Batiuk, M. Y. et al. Identification of region-specific astrocyte subtypes at single cell resolution. Nat. Commun. 11, 1220 (2020).
Johnson, M. B. & Walsh, C. A. Cerebral cortical neuron diversity and development at single-cell resolution. Curr. Opin. Neurobiol. 42, 9–16 (2017).
Pala, A. & Petersen, C. C. State-dependent cell-type-specific membrane potential dynamics and unitary synaptic inputs in awake mice. eLife 7, e35869 (2018).
Deemyad, T., Lüthi, J. & Spruston, N. Astrocytes integrate and drive action potential firing in inhibitory subnetworks. Nat. Commun. 9, 4336 (2018).
Zucca, S. et al. An inhibitory gate for state transition in cortex. eLife 6, e26177 (2017).
Rasmussen, R. et al. Cortex-wide changes in extracellular potassium ions parallel brain state transitions in awake behaving mice. Cell Rep. 28, 1182–1194.e4 (2019).
Tewari, B. P. et al. Perineuronal nets decrease membrane capacitance of peritumoral fast spiking interneurons in a model of epilepsy. Nat. Commun. 9, 4724 (2018).
Ding, F. et al. Changes in the composition of brain interstitial ions control the sleep–wake cycle. Science 352, 550–555 (2016).
Verkhratsky, A. Physiology of neuronal–glial networking. Neurochem. Int. 57, 332–343 (2010).
Mazaud, D., Capano, A. & Rouach, N. The many ways astroglial connexins regulate neurotransmission and behavior. Glia 69, 2527–2545 (2021).
Goldey, G. J. et al. Removable cranial windows for long-term imaging in awake mice. Nat. Protoc. 9, 2515–2538 (2014).
Watson, B. O., Yuste, R. & Packer, A. M. PackIO and EphysViewer: software tools for acquisition and analysis of neuroscience data. Preprint at bioRxiv https://doi.org/10.1101/054080 (2016).
Saravanan, V., Berman, G. J. & Sober, S. J. Application of the hierarchical bootstrap to multi-level data in neuroscience. Neuron. Behav. Data Anal. Theory 3, (2020).
Bokil, H., Andrews, P., Kulkarni, J. E., Mehta, S. & Mitra, P. P. Chronux: a platform for analyzing neural signals. J. Neurosci. Methods 192, 146–151 (2010).
Pachitariu, M. et al. Suite2p: beyond 10,000 neurons with standard two-photon microscopy. Preprint at bioRxiv https://doi.org/10.1101/061507 (2016).
Watson, B. O., Levenstein, D., Greene, J. P., Gelinas, J. N. & Buzsáki, G. Network homeostasis and state dynamics of neocortical sleep. Neuron 90, 839–852 (2016).
Acknowledgements
We thank G. Chin and S. Yokoyama for technical assistance, K. Roberts and T. Li for help with smFISH data generation, J. Thompson for administrative assistance, and R. Reitman for assistance in data analysis pipeline construction. We also thank the Poskanzer lab for helpful discussions about the project, and S. Lavrentyeva and E. Kish for comments on the paper. We thank E. Feinberg and C. Kirst for helpful discussions on the resubmitted paper and experiments, and J. Reimer and C. Smith for discussions related to neuron-astrocyte imaging. This work was funded by the UCSF Genentech Fellowship (M.E.R.); National Institute of Health R01MH110504 (G.Y.), U19NS123719 (G.Y.), R01NS099254 (K.E.P.), R01MH121446 (K.E.P.) and R01HL31113-27 (P.C.S.); National Science Foundation 1750931 (G.Y.) and CAREER 1942360 (K.E.P.); Veteran Affairs BX004314 (P.C.S.); and the UCSF Program for Breakthrough Biomedical Research, which is funded in part by the Sandler Foundation (K.E.P.). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the paper.
Author information
Authors and Affiliations
Contributions
M.E.R. and K.E.P. conceptualized and designed the experiments. M.E.R. performed all 2P imaging and physiology experiments, and carried out data analyses for these experiments. V.T. performed immunostaining experiments and analyses. X.M. and G.Y. worked with M.E.R. and K.E.P. to develop the GRABNE analysis method. D.D.W. performed and analyzed the fiber photometry experiments. A.P. collaborated on the analysis of 2P imaging and physiology data. A.A. and O.A.B. performed and analyzed smFISH experiments. B.-E.M. and P.C.S. generated Adra1a floxed mice. M.E.R. and K.E.P wrote the paper with input from other authors. K.E.P. supervised all phases of the project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Neuroscience thanks Inbal Goshen, Nathan Smith and the other, anonymous reviewer(s) for their contribution to the peer review of this work. This article has been peer reviewed as part of Springer Nature’s Guided Open Access initiative.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Dissection of astrocyte Ca2+ and behavioral state.
(a) Representative 2P mean projection image from one ten-minute recording of in vivo astrocyte GcaMP6f (left, scale bar = 100 µm). AQuA detected both large (middle) and small (right) astrocyte Ca2+ events within the entire movie, even when they were spatially overlapping. Arrows indicate two pairs of spatially overlapping events (arrows 1 and 3, and arrows 2 and 4).(b) Traces from the AQuA events shown in (a), with the time period of the AQuA-detected event highlighted in red. (c) Related to Fig. 1c: Individual astrocyte Ca2+ events correlated better with pupil diameter (left, n = 1.2e4 Ca2+ events, One-sided Kruskal-Wallis test) and had a shorter lag with pupil diameter than wheel speed (right, pupil n = 9.6e3, wheel n = 8.3e3, rank-sum test). (d) Related to Fig. 1d: average pupil diameter during movement (n = 100) and stationary periods (n = 76). (e) Classification of behavioral state by both pupil diameter and movement. (f) Pupil dilation is smaller (left) and shorter (right, rank-sum tests) during stationary periods (n = 261 dilations, blue) compared with movement-associated dilations (n = 136 dilations, red, boxplots show median and IQR with whiskers to 1.5 * IQR, two-sided Rank Sum test). (g) Related to Fig. 1l: Left: Movement duration (n = 104) was related to the maximum wheel speed (top) and pupil diameter (middle), but not to the maximum astrocyte Ca2+ (bottom). Right: the latency to the maximum wheel speed (top), pupil diameter (middle), and astrocyte Ca2+ (bottom) were strongly linked to movement duration. (h) Heatmap summary of the r2 between the latencies in (g) right, and movement bout duration.
Extended Data Fig. 2 Hemodynamic correction of 2P GRABNE signals.
(a) Left: Average projection of a single GRABNE recording with four regions-of-interest (ROI) in areas with clear GRABNE fluorescence (black squares, labeled 1–4) and one ROI in a blood vessel (red square, labeled BV). Middle: standard deviation projection. Right: average fluorescence in each ROI before hemodynamic correction. (b) Left: Estimated hemodynamic signal present in each pixel. Middle: recovered average projection after hemodynamic correction. Note average fluorescence after hemodynamic correction is broadly similar the uncorrected data in (a) with some blurring due to Gaussian smoothing in preprocessing. Right: average ROI fluorescence after hemodynamic correction. (c) ROI-free methodology for obtaining GRABNE signal. Left: Following hemodynamic correction, the bottom quartile of pixels with the lowest hemodynamic weights, excluding the bottom 1% which often had artefactual signals, were kept. Right: These pixels were averaged together to produce the corrected GRABNE signal (bottom) which had substantially less hemodynamic contamination than the uncorrected field fluorescence (top). (d) Correlation between the corrected GRABNE signal and the uncorrected ROIs in (a) and (b). (e) Left: Correlation between uncorrected GRABNE fluorescence and pupil diameter. Right: Example traces from the average of pixels that were positively correlated (top), negatively correlated (bottom), or showed little relationship (middle) with pupil diameter. (f) The corrected GRABNE signal is not similar to the uncorrected GRABNE signal (red dot) but instead reflects the GRABNE signal of binned pixels (black dots) correlated to pupil diameter (R2 = 0.62, p = 1.55−5, two-sided t-test).
Extended Data Fig. 3 Freely moving fiber photometry recordings of GRABNE and astrocyte Ca2+.
(a) Schematic of fiber photometry recording set-up. (b) A 5-minute example fiber photometry recording of GRABNE signal. (c) GRABNE power spectrum. n = 4 mice, 19 recordings over multiple days, 10–30 minutes/recording. Error lines are ± s.e.m. across all mice.) (d) GRABNE (green) and astrocyte jRGECO1b (magenta) signals evoked by startle responses due to tail lifts (grey arrow heads). (e) Average startle-response evoked traces for GRABNE and jRGECO, aligned to onset of astrocyte Ca2+. n = 4 mice, 4 tail lifts per mouse. Error is ± s.e.m. across mice.
Extended Data Fig. 4 Analysis of contributions to astrocyte Ca2+.
(a) Left: A Random Forest Regression model was trained (80% of data, 2.3e4 samples) to predict (20% of data, 5.63 samples) average astrocyte Ca2+ fluorescence accurately (mean r2 = 0.85 ± 4.5e-3 std, n = 10 cross-validations). Right: Relative feature importance based on permutation testing of each predictor. Randomly generated data was included as a negative control and did not inform model predictions. (b) Same analysis as in (a), using the neuronal arousal PC rather than average neuronal activity. Random Forest Regression using the neuronal arousal PC showed a similar accuracy to that using mean neuronal fluorescence (mean r2 = 0.87 ± 6.4e-3 std, n = 10 cross-validations). Boxplots show median and IQR with whiskers to 1.5 * IQR.
Extended Data Fig. 5 Cortical astrocyte expression of adrenergic receptors.
(a) Ribosomal-mRNA expression in visual cortex astrocytes of P120 mice from the Farhy-Tselnicker et. al. publicly available dataset. Visual cortex astrocytes show expression of many adrenergic receptors (left) but preferentially express the Adra1a receptor relative to input control (right). Boxplots show median and IQR with whiskers to 1.5 * IQR (N = 3 mice for astrocyte ribosomal-mRNA and 1 input control). (b) Left: Representative brain section with Adra1a (green), Adra1b (red), and Adra2a (yellow) mRNA labeled by smFISH using the LaST map pipeline34. SLC1a3 (GLAST) mRNA expression (purple) was used to define the location of astrocytes. The white square shows a representative ROI used to quantify visual cortex expression. Right: High-magnification image of visual cortex, showing receptor mRNA both within astrocytes marked by high GLAST mRNA (purple), and outside of astrocytes. (c) Quantification of NE-receptor mRNA expression in visual cortex astrocytes, quantified by relative cortical depth. Line plots show mean ± std (n = 3 mice, 7 ROIs).
Extended Data Fig. 6 Generation of Adra1afl/fl mice.
Top: schematic of the genetic strategy used to generate conditional deletion of Adra1a. Primers (green arrows) were designed to identify and distinguish between the endogenous and knock-in Adra1a alleles. Bottom: Numbers are in base pairs. Validation of the knock-in strategy used to generate Adra1afl/fl mice. PCR of Adra1afl/+ mice generates both a 154 base pair band corresponding to the wild type allele and a 235 base pair band corresponding to successful LoxP knock-in, which is not found in wild-type mice. N = 5 Adra1afl/+ and 4 wild type mice.
Extended Data Fig. 7 Astrocyte-specific Cre expression.
(a) Representative average 2 P image of in vivo neuronal jRGECO1b (gray) and astrocytes expressing Cre-GFP (magenta) (b) Example images show astrocyte-specific Cre-GFP (magenta) expression overlaps with the astrocyte marker S100β (left, green) but not the neuronal marker NeuN (right, grey), scale bar = 100 µm. (c) Representative mean-projection of a confocal 60x z-stack used for cell counting. Each animal had two sections with each section having six distinct field-of-views containing 20 z-planes. GFAP(0.7)-Cre-GFP expression (left) was highly astrocytic and the neuronal marker NeuN (middle) was rarely colocalized (right, scale bars = 50 µm). (d) Cre expression in neurons (grey bars) was low across genotypes (overall = 2.2 ± 0.39 neurons/50 µm3) and no difference was found between Cre expression in Adra1afl/fl mice and control mice or the cohort as a whole (One-sided Kruskal-Wallis test). (e) Cre-expressing neurons were rare in both wild-type (black, 4.0% ± 0.75%) and Adra1afl/fl mice (green, 4.1% ± 0.65%, two-sided ranked-sum test). (n = 7 Adra1afl/fl and n = 5 wild-type mice for all graphs. Data are presented as mean ± S.E.M.).
Extended Data Fig. 8 Resonant galvo imaging (7.5 Hz effective frame rate) confirms increased neuronal activity in Adra1afl/fl mice.
(a–c) Histograms show neuronal activity in Adra1afl/fl (green) and wild-type littermate mice (grey) based on HB. (a) Neuronal Ca2+ event rate was increased in Adra1afl/fl (green, n = 4 mice) compared to wild-type mice (grey, n = 4 mice). (b) Left: power spectrum of Adra1afl/fl (green) and wild-type (black) Ca2+ activity between 0–0.05 Hz. Right: Total power in 0–0.05 Hz neuronal Ca2+ activity, comparing Adra1afl/fl and wild-type mice. (c) Same analysis as (b), comparing power in neuronal Ca2+ fluctuations > 0.05 Hz. All p-values from one-sided HB tests.
Extended Data Fig. 9 Quantification of arousal-associated neuronal activity.
(a) Cumulative distribution plots of the duration of movement bouts (left) and stationary pupil dilations (right) for wild-type (black, n = 400 movement bouts and 1396 pupil dilations) and Adra1afl/fl (green, n = 177 movement bouts and n = 923 pupil dilations) mice. (b) Analysis range (red) used to quantify the normalized arousal PC response to movement in wild-type (left) and Adra1afl/fl (right) mice. Each row represents one movement event, and the movements from all recordings and mice are concatenated to show the entire dataset (n = 4 wild-type mice and n = 4 Adra1afl/fl mice). (c) Same analysis as in (b), showing stationary pupil dilations in Adra1afl/fl mice compared to wild-type.
Supplementary information
Supplementary Information
Supplementary Tables 1–5.
2P imaging of GRABNE and astrocyte jRGECO1b. Thirty-minute recording of spontaneous GRABNE (green) and astrocyte jRGECO1b (magenta) fluctuations in layer 2/3 of visual cortex of an awake mouse. Recording was acquired with a ~410 µm × 410 µm field of view, 10× averaged, and a one-pixel median filter was applied. Video is played at a 10 Hz frame rate.
2P imaging of neuronal GCaMP6f and astrocyte jRGECO1b. Ten-minute recording of spontaneous neuronal GCaMP6f (gray) and astrocyte jRGECO1b (magenta) fluctuations in layer 2/3 of the awake cortex. Recording was acquired with a ~410 µm × 410 µm field of view, 10× averaged, and a 0.5-pixel median filter was applied. Video is played at a 10 Hz frame rate.
Neuronal and astrocyte Ca2+ responses to A61603. Neuronal GCaMP6f (gray, left) and astrocyte jRGECO1b (magenta, right) recorded for 30 min at baseline and for 40 min post-injection of A61603 (10 µg kg−1, i.p.) Labels on the top left indicate pharmacological condition. Recording was acquired with a ~410 µm × 410 µm field of view, 10× averaged, and a 0.5-pixel median filter was applied. Video is played at a 60 Hz frame rate.
2P z-stack showing neuronal hM4Di and astrocyte GCaMP6f expression in same tissue region. An ~300 µm z-stack of in vivo expression of neuronal hM4Di-mCherry (gray) and astrocyte jRGECO1b (magenta). The z-stack was acquired with a 5 µm step size. A four-frame moving average projection (astrocytes) or s.d. projection (neurons) was calculated for a display step size of 20 µm per frame. Neuronal data were background-subtracted to aid visualization of soma, and astrocyte data were attenuation-corrected.
Supplementary Data
Editorial assessment report.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Reitman, M.E., Tse, V., Mi, X. et al. Norepinephrine links astrocytic activity to regulation of cortical state. Nat Neurosci 26, 579–593 (2023). https://doi.org/10.1038/s41593-023-01284-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41593-023-01284-w
This article is cited by
-
Serotonergic modulation of vigilance states in zebrafish and mice
Nature Communications (2024)
-
Microglia regulate sleep through calcium-dependent modulation of norepinephrine transmission
Nature Neuroscience (2024)
-
A midbrain GABAergic circuit constrains wakefulness in a mouse model of stress
Nature Communications (2024)
-
Multicore fiber optic imaging reveals that astrocyte calcium activity in the mouse cerebral cortex is modulated by internal motivational state
Nature Communications (2024)
-
Network-level encoding of local neurotransmitters in cortical astrocytes
Nature (2024)