Abstract
Benzodiazepines are widely administered drugs to treat anxiety and insomnia. In addition to tolerance development and abuse liability, their chronic use may cause cognitive impairment and increase the risk for dementia. However, the mechanism by which benzodiazepines might contribute to persistent cognitive decline remains unknown. Here we report that diazepam, a widely prescribed benzodiazepine, impairs the structural plasticity of dendritic spines, causing cognitive impairment in mice. Diazepam induces these deficits via the mitochondrial 18 kDa translocator protein (TSPO), rather than classical γ-aminobutyric acid type A receptors, which alters microglial morphology, and phagocytosis of synaptic material. Collectively, our findings demonstrate a mechanism by which TSPO ligands alter synaptic plasticity and, as a consequence, cause cognitive impairment.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
Raw data that support the findings of this study are available from the corresponding authors upon reasonable request. Source data are provided with this paper.
References
Rudolph, U. & Knoflach, F. Beyond classical benzodiazepines: novel therapeutic potential of GABAA receptor subtypes. Nat. Rev. Drug Discov. 10, 685–697 (2011).
Rupprecht, R. et al. Translocator protein (18 kDa) (TSPO) as a therapeutic target for neurological and psychiatric disorders. Nat. Rev. Drug Discov. 9, 971–988 (2010).
Papadopoulos, V. et al. Translocator protein (18 kDa): new nomenclature for the peripheral-type benzodiazepine receptor based on its structure and molecular function. Trends Pharmacol. Sci. 27, 402–409 (2006).
Liu, G.-J. et al. Functional gains in energy and cell metabolism after TSPO gene insertion. Cell Cycle 16, 436–447 (2017).
Gut, P., Zweckstetter, M. & Banati, R. B. Lost in translocation: the functions of the 18-kD translocator protein. Trends Endocrinol. Metab. 26, 349–356 (2015).
Barker, M. J., Greenwood, K. M., Jackson, M. & Crowe, S. F. Cognitive effects of long-term benzodiazepine use. CNS Drugs 18, 37–48 (2004).
Arolfo, M. P. & Brioni, J. D. Diazepam impairs place learning in the Morris water maze. Behav. Neural Biol. 55, 131–136 (1991).
Gallacher, J. et al. Benzodiazepine use and risk of dementia: evidence from the Caerphilly Prospective Study (CaPS). J. Epidemiol. Community Health 66, 869–873 (2012).
de Gage, S. B. et al. Benzodiazepine use and risk of Alzheimer’s disease: case–control study. BMJ 349, g5205 (2014).
Penninkilampi, R. & Eslick, G. D. A systematic review and meta-analysis of the risk of dementia associated with benzodiazepine use, after controlling for protopathic bias. CNS Drugs 32, 485–497 (2018).
Gray, S. L. et al. Benzodiazepine use and risk of incident dementia or cognitive decline: prospective population based study. BMJ 352, i90 (2016).
Biétry, F. A., Pfeil, A. M., Reich, O., Schwenkglenks, M. & Meier, C. R. Benzodiazepine use and risk of developing Alzheimer’s disease: a case–control study based on Swiss claims data. CNS Drugs 31, 245–251 (2017).
Kasai, H., Fukuda, M., Watanabe, S., Hayashi-Takagi, A. & Noguchi, J. Structural dynamics of dendritic spines in memory and cognition. Trends Neurosci. 33, 121–129 (2010).
Zou, C. et al. Neuroinflammation impairs adaptive structural plasticity of dendritic spines in a preclinical model of Alzheimer’s disease. Acta Neuropathol. 131, 235–246 (2016).
Zuo, Y., Lin, A., Chang, P. & Gan, W.-B. Development of long-term dendritic spine stability in diverse regions of cerebral cortex. Neuron 46, 181–189 (2005).
Grutzendler, J., Kasthuri, N. & Gan, W.-B. Long-term dendritic spine stability in the adult cortex. Nature 420, 812–816 (2002).
Feng, G. et al. Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron 28, 41–51 (2000).
Rudolph, U. et al. Benzodiazepine actions mediated by specific γ-aminobutyric acidA receptor subtypes. Nature 401, 796–800 (1999).
Richter, L. et al. Diazepam-bound GABAA receptor models identify new benzodiazepine binding-site ligands. Nat. Chem. Biol. 8, 455–464 (2012).
Ralvenius, W. T., Benke, D., Acuna, M. A., Rudolph, U. & Zeilhofer, H. U. Analgesia and unwanted benzodiazepine effects in point-mutated mice expressing only one benzodiazepine-sensitive GABAA receptor subtype. Nat. Commun. 6, 6803 (2015).
Sohal, V. S., Zhang, F., Yizhar, O. & Deisseroth, K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature 459, 698–702 (2009).
Hu, H., Gan, J. & Jonas, P. Fast-spiking, parvalbumin+ GABAergic interneurons: from cellular design to microcircuit function. Science 345, 1255263 (2014).
Pritchett, D. B. & Seeburg, P. H. γ‐Aminobutyric acidA receptor α5‐subunit creates novel type II benzodiazepine receptor pharmacology. J. Neurochem. 54, 1802–1804 (1990).
Romeo, E. et al. 2-Aryl-3-indoleacetamides (FGIN-1): a new class of potent and specific ligands for the mitochondrial DBI receptor (MDR). J. Pharmacol. Exp. Ther. 262, 971–978 (1992).
Löw, K. et al. Molecular and neuronal substrate for the selective attenuation of anxiety. Science 290, 131–134 (2000).
Crestani, F. et al. Trace fear conditioning involves hippocampal α5 GABAA receptors. Proc. Natl Acad. Sci. USA 99, 8980–8985 (2002).
Banati, R. B. et al. Positron emission tomography and functional characterization of a complete PBR/TSPO knockout. Nat. Commun. 5, 5452 (2014).
Brendel, M. et al. Glial activation and glucose metabolism in a transgenic amyloid mouse model: a triple-tracer PET study. J. Nucl. Med. 57, 954–960 (2016).
Kreisl, W. C. et al. In vivo radioligand binding to translocator protein correlates with severity of Alzheimer’s disease. Brain 136, 2228–2238 (2013).
Cagnin, A. et al. In-vivo measurement of activated microglia in dementia. Lancet 358, 461–467 (2001).
Wolf, L. et al. Enhancing neurosteroid synthesis—relationship to the pharmacology of translocator protein (18 kDa)(TSPO) ligands and benzodiazepines. Pharmacopsychiatry 48, 72–77 (2015).
Salter, M. W. & Stevens, B. Microglia emerge as central players in brain disease. Nat. Med. 23, 1018–1027 (2017).
Wake, H., Moorhouse, A. J., Miyamoto, A. & Nabekura, J. Microglia: actively surveying and shaping neuronal circuit structure and function. Trends Neurosci. 36, 209–217 (2013).
Hong, S. et al. Complement and microglia mediate early synapse loss in Alzheimer mouse models. Science 352, 712–716 (2016).
Chung, W.-S., Welsh, C. A., Barres, B. A. & Stevens, B. Do glia drive synaptic and cognitive impairment in disease? Nat. Neurosci. 18, 1539–1545 (2015).
Elmore, M. R. et al. Colony-stimulating factor 1 receptor signaling is necessary for microglia viability, unmasking a microglia progenitor cell in the adult brain. Neuron 82, 380–397 (2014).
Paolicelli, R. C. et al. Synaptic pruning by microglia is necessary for normal brain development. Science 333, 1456–1458 (2011).
Neniskyte, U. & Gross, C. T. Errant gardeners: glial-cell-dependent synaptic pruning and neurodevelopmental disorders. Nat. Rev. Neurosci. 18, 658–670 (2017).
Dejanovic, B. et al. Changes in the synaptic proteome in tauopathy and rescue of tau-induced synapse loss by C1q antibodies. Neuron 100, 1322–1336 (2018).
Liu, Y. U. et al. Neuronal network activity controls microglial process surveillance in awake mice via norepinephrine signaling. Nat. Neurosci. 22, 1771–1781 (2019).
Umpierre, A. D. & Wu, L. J. How microglia sense and regulate neuronal activity. Glia 69, 1637–1653 (2021).
Zhang, Y., Zhou, X.-h, Meranus, D. H., Wang, L. & Kukull, W. A. Benzodiazepine use and cognitive decline in elderly with normal cognition. Alzheimer Dis. Assoc. Disord. 30, 113–117 (2016).
Pariente, A., de Gage, S. B., Moore, N. & Bégaud, B. The benzodiazepine–dementia disorders link: current state of knowledge. CNS Drugs 30, 1–7 (2016).
Filipello, F. et al. The microglial innate immune receptor TREM2 is required for synapse elimination and normal brain connectivity. Immunity 48, 979–991 (2018).
Paolicelli, R. C. et al. TDP-43 depletion in microglia promotes amyloid clearance but also induces synapse loss. Neuron 95, 297–308 (2017).
Lechin, F. et al. Peripheral blood immunological parameters in long-term benzodiazepine users. Clin. Neuropharmacol. 17, 63–72 (1994).
Covelli, V. et al. Effects of benzodiazepines on the immune system. Acta Neurologica 13, 418–423 (1991).
Colasanti, A. et al. Bipolar disorder is associated with the rs6971 polymorphism in the gene encoding 18 kDa translocator protein (TSPO). Psychoneuroendocrinology 38, 2826–2829 (2013).
Jung, S. et al. Analysis of fractalkine receptor CX3CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol. Cell. Biol. 20, 4106–4114 (2000).
Fuhrmann, M., Mitteregger, G., Kretzschmar, H. & Herms, J. Dendritic pathology in prion disease starts at the synaptic spine. J. Neurosci. 27, 6224–6233 (2007).
Mrsic-Flogel, T. D. et al. Homeostatic regulation of eye-specific responses in visual cortex during ocular dominance plasticity. Neuron 54, 961–972 (2007).
Deussing, M. et al. Coupling between physiological TSPO expression in brain and myocardium allows stabilization of late-phase cerebral [18F]GE180 PET quantification. Neuroimage 165, 83–91 (2018).
Kleinberger, G. et al. The FTD‐like syndrome causing TREM2 T66M mutation impairs microglia function, brain perfusion, and glucose metabolism. EMBO J. 36, 1837–1853 (2017).
Leger, M. et al. Object recognition test in mice. Nat. Protoc. 8, 2531–2537 (2013).
Acknowledgements
We would like to thank G. Mitteregger, M. Miller, A. von Thaden, M. Schneider, C. Ihbe, E. Göcmenoglu, N. Lachner, F. Zhang, M. Matt and R. Trevisi for their technical support and animal care. We also thank A. Giese for support in discussing and preparing this manuscript. The DZNE Brain Bank provided advice on human tissue use. We appreciate the help of S. Reinert and T. Bonhoeffer in establishing in vivo imaging using microprism. We are grateful to B. von Ungern-Sternberg and K. Bormann-Giglmaier for technical assistance in PET scanning. The authors thank Plexxikon Inc. for providing PLX5622. This work was funded by Deutsche Forschungsgemeinschaft grants (FOR 2858 / ID 422181340 to J.H. and M.M.D., ID 420462116 to R.R. and EXC 2145 / ID 390857198 to J.H.).
Author information
Authors and Affiliations
Contributions
Y.S., M.M.D. and J.H. conceived and designed this study. J.H. provided project oversight. Y.S. performed all the in vivo two-photon imaging experiments, stereotactic injection and immunohistochemistry and interpreted the results. M.C. performed the Golgi staining, immunoblotting, ELISA, 3D reconstruction and data analysis. C.Z. and M.C. performed the behavioral experiments. F.L.S. performed bioinformatic data analysis. K.O. performed immunoblotting. N.B. performed the imaging of Golgi staining with M.C. M.B. and F.E. performed the PET imaging and PET data analysis. R.B.B., G.-J.L. and R.J.M. provided global Tspo−/− knockout mice (C57BL/6-Tspotm1GuMu(GuwiyangWurra)), designed experiments and co-wrote the manuscript. U.R. and H.U.Z. provided the Gabra RRRR quadruple point-mutated mice, designed experiments and co-wrote the manuscript. R.R. and G.R. helped design the experiments and contributed to the conception and co-wrote the manuscript. Y.S. wrote the manuscript with critical input from R.R., R.B.B., H.U.Z., U.R., M.C., M.M.D. and J.H. All authors reviewed and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Peer review
Peer review information
Nature Neuroscience thanks Graziano Pinna, Long-Jun Wu and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
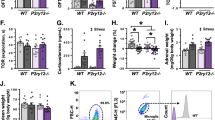
Extended Data Fig. 1 Effects of various compounds on mouse locomotor activity.
a, Representative traces of mouse movement during the 10-minute open field test. b, Respective effects of diazepam (1.25-20 mg/kg, i.g.) on mouse locomotor activity. Diazepam or vehicle was administered i.g. 15 min before locomotor activity tests. c & d Non-sedating dose of diazepam (1 mg/kg) is not able to induce locomotor sedation (c), while markedly increases the time spent in the light box of mice in the light dark-box test (d), suggesting the anxiolytic effect of diazepam (1 mg/kg). e, Representative traces of mouse movement during the 10-minute open field test in the vehicle, diazepam, flumazenil + diazepam, and zolpidem groups. f, Flumazenil blocks the observed effects of diazepam on the mouse locomotor activity, while zolpidem is able to induce similar locomotor sedation as diazepam. Mice were pretreated with flumazenil 15 min before diazepam and 30 min before locomotor activity tests. n = 4-8 animals per group (b); n = 15 and 16 animals per group (c, d); n = 8-11 animals per group (f). Box-and-whiskers plots show median, 25th and 75th percentiles, with maximum and minimum values as whiskers. One-way ANOVA followed by Dunnett’s or Bonferroni’s multiple comparisons test (b: F(5, 34) = 35.60, p < 0.0001; f: F(3, 32) = 47.00, p < 0.0001); two-sided unpaired Student’s t test (c: t(29) = 1.380, p = 0.1781); two-sided Mann-Whitney test (d: U = 20.50, p < 0.0001). **p < 0.01, ***p < 0.001. n.s. – no significant difference.
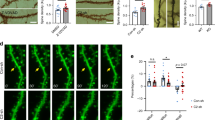
Extended Data Fig. 2 Prolonged diazepam administration alters the structural plasticity of dendritic spines in vivo.
a, Schematic of the experimental design. b, Representative in vivo two-photon micrographs of eGFP-labelled apical dendritic tufts originating from layer V pyramidal neurons in the somatosensory cortex. c-e, Spine density (c), and the fractions of gained (d) and lost (e) spines in four-week diazepam-treated mice. f, Total distance travelled during a 10-min open field test. g, Ratios of time spent exploring the novel and familiar objects in the novel object recognition task. h & i, Total number of arm entries (h) and percentage of spontaneous arm-alternations (i) in the Y-maze task. n = 5 animals in diazepam-treated group, ~1511 spines from 50 dendrites were analyzed (c, d, e); n = 11 animals per group (f, g); n = 11 and 10 animals per group (h, i). Dot plots represent mean ± s.e.m.; box-and-whiskers plots show median, 25th and 75th percentiles, with maximum and minimum values as whiskers. Repeated measures ANOVA with Geisser-Greenhouse correction followed by Dunnett’s multiple comparisons test (c: F(1.877, 7.510) = 30.62, p = 0.0003; d: F(2.678, 10.71) = 4.325, p = 0.0341; e: F(2.720, 10.88) = 9.342, p = 0.0028); two-sided unpaired Student’s t test (f: t(20) = 3.461, p = 0.0025; h: t(19) = 0.2861, p = 0.7779; i: t(19) = 2.074, p = 0.0519); two-sided Wilcoxon signed-rank test: compared to the hypothetical value 1 (g: pveh = 0.0020; pDz = 0.9609). *p < 0.05, **p < 0.01, ***p < 0.001. n.s. - no significant difference. Scale bar = 5 μm.
Extended Data Fig. 3 Diazepam alters the structural plasticity of dendritic spines of medial prefrontal cortex in the Thy1-eGFP, but not Tspo−/−: Thy1-eGFP mice in vivo.
a, Schematic of the experimental design. b,f, j, n, Representative in vivo two-photon micrographs of eGFP-labelled apical dendritic tufts in the medial prefrontal cortex. c-e & g-i, Spine density is reduced in one-week diazepam-treated mice (c), with the fractions of both gained (d) and lost (e) spines altered, which is not observed in the vehicle-treated mice (g-i). k-m & o-q, Spine density (k), the fraction of gained spines (l), and the fraction of lost spines (m) remain unchanged in one-week diazepam-treated Tspo−/−: Thy1-eGFP mice, which are comparable to the vehicle-treated Tspo−/−: Thy1-eGFP mice (o-q). n = 4 animals in the diazepam-treated group, ~693 spines from 33 dendrites were analyzed (c, d, e); n = 4 animals in the vehicle-treated group, ~673 spines from 30 dendrites were analyzed (g, h, i); n = 3 animals in the diazepam-treated group, ~604 spines from 25 dendrites were analyzed (k, l, m); n = 3 animals in vehicle-treated group, ~634 spines from 28 dendrites were analyzed (o, p, q). Data are presented as mean ± s.e.m. Repeated measures ANOVA with Geisser-Greenhouse correction followed by Dunnett’s multiple comparisons test (c: F(1.302, 3.907) = 32.95, p = 0.0043; d: F(1.775, 5.326) = 11.55, p = 0.0124; e: F(1.687, 5.060) = 19.49, p = 0.0047; g: F(1.561, 4.684) = 1.553, p = 0.2938; h: F(1.656, 4.967) = 3.397, p = 0.1202; i: F(1.419, 4.258) = 0.02780, p = 0.9361; k: F(1.114, 2.229) = 1.154, p = 0.3968; l: F(1.110, 2.220) = 0.2042, p = 0.7150; m: F(1.813, 3.626) = 1.109, p = 0.4129; o: F(1.133, 2.266) = 2.847, p = 0.2235; p: F(1.129, 2.259) = 3.227, p = 0.2034; q: F(1.923, 3.846) = 0.03459, p = 0.9627). *p < 0.05. Scale bars: b, f, j, n = 5 μm.
Extended Data Fig. 4 Diazepam alters the dendritic spine density and morphology in the somatosensory and prefrontal cortices via TSPO.
a, f, k, p, Representative confocal micrographs and 3D reconstruction of apical dendritic tufts in the somatosensory (a, k) and prefrontal (f, p) cortices in one-week diazepam or vehicle-treated Thy1-eGFP (a, f) or Tspo−/−: Thy1-eGFP (k, p) mice. b, g, l, q, Quantifications of dendritic spine density in the somatosensory (b, l) and prefrontal (g, q) cortices (median values and interquartile ranges are plotted). c, h, m, r, Quantifications of dendritic spine morphology in the somatosensory (c, m) and prefrontal (h, r) cortices. d, i, n, s, Spine head volume in the somatosensory (d, n) and prefrontal (i, s) cortices (bars show mean and s.e.m., dots represent individual spine head volumes). e, j, o, t, Spine head volume cumulative probability in the somatosensory (e, o) and prefrontal (j, t) cortices. n = 5 animals per group, SSC + Veh: ~1435 spines from 40 dendrites, SSC + Dz: ~1197 spines from 41 dendrites (b, c, d, e); n = 5 animals per group, PFC + Veh: ~1824 spines from 42 dendrites, PFC + Dz: ~1454 spines from 45 dendrites (g, h, i, j); n = 5 animals per group, SSC + Veh: ~1560 spines from 40 dendrites, SSC + Dz: ~1471 spines from 40 dendrites (l, m, n, o); n = 5 animals per group, PFC + Veh: ~1863 spines from 40 dendrites, PFC + Dz: ~1885 spines from 40 dendrites (q, r, s, t). Two-sided Mann-Whitney test (b: U = 559, p = 0.0133; d: U = 805339, p = 0.0058; i: U = 1156228, p < 0.0001; n: U = 1147338, p = 0.9986; s: U = 1702871, p = 0.1095); χ2 test (c: χ2(2) = 5.268, p = 0.0718; h: χ2(2) = 4.487, p = 0.1061; m: χ2(2) = 0.06183, p = 0.9696; r: χ2(2) = 0.05790, p = 0.9715); Kolmogorov-Smirnov test (e: D = 0.05713, p = 0.0283; j: D = 0.1051, p < 0.0001; o: D = 0.04572, p = 0.0844; t: D = 0.03359, p = 0.2411); two-sided unpaired Student’s t test (g: t(85) = 3.437, p = 0.0009; l: t(78) = 0.2700, p = 0.7878; q: t(78) = 0.02585, p = 0.9794). *p < 0.05, **p < 0.01, ***p < 0.001. n.s. - no significant difference. Scale bars: a, f, k, p = 5 µm.
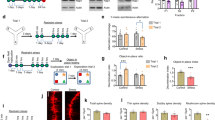
Extended Data Fig. 5 CNO-enhanced GABAergic inhibition fails to phenocopy diazepam-impaired structural plasticity of dendritic spines in vivo.
a, Construct of hM3D(Gq)-mCherry AAV and PV-immunostaining in the somatosensory cortex of PVCre/+ mice expressing hM3D(Gq)-mCherry. b, CNO increases the expression of c-fos in the somatosensory cortex of PVCre/+ mice expressing hM3D(Gq)-mCherry. c, Schematic of the experimental design. d & h, Representative in vivo two-photon micrographs of eGFP-labelled apical dendritic tufts in the somatosensory cortex imaged before and after administration of CNO in PVCre/+: Thy1-eGFP mice injected with the AAV vector containing pAAV-hSyn-DIO-hM3D(Gq)-mCherry (d) or control AAV vector containing pAAV-hSyn-DIO-mCherry (h). CNO treatment started 14 days after the first imaging time point and was continued over 7 days in a 24-h interval. e & i, Spine density is increased upon CNO treatment in animals injected with the AAV vector containing pAAV-hSyn-DIO-hM3D(Gq)-mCherry (e), while remains unchanged in animals injected with control AAV vector containing pAAV-hSyn-DIO-mCherry (i). f & j, Quantifications of the fractions of gained spines in animals injected with the AAV vector containing pAAV-hSyn-DIO-hM3D(Gq)-mCherry (f) or with control AAV vector containing pAAV-hSyn-DIO-mCherry (j). g & k, Quantifications of the fractions of lost spines in animals injected with the AAV vector containing pAAV-hSyn-DIO-hM3D(Gq)-mCherry (g) or with control AAV vector containing pAAV-hSyn-DIO-mCherry (k). n = 6 animals injected with the AAV vector containing pAAV-hSyn-DIO-hM3D(Gq)-mCherry, ~1941 spines from 60 dendrites were analyzed (e, f, g); n = 4 animals injected with control AAV vector containing pAAV-hSyn-DIO-mCherry, ~1314 spines from 40 dendrites were analyzed (i, j, k). Data are presented as mean ± s.e.m. Repeated measures ANOVA with Geisser-Greenhouse correction followed by Dunnett’s multiple comparisons test (e: F(2.821, 14.11) = 7.783, p = 0.0029; f: F(2.222, 11.11) = 1.061, p = 0.3858; g: F(2.685, 13.43) = 2.264, p = 0.1320; i: F(2.515, 7.544) = 0.5128, p = 0.6568; j: F(2.253, 6.758) = 1.235, p = 0.3553; k: F(2.275, 6.824) = 1.829, p = 0.2317). *p < 0.05, **p < 0.01. Scale bars: a = 100 μm (upper right), 5 μm (lower panel); b, d, h = 5 μm.
Extended Data Fig. 6 Effects of Zolpidem, XBD173 and allopregnanolone on structural plasticity of dendritic spines.
a, Schematic of the experimental design. b, j, n, Representative in vivo two-photon micrographs of eGFP-labelled apical dendritic tufts originating from layer V pyramidal neurons in the somatosensory cortex imaged before and after administration of zolpidem (b), XBD 173 (j), and allopregnanolone (n). c-e Spine density (c), and the fractions of gained (d) and lost (e) spines in zolpidem-treated mice. f & g, Representative immunoblots for the indicated proteins from one-week zolpidem or vehicle -treated mouse whole-brain homogenates (f), and quantitation of GABAA R α1 immunoblots, normalized to GAPDH (g). h & i, Representative immunoblots for the indicated proteins from one-week diazepam or vehicle -treated mouse whole-brain homogenates (h), and quantitation of GABAA R α1 immunoblots, normalized to GAPDH (i). k-m, Spine density (k), the fractions of gained spines (l) and lost (m) spines in XBD173-treated mice. o-q Spine density (o), the fractions of gained (p) and lost (q) spines in allopregnanolone-treated mice. n = 6 animals in zolpidem-treated group, ~1891 spines from 60 dendrites were analyzed (c, d, e); n = 3 animals per group, samples were immunoblotted in triplicate (g, i); n = 5 animals in XBD173-treated group, ~1679 spines from 50 dendrites were analyzed (k, l, m); n = 6 animals in the allopregnanolone-treated group, ~2080 spines from 60 dendrites were analyzed (o, p, q). Data are presented as mean ± s.e.m. Repeated measures ANOVA with Geisser-Greenhouse correction followed by Dunnett’s multiple comparisons test (c: F(2.588, 12.94) = 1.328, p = 0.3054; d: F(2.573, 12.87) = 1.801, p = 0.2004; e: F(2.667, 13.34) = 1.128, p = 0.3676; k: F(2.202, 8.806) = 12.39, p = 0.0024; l: F(2.742, 10.97) = 6.341, p = 0.0104; m: F(2.458, 9.831) = 9.659, p = 0.0036; o: F(2.859, 14.29) = 6.095, p = 0.0074; p: F(2.641, 13.21) = 9.895, p = 0.0014; q: F(2.683, 13.42) = 2.234, p = 0.1356); two-sided Student’s t test (g: t(4) = 6.221, p = 0.0034; i: t(4) = 1.121, p = 0.3249). *p < 0.05, **p < 0.01. n.s. - no significant difference. Scale bars: b, j, n = 5 μm.
Extended Data Fig. 7 Characterization of TSPO expression and microglial morphology.
a, Immunoblots showing the complete absence of TSPO in microglia from the Tspo−/− mice. b & c, Representative confocal micrographs of cortical Iba1-positive microglia (b) with quantification of microglial numbers in drug-naïve animals (c). d-i, Representative confocal micrographs and 3D reconstruction of cortical Iba1-positive microglia in drug-naïve animals (d), with Imaris-based semi-automatic quantification of microglial morphology (e-i). j-p, Representative confocal micrographs and 3D reconstruction of cortical Iba1-positive microglia in eight-week diazepam (1 mg/kg, i.g., q.d.) or vehicle -treated WT or Tspo−/− mice (j), with Imaris-based semi-automatic quantification of microglia morphology (k-p). q-w, Representative confocal micrographs and 3D reconstruction of cortical Iba1-positive microglia in one-week diazepam or vehicle -treated Gabra RRRR mice (q), with Imaris-based semi-automatic quantification of microglial morphology (r-w). n = 4 animals per group (c, e, f, g, h, i); n = 3 animals per group (k, l, m, n, o, p); n = 3 animals per group (r, s, t, u, v, w). Data are presented as mean ± s.e.m. One-way ANOVA followed by Dunnett’s or Bonferroni’s multiple comparisons test (c: F(2, 9) = 3.774, p = 0.0645; e: F(2, 9) = 15.98, p = 0.0011; f: F(2, 9) = 18.89, p = 0.0006; g: F(2, 9) = 10.66, p = 0.0042; h: F(2, 9) = 15.72, p = 0.0012; i: F(2, 9) = 16.20, p = 0.0010); two-way ANOVA with Bonferroni’s multiple comparisons test (k: Finteraction: drug treatment x genotype (1, 8) = 0.007812, p = 0.9317; l: Finteraction: drug treatment x genotype (1, 8) = 24.33, p = 0.0011; m: Finteraction: drug treatment x genotype (1, 8) = 78.88, p < 0.0001; n: Finteraction: drug treatment x genotype (1, 8) = 20.78, p = 0.0019; o: Finteraction: drug treatment x genotype (1, 8) = 23.99, p = 0.0012; p: Finteraction: drug treatment x genotype (1, 8) = 22.88, p = 0.0014); two-sided unpaired Student’s t test (r: t(4) = 0.07773, p = 0.9418; s: t(4) = 9.019, p = 0.0008; t: t(4) = 11.54, p = 0.0003; u: t(4) = 5.345, p = 0.0059; v: t(4) = 9.160, p = 0.0008; w: t(4) = 8.813, p = 0.0009). *p < 0.05, **p < 0.01, ***p < 0.001. n.s. - no significant difference. Scale bars: b = 20 μm; d, j, q = 5 μm.
Extended Data Fig. 8 The anxiolytic dose of diazepam fails to impair the dendritic spine dynamics in Tspo−/−: Thy1-eGFP mice in vivo and ex vivo.
a, Schematic of the experimental design. b, Representative in vivo two-photon micrographs of eGFP-labelled apical dendritic tufts originating from layer V pyramidal neurons in the somatosensory cortex in Tspo−/−: Thy1-eGFP mice. c, Spine density in the anxiolytic dose of diazepam (1 mg/kg)-treated Tspo−/−: Thy1-eGFP mice. d & e, The fractions of gained (d) and lost spines (e) in the anxiolytic dose of diazepam-treated mice. f, Representative confocal micrographs and 3D reconstruction of eGFP-labelled apical dendritic tufts in the somatosensory cortex in the eight-week anxiolytic dose of diazepam (1 mg/kg, i.g., q.d.) or vehicle-treated Tspo−/−: Thy1-eGFP mice. g, Quantification of dendritic spine density (median values and interquartile ranges are plotted). h, Quantification of dendritic spine morphology. i & j, Spine head volume (i) (bars show mean and s.e.m., dots represent individual spine head volumes) and the cumulative probability (j) after eight-week diazepam (1 mg/kg) or vehicle administration in Tspo−/−: Thy1-eGFP mice. n = 3 animals in the diazepam-treated group, ~1486 spines from 28 dendrites were analyzed (c, d, e); n = 4 animals per group, 1195 spines from 32 dendrites in the vehicle-treated group, 1216 spines from 32 dendrites in the diazepam-treated group were analyzed (g, h, i, j). For dot plots, data are presented as mean ± s.e.m. Repeated measures ANOVA with Geisser-Greenhouse correction followed by Dunnett’s multiple comparisons test (c: F(1.640, 3.281) = 1.319, p = 0.3665; d: F(1.776, 3.553) = 0.5656, p = 0.5938; e: F(1.549, 3.098) = 0.7134, p = 0.5220); two-sided Mann-Whitney test (g: U = 479, p = 0.6621; i: U = 693229, p = 0.0511); χ2 test (h: χ2(2) = 3.097, p = 0.2126); Kolmogorov-Smirnov test (j: D = 0.05227, p = 0.0743). n.s. - no significant difference. Scale bars: b & f = 5 μm.
Extended Data Fig. 9 Effects of diazepam on TSPO expression in different cell types.
a-j, Representative confocal micrographs and 3D reconstruction of TSPO inside cortical Iba1-positive microglia upon vehicle (a-e) or diazepam (f-j) treatment. k, Quantification of TSPO positive volume per microglia. l-u, Representative confocal micrographs, 3D reconstruction of TSPO inside cortical GFAP and S100B double positive astrocytes upon one-week vehicle (l-p) or diazepam (q-u) treatment. v, Quantification of TSPO positive volume per astrocyte. w-af, Representative confocal micrographs, 3D reconstruction of TSPO inside cortical NeuN-positive neuronal soma upon one-week vehicle (w-aa) or diazepam (ab-af) treatment. ag, Quantification of TSPO positive volume per neuronal soma. ah-aq, Representative confocal micrographs, 3D reconstruction of TSPO inside cortical GLUT1-positive vascular endothelium upon one-week vehicle (ah-al) or diazepam (am-aq) treatment. ar, Quantification of TSPO positive volume per vascular segment. n = 5 animals per group (k, v, ag, ar). Data are presented as mean ± s.e.m. Two-sided Mann-Whitney test (k: U = 0, p = 0.0079); two-sided unpaired Student’s t test (v: t(8) = 0.1226, p = 0.9054; ag: t(8) = 4.223, p = 0.0029; ar: t(8) = 0.2850, p = 0.7829). **p < 0.01. n.s. - no significant difference. Scale bars = 5 μm.
Extended Data Fig. 10 Long-term microglia elimination does not affect astrocytes or neurons.
a & b, Representative confocal micrographs show that animals treated with control diet (a) or PLX5622 (b) for 6 weeks to fully eliminate microglia. c, Quantification of Iba1-positive cell bodies in the somatosensory cortex as shown were performed via Imaris spot counts and shown in (c), revealing robust decreases in microglial numbers. d-i, Brain sections from 3-4 month-old WT mice fed with six-week PLX5622 or control diet were stained with the astrocytic markers GFAP and S100B. Representative confocal micrographs from control (d-f) and PLX5622 (g-i) treatement groups are shown. j, Quantification of GFAP and S100B double-positive cells encompassing the hippocampus as shown were performed via Imaris spot counts and shown in (j), revealing no changes. k, Sholl analysis. l & m, Brain sections were stained with the neuronal marker NeuN. Representative confocal micrographs from control (l) and PLX5622 (m) treatment groups are shown. n, Relative numbers of NeuN-positive cells per field in the somatosensory cortex as shown were performed via Imaris spot counts and shown in (n), revealing no changes. n = 5 animals per group (c); n = 3 animals per group (j, k, n). Data are presented as mean ± s.e.m. Two-sided unpaired Student’s t test (c: t(8) = 37.57, p < 0.0001; j: t(4) = 0.4009, p = 0.7090; n: t(4) = 0.2562, p = 0.8104); two-sided Mann-Whitney test (k: U = 1132, p = 0.4081). ***p < 0.001. n.s. - no significant difference. Scale bars = 20 μm.
Supplementary information
Supplementary Information
Supplementary Figs. 1 and 2 and Supplementary Methods
Source data
Source Data Fig. 1
Statistical Source Data
Source Data Fig. 2
Statistical Source Data
Source Data Fig. 3
Statistical Source Data
Source Data Fig. 4
Statistical Source Data
Source Data Fig. 4
Uncropped blots
Source Data Fig. 5
Statistical Source Data
Source Data Fig. 5
Uncropped blots
Source Data Fig. 6
Statistical Source Data
Source Data Fig. 7
Statistical Source Data
Source Data Fig. 8
Statistical Source Data
Source Data Extended Data Fig. 1
Statistical Source Data
Source Data Extended Data Fig. 2
Statistical Source Data
Source Data Extended Data Fig. 3
Statistical Source Data
Source Data Extended Data Fig. 4
Statistical Source Data
Source Data Extended Data Fig. 5
Statistical Source Data
Source Data Extended Data Fig. 6
Statistical Source Data
Source Data Extended Data Fig. 6
Uncropped blots
Source Data Extended Data Fig. 7
Statistical Source Data
Source Data Extended Data Fig. 7
Uncropped blots
Source Data Extended Data Fig. 8
Statistical Source Data
Source Data Extended Data Fig. 9
Statistical Source Data
Source Data Extended Data Fig. 10
Statistical Source Data
Rights and permissions
About this article
Cite this article
Shi, Y., Cui, M., Ochs, K. et al. Long-term diazepam treatment enhances microglial spine engulfment and impairs cognitive performance via the mitochondrial 18 kDa translocator protein (TSPO). Nat Neurosci 25, 317–329 (2022). https://doi.org/10.1038/s41593-022-01013-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41593-022-01013-9
This article is cited by
-
Microglia govern the extinction of acute stress-induced anxiety-like behaviors in male mice
Nature Communications (2024)
-
Chronic administration of XBD173 ameliorates cognitive deficits and neuropathology via 18 kDa translocator protein (TSPO) in a mouse model of Alzheimer’s disease
Translational Psychiatry (2023)
-
A molecular characterization and clinical relevance of microglia-like cells derived from patients with panic disorder
Translational Psychiatry (2023)
-
Neurosteroids and translocator protein 18 kDa (TSPO) in depression: implications for synaptic plasticity, cognition, and treatment options
European Archives of Psychiatry and Clinical Neuroscience (2023)
-
Depletion and activation of microglia impact metabolic connectivity of the mouse brain
Journal of Neuroinflammation (2023)