Abstract
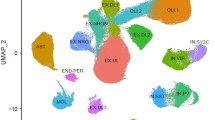
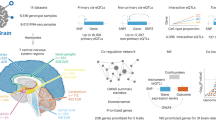
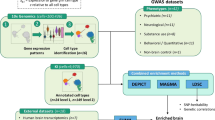
To date, most expression quantitative trait loci (eQTL) studies, which investigate how genetic variants contribute to gene expression, have been performed in heterogeneous brain tissues rather than specific cell types. In this study, we performed an eQTL analysis using single-nuclei RNA sequencing from 192 individuals in eight brain cell types derived from the prefrontal cortex, temporal cortex and white matter. We identified 7,607 eGenes, a substantial fraction (46%, 3,537/7,607) of which show cell-type-specific effects, with strongest effects in microglia. Cell-type-level eQTLs affected more constrained genes and had larger effect sizes than tissue-level eQTLs. Integration of brain cell type eQTLs with genome-wide association studies (GWAS) revealed novel relationships between expression and disease risk for neuropsychiatric and neurodegenerative diseases. For most GWAS loci, a single gene co-localized in a single cell type, providing new clues into disease etiology. Our findings demonstrate substantial contrast in genetic regulation of gene expression among brain cell types and reveal potential mechanisms by which disease risk genes influence brain disorders.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
A shinyApp to browse the result of this study is available at https://malhotralab.shinyapps.io/brain_cell_type_eqtl/. The full eQTL summary statistics are available on Zenodo at https://doi.org/10.5281/zenodo.5543734. Single-nuclei RNA-seq data and genotype data for the Roche datasets have been deposited at the European Genome-phenome Archive, which is hosted by the European Bioinformatics Institute and the Centre for Genomic Regulation, under accession number EGAS00001006345. Genotypes for the ROSMAP datasets are available at https://www.synapse.org/#!Synapse:syn10901595. Single-nuclei RNA-seq data for the ROSMAP datasets are available at https://www.synapse.org/#!Synapse:syn18485175, https://www.synapse.org/Portal.html#!Synapse:syn3157322, https://adknowledgeportal.synapse.org/Explore/Studies?Study=syn21670836 and https://www.synapse.org/#!Synapse:syn16780177. GRCh38 reference human genome: http://ftp.ensembl.org/pub/release-96/fasta/homo_sapiens/dna/. Ensembl Homo_sapiens GRCh38.96 reference annotation: http://ftp.ensembl.org/pub/release-96/gtf/homo_sapiens/. Ensembl Homo_sapiens GRCh38.91 reference annotation: http://ftp.ensembl.org/pub/release-91/gtf/homo_sapiens/. GWAS summary statistics are available here: Alzheimer’s disease: http://ftp.ebi.ac.uk/pub/databases/gwas/summary_statistics/GCST90012001-GCST90013000/GCST90012877/GCST90012877_buildGRCh37.tsv.gz; Parkinson’s disease: https://drive.google.com/drive/folders/10bGj6HfAXgl-JslpI9ZJIL_JIgZyktxn; multiple sclerosis: https://imsgc.net/?page_id=31; and schizophrenia: https://www.med.unc.edu/pgc/download-results.
Code availability
The code used to perform the analysis described in this study is available at https://jbryois.github.io/snRNA_eqtl/.
References
Edwards, S. L., Beesley, J., French, J. D. & Dunning, M. Beyond GWASs: illuminating the dark road from association to function. Am. J. Hum. Genet. 93, 779–797 (2013).
GTEx Consortium. The GTEx Consortium atlas of genetic regulatory effects across human tissues. Science 369, 1318–1330 (2020).
Giambartolomei, C. et al. Bayesian test for colocalisation between pairs of genetic association studies using summary statistics. PLoS Genet. 10, e1004383 (2014).
Schwartzentruber, J. et al. Genome-wide meta-analysis, fine-mapping and integrative prioritization implicate new Alzheimer’s disease risk genes. Nat. Genet. 53, 392–402 (2021).
Neavin, D. et al. Single cell eQTL analysis identifies cell type-specific genetic control of gene expression in fibroblasts and reprogrammed induced pluripotent stem cells. Genome Biol. 22, 76 (2021).
Werling, D. M. et al. Whole-genome and RNA sequencing reveal variation and transcriptomic coordination in the developing human prefrontal cortex. Cell Rep. 31, 107489 (2020).
Trevino, A. E. et al. Chromatin and gene-regulatory dynamics of the developing human cerebral cortex at single-cell resolution. Cell 184, 5053–5069 (2021).
Jerber, J. et al. Population-scale single-cell RNA-seq profiling across dopaminergic neuron differentiation. Nat. Genet. 53, 304–312 (2021).
Young, A. M. H. et al. A map of transcriptional heterogeneity and regulatory variation in human microglia. Nat. Genet. 53, 861–868 (2021).
Lopes, K. P., Snijders, G. J. L., Humphrey, J., de Witte, L. D. & Raj, T. Atlas of genetic effects in human microglia transcriptome across brain regions, aging and disease pathologies. Alzheimers Dement. 17, e050942 (2021).
Mathys, H. et al. Single-cell transcriptomic analysis of Alzheimer’s disease. Nature 570, 332–337 (2019).
Zhou, Y. et al. Human and mouse single-nucleus transcriptomics reveal TREM2-dependent and TREM2-independent cellular responses in Alzheimer’s disease. Nat. Med. 26, 131–142 (2020).
Cain, A. et al. Multi-cellular communities are perturbed in the aging human brain and with Alzheimer’s disease. Preprint at https://www.biorxiv.org/content/10.1101/2020.12.22.424084v1 (2020).
de Klein, N. et al. Brain expression quantitative trait locus and network analysis reveals downstream effects and putative drivers for brain-related diseases. Preprint at https://www.biorxiv.org/content/10.1101/2021.03.01.433439v2 (2021).
Qi, T. et al. Identifying gene targets for brain-related traits using transcriptomic and methylomic data from blood. Nat. Commun. 9, 2282 (2018).
Nott, A. et al. Brain cell type-specific enhancer–promoter interactome maps and disease-risk association. Science 366, 1134–1139 (2019).
Corces, M. R. et al. Single-cell epigenomic analyses implicate candidate causal variants at inherited risk loci for Alzheimer’s and Parkinson’s diseases. Nat. Genet. 52, 1158–1168 (2020).
Ferland, R. J. et al. Abnormal cerebellar development and axonal decussation due to mutations in AHI1 in Joubert syndrome. Nat. Genet. 36, 1008–1013 (2004).
Fossati, M. et al. Trans-synaptic signaling through the glutamate receptor delta-1 mediates inhibitory synapse formation in cortical pyramidal neurons. Neuron 104, 1081–1094 (2019).
Fullard, J. F. et al. An atlas of chromatin accessibility in the adult human brain. Genome Res. 28, 1243–1252 (2018).
Storey, J. D., Bass, A. J., Dabney, A. & Robinson, D. qvalue: Q-value estimation for false discovery rate control. R package 2.28.0. https://github.com/StoreyLab/qvalue (2022).
Nalls, M. A. et al. Large-scale meta-analysis of genome-wide association data identifies six new risk loci for Parkinson’s disease. Nat. Genet. 46, 989–993 (2014).
Nalls, M. A. et al. Identification of novel risk loci, causal insights, and heritable risk for Parkinson’s disease: a meta-analysis of genome-wide association studies. Lancet Neurol. 18, 1091–1102 (2019).
Trubetskoy, V. et al. Mapping genomic loci implicates genes and synaptic biology in schizophrenia. Nature 604, 502–508 (2022).
International Multiple Sclerosis Genetics Consortium. Multiple sclerosis genomic map implicates peripheral immune cells and microglia in susceptibility. Science 365, eaav7188 (2019).
Leng, F. & Edison, P. Neuroinflammation and microglial activation in Alzheimer disease: where do we go from here? Nat. Rev. Neurol. 17, 157–172 (2020).
Podleśny-Drabiniok, A., Marcora, E. & Goate, A. M. Microglial phagocytosis: a disease-associated process emerging from Alzheimer’s disease genetics. Trends Neurosci. 43, 965–979 (2020).
Rouka, E. et al. Differential recognition preferences of the three Src homology 3 (SH3) domains from the adaptor CD2-associated protein (CD2AP) and direct association with Ras and Rab interactor 3 (RIN3). J. Biol. Chem. 290, 25275–25292 (2015).
Kajiho, H. et al. RIN3: a novel Rab5 GEF interacting with amphiphysin II involved in the early endocytic pathway. J. Cell Sci. 116, 4159–4168 (2003).
Walter, S. et al. The metalloprotease ADAMTS4 generates N-truncated Aβ4-x species and marks oligodendrocytes as a source of amyloidogenic peptides in Alzheimer’s disease. Acta Neuropathol. 137, 239–257 (2019).
Brady, O. A., Zhou, X. & Hu, F. Regulated intramembrane proteolysis of the frontotemporal lobar degeneration risk factor, TMEM106B, by signal peptide peptidase-like 2a (SPPL2a)*. J. Biol. Chem. 289, 19670–19680 (2014).
Van Deerlin, V. M. et al. Common variants at 7p21 are associated with frontotemporal lobar degeneration with TDP-43 inclusions. Nat. Genet. 42, 234–239 (2010).
Rao, S. et al. An APOE-independent cis-eSNP on chromosome 19q13.32 influences tau levels and late-onset Alzheimer’s disease risk. Neurobiol. Aging 66, 178.e1–178.e8 (2018).
Skipper, L. et al. Linkage disequilibrium and association of MAPT H1 in Parkinson disease. Am. J. Hum. Genet. 75, 669–677 (2004).
Vitner, E. B. et al. Induction of the type I interferon response in neurological forms of Gaucher disease. J. Neuroinflammation 13, 104 (2016).
Sanchez, V. B., Ali, S., Escobar, A. & Cuajungco, M. P. Transmembrane 163 (TMEM163) protein effluxes zinc. Arch. Biochem. Biophys. 677, 108166 (2019).
Moloney, E. B., Moskites, A., Ferrari, E. J., Isacson, O. & Hallett, P. J. The glycoprotein GPNMB is selectively elevated in the substantia nigra of Parkinson’s disease patients and increases after lysosomal stress. Neurobiol. Dis. 120, 1–11 (2018).
Schmiedel, B. J. et al. Impact of genetic polymorphisms on human immune cell gene expression. Cell 175, 1701–1715 (2018).
Li, G. et al. Human genetics in rheumatoid arthritis guides a high-throughput drug screen of the CD40 signaling pathway. PLoS Genet. 9, e1003487 (2013).
Skene, N. G. et al. Genetic identification of brain cell types underlying schizophrenia. Nat. Genet. 50, 825–833 (2018).
Fromer, M. et al. Gene expression elucidates functional impact of polygenic risk for schizophrenia. Nat. Neurosci. 19, 1442–1453 (2016).
Dong, Z. et al. CUL3 deficiency causes social deficits and anxiety-like behaviors by impairing excitation–inhibition balance through the promotion of cap-dependent translation. Neuron 105, 475–490 (2020).
Prox, J. et al. Postnatal disruption of the disintegrin/metalloproteinase ADAM10 in brain causes epileptic seizures, learning deficits, altered spine morphology, and defective synaptic functions. J. Neurosci. 33, 12915–12928 (2013).
Li, W. et al. Independent replications and integrative analyses confirm TRANK1 as a susceptibility gene for bipolar disorder. Neuropsychopharmacology 46, 1103–1112 (2020).
Dowler, S. et al. Identification of pleckstrin-homology-domain-containing proteins with novel phosphoinositide-binding specificities. Biochem. J. 351, 19–31 (2000).
Wingo, A. P. et al. Integrating human brain proteomes with genome-wide association data implicates new proteins in Alzheimer’s disease pathogenesis. Nat. Genet. 53, 143–146 (2021).
Kumar, S., Ambrosini, G. & Bucher, P. SNP2TFBS—a database of regulatory SNPs affecting predicted transcription factor binding site affinity. Nucleic Acids Res. 45, D139–D144 (2017).
Kaushik, D. K., Gupta, M., Das, S. & Basu, A. Krüppel-like factor 4, a novel transcription factor regulates microglial activation and subsequent neuroinflammation. J. Neuroinflammation 7, 68 (2010).
Ballas, N., Grunseich, C., Lu, D. D., Speh, J. C. & Mandel, G. REST and its corepressors mediate plasticity of neuronal gene chromatin throughout neurogenesis. Cell 121, 645–657 (2005).
Zhang, Q. et al. Risk prediction of late-onset Alzheimer’s disease implies an oligogenic architecture. Nat. Commun. 11, 4799 (2020).
Karczewski, K. J. et al. The mutational constraint spectrum quantified from variation in 141,456 humans. Nature 581, 434–443 (2020).
Germain, P.-L., Lun, A., Macnair, W. & Robinson, M. D. Doublet identification in single-cell sequencing data using scDblFinder. F1000Res. 10, 979 (2021).
Macnair, W. & Robinson, M. D. SampleQC: robust multivariate, multi-celltype, multi-sample quality control for single cell data. Preprint at https://www.biorxiv.org/content/10.1101/2021.08.28.458012v1 (2021).
Barkas, N. et al. Joint analysis of heterogeneous single-cell RNA-seq dataset collections. Nat. Methods 16, 695–698 (2019).
Korsunsky, I. et al. Fast, sensitive and accurate integration of single-cell data with Harmony. Nat. Methods 16, 1289–1296 (2019).
Robinson, M. D. & Oshlack, A. A scaling normalization method for differential expression analysis of RNA-seq data. Genome Biol. 11, R25 (2010).
McCarthy, S. et al. A reference panel of 64,976 haplotypes for genotype imputation. Nat. Genet. 48, 1279–1283 (2016).
Purcell, S. et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 81, 559–575 (2007).
De Jager, P. L. et al. A multi-omic atlas of the human frontal cortex for aging and Alzheimer’s disease research. Sci. Data 5, 180142 (2018).
Auton, A. et al. A global reference for human genetic variation. Nature 526, 68–74 (2015).
Fort, A. et al. MBV: a method to solve sample mislabeling and detect technical bias in large combined genotype and sequencing assay datasets. Bioinformatics 33, 1895–1897 (2017).
Lun, A. T. L. et al. EmptyDrops: distinguishing cells from empty droplets in droplet-based single-cell RNA sequencing data. Genome Biol. 20, 63 (2019).
Ongen, H., Buil, A., Brown, A. A., Dermitzakis, E. T. & Delaneau, O. Fast and efficient QTL mapper for thousands of molecular phenotypes. Bioinformatics 32, 1479–1485 (2016).
Delaneau, O. et al. A complete tool set for molecular QTL discovery and analysis. Nat. Commun. 8, 15452 (2017).
Benjamini, Y. & Hochberg, Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B Stat. Methodol. 57, 289–300 (1995).
Brooks, M. E. et al. glmmTMB: balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. The R Journal 9, 378–400 (2017).
Brown, A. A. et al. Predicting causal variants affecting expression by using whole-genome sequencing and RNA-seq from multiple human tissues. Nat. Genet. 49, 1747–1751 (2017).
Willer, C. J., Li, Y. & Abecasis, G. R. METAL: fast and efficient meta-analysis of genomewide association scans. Bioinformatics 26, 2190 (2010).
de Leeuw, C. A., Mooij, J. M., Heskes, T. & Posthuma, D. MAGMA: generalized gene-set analysis of GWAS data. PLoS Comput. Biol. 11, 1004219 (2015).
Myers, T. A., Chanock, S. J. & Machiela, M. J. LDlinkR: an R package for rapidly calculating linkage disequilibrium statistics in diverse populations. Front. Genet. 11, 157 (2020).
Acknowledgements
The results presented here are, in whole or in part, based on data obtained from the AD Knowledge Portal (https://adknowledgeportal.org). Study data were provided by the Rush Alzheimer’s Disease Center, Rush University Medical Center. Data collection was supported through funding by National Institute on Aging grants P30AG10161 (ROS), R01AG15819 (ROSMAP; genomics and RNA-seq), R01AG17917 (MAP), R01AG30146, R01AG36042 (5hC methylation, ATAC-seq), RC2AG036547 (H3K9Ac), R01AG36836 (RNA-seq), R01AG48015 (monocyte RNA-seq) RF1AG57473 (single-nuclei RNA-seq), U01AG32984 (genomic and whole-exome sequencing), U01AG46152 (ROSMAP AMP-AD, targeted proteomics), U01AG46161 (TMT proteomics), U01AG61356 (whole-genome sequencing, targeted proteomics, ROSMAP AMP-AD), the Illinois Department of Public Health (ROSMAP) and the Translational Genomics Research Institute (genomic). Additional phenotypic data can be requested at www.radc.rush.edu.
Author information
Authors and Affiliations
Contributions
J.B. and D.M. designed the study and wrote the manuscript. J.B. mapped raw sequencing data, performed quality control on the AD datasets and performed the eQTL, Coloc and fine-mapping analysis. D.C. generated single-nuclei RNA-seq data on AD and MS samples. W.M. performed quality control on the MS dataset and provided critical statistical comments. L.F. provided AD samples. A.W., E.U., E.N., M.M., G.C.-B. and S.A. provided MS samples. W.O., V.A.I. and S.S. genotyped MS and a subset of AD samples and performed imputation of the genotype data. V.M. and P.D.J. provided single-nuclei RNA-seq and whole-genome sequencing data on a subset of the AD samples.
Corresponding authors
Ethics declarations
Competing interests
J.B., D.C., W.M., L.F., E.U., W.O., V.A.I., S.S. and D.M. are employees of Roche/Genentech. The authors received internal funding for this work. All other authors declare no competing interests.
Peer review
Peer review information
Nature Neuroscience thanks Jason Stein and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–24
Supplementary Table
Supplementary Tables 1–7
Rights and permissions
About this article
Cite this article
Bryois, J., Calini, D., Macnair, W. et al. Cell-type-specific cis-eQTLs in eight human brain cell types identify novel risk genes for psychiatric and neurological disorders. Nat Neurosci 25, 1104–1112 (2022). https://doi.org/10.1038/s41593-022-01128-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41593-022-01128-z
This article is cited by
-
PICALO: principal interaction component analysis for the identification of discrete technical, cell-type, and environmental factors that mediate eQTLs
Genome Biology (2024)
-
Single-cell multiplex chromatin and RNA interactions in ageing human brain
Nature (2024)
-
Genomic insights into the comorbidity between type 2 diabetes and schizophrenia
Schizophrenia (2024)
-
Decreased CNNM2 expression in prefrontal cortex affects sensorimotor gating function, cognition, dendritic spine morphogenesis and risk of schizophrenia
Neuropsychopharmacology (2024)
-
Brain tissue- and cell type-specific eQTL Mendelian randomization reveals efficacy of FADS1 and FADS2 on cognitive function
Translational Psychiatry (2024)