Abstract
Neuroscientists today can measure activity from more neurons than ever before, and are facing the challenge of connecting these brain-wide neural recordings to computation and behavior. In the present review, we first describe emerging tools and technologies being used to probe large-scale brain activity and new approaches to characterize behavior in the context of such measurements. We next highlight insights obtained from large-scale neural recordings in diverse model systems, and argue that some of these pose a challenge to traditional theoretical frameworks. Finally, we elaborate on existing modeling frameworks to interpret these data, and argue that the interpretation of brain-wide neural recordings calls for new theoretical approaches that may depend on the desired level of understanding. These advances in both neural recordings and theory development will pave the way for critical advances in our understanding of the brain.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
All data used to generate Fig. 2 are available at https://github.com/anne-urai/largescale_recordings under a CC-BY 4.0 license.
Code availability
All code used to generate Fig. 2 are available at https://github.com/anne-urai/largescale_recordings under a CC-BY 4.0 license.
References
Perkel, D. H., Gerstein, G. L. & Moore, G. P. Neuronal spike trains and stochastic point processes. Biophys. J. 7, 391–418 (1967).
Abbott, L. F. & Dayan, P. The effect of correlated variability on the accuracy of a population code. Neural Comput. 11, 91–101 (1999).
Sompolinsky, H., Yoon, H., Kang, K. & Shamir, M. Population coding in neuronal systems with correlated noise. Phys. Rev. E 64, 051904 (2001).
Hebb, D. O. The Organization of Behavior: A Neuropsychological Theory. (Wiley, 1949).
Abeles, M. Corticonics: Neural Circuits of the Cerebral Cortex. (Cambridge Univ. Press, 1991).
Doiron, B., Litwin-Kumar, A., Rosenbaum, R., Ocker, G. K. & Josić, K. The mechanics of state-dependent neural correlations. Nat. Neurosci. 19, 383–393 (2016).
Kohn, A., Coen-Cagli, R., Kanitscheider, I. & Pouget, A. Correlations and neuronal population information. Annu. Rev. Neurosci. 39, 237–256 (2016).
Barlow, H. in Sensory Communication (ed. Rosenblith, W. A.) 217–234 (MIT Press, 1961).
Britten, K. H., Shadlen, M. N., Newsome, W. T. & Movshon, A. Responses of neurons in macaque MT to stochastic motion signals. Vis. Neurosci. 10, 1157–1169 (1993).
Parker, A. J. & Newsome, W. T. Sense and the single neuron: probing the physiology of perception. Annu. Rev. Neurosci. 21, 227–277 (1998).
Wilson, H. R. & Cowan, J. D. Excitatory and inhibitory interactions in localized populations of model neurons. Biophys. J. 12, 1–24 (1972).
van Vreeswijk, C. & Sompolinsky, H. Chaos in neuronal networks with balanced excitatory and inhibitory activity. Science 274, 1724–1726 (1996).
Amit, D. J. & Brunel, N. Model of global spontaneous activity and local structured activity during delay periods in the cerebral cortex. Cereb. Cortex 7, 237–252 (1997).
Shadlen, M. N. & Newsome, W. T. The variable discharge of cortical neurons: implications for connectivity, computation, and information coding. J. Neurosci. 18, 3870–3896 (1998).
Li, P. H. et al. Anatomical identification of extracellularly recorded cells in large-scale multielectrode recordings. J. Neurosci. 35, 4663–4675 (2015).
Steinmetz, N. A., Zatka-Haas, P., Carandini, M. & Harris, K. D. Distributed coding of choice, action and engagement across the mouse brain. Nature 576, 266–273 (2019).
Siegle, J. H. et al. Survey of spiking in the mouse visual system reveals functional hierarchy. Nature 592, 86–92 (2021).
Siegle, J. H. et al. Reconciling functional differences in populations of neurons recorded with two-photon imaging and electrophysiology. eLife 10, e69068 (2021).
Humphries, M. The Spike: An Epic Journey through the Brain in 2.1 Seconds (Princeton Univ. Press, 2021).
de Vries, S. E. J. et al. A large-scale standardized physiological survey reveals functional organization of the mouse visual cortex. Nat. Neurosci. 23, 138–151 (2020).
Shoham, S., O’Connor, D. H. & Segev, R. How silent is the brain: is there a ‘dark matter’ problem in neuroscience? J. Comp. Physiol. A 192, 777–784 (2006).
Musall, S., Kaufman, M. T., Juavinett, A. L., Gluf, S. & Churchland, A. K. Single-trial neural dynamics are dominated by richly varied movements. Nat. Neurosci. 22, 1677–1686 (2019).
Stringer, C. et al. Spontaneous behaviors drive multidimensional, brainwide activity. Science 364, eaav7893 (2019).
Salkoff, D. B., Zagha, E., McCarthy, E. & McCormick, D. A. Movement and performance explain widespread cortical activity in a visual detection task. Cereb. Cortex 30, 421–437 (2020).
Niell, C. M. & Stryker, M. P. Modulation of visual responses by behavioral state in mouse visual cortex. Neuron 65, 472–479 (2010).
Erisken, S. et al. Effects of locomotion extend throughout the mouse early visual system. Curr. Biol. 24, 2899–2907 (2014).
Vinck, M., Batista-Brito, R., Knoblich, U. & Cardin, J. A. Arousal and locomotion make distinct contributions to cortical activity patterns and visual encoding. Neuron 86, 740–754 (2015).
Roth, M. M. et al. Thalamic nuclei convey diverse contextual information to layer 1 of visual cortex. Nat. Neurosci. 19, 299–307 (2016).
Dolensek, N., Gehrlach, D. A., Klein, A. S. & Gogolla, N. Facial expressions of emotion states and their neuronal correlates in mice. Science 368, 89–94 (2020).
Keller, G. B. & Mrsic-Flogel, T. D. Predictive processing: a canonical cortical computation. Neuron 100, 424–435 (2018).
Cunningham, J. P. & Yu, B. M. Dimensionality reduction for large-scale neural recordings. Nat. Neurosci. 17, 1500–1509 (2014).
Pang, R., Lansdell, B. J. & Fairhall, A. L. Dimensionality reduction in neuroscience. Curr. Biol. 26, R656–R660 (2016).
Ni, A., Ruff, D. A., Alberts, J. J., Symmonds, J. & Cohen, M. R. Learning and attention reveal a general relationship between population activity and behavior. Science 359, 463–465 (2018).
Aoi, M. C., Mante, V. & Pillow, J. W. Prefrontal cortex exhibits multidimensional dynamic encoding during decision-making. Nat. Neurosci. 23, 1410–1420 (2020).
Churchland, M. M. et al. Neural population dynamics during reaching. Nature 487, 51–56 (2012).
Kato, S. et al. Global brain dynamics embed the motor command sequence of Caenorhabditis elegans. Cell 163, 656–669 (2015).
Briggman, K. L., Abarbanel, H. D. I. & Kristan, W. B. Optical imaging of neuronal populations during decision-making. Science 307, 896–901 (2005).
Fusi, S., Miller, E. K. & Rigotti, M. Why neurons mix: high dimensionality for higher cognition. Curr. Opin. Neurobiol. 37, 66–74 (2016).
Gao, P. et al. A theory of multineuronal dimensionality, dynamics and measurement. Preprint at bioRxiv https://doi.org/10.1101/214262 (2017).
Humphries, M. D. Strong and weak principles of neural dimension reduction. Neurons Behav. Data Anal. Theory 5, 1–28 (2021).
Stringer, C., Pachitariu, M., Steinmetz, N., Carandini, M. & Harris, K. D. High-dimensional geometry of population responses in visual cortex. Nature 1, 197 (2019).
Georgopoulos, A., Schwartz, A. B. & Kettner, R. E. Neuronal population coding of movement direction. Science 233, 1416–1419 (1986).
Averbeck, B. B., Latham, P. E. & Pouget, A. Neural correlations, population coding and computation. Nat. Rev. Neurosci. 7, 358–366 (2006).
Shenoy, K. V., Sahani, M. & Churchland, M. M. Cortical control of arm movements: a dynamical systems perspective. Annu. Rev. Neurosci. 36, 337–359 (2013).
Russo, A. A. et al. Neural trajectories in the supplementary motor area and motor cortex exhibit distinct geometries, compatible with different classes of computation. Neuron 107, 745–758.e6 (2020).
Saxena, S., Russo, A. A., Cunningham, J. P. & Churchland, M. M. Motor cortex activity across movement speeds is predicted by network-level strategies for generating muscle activity. Preprint at bioRxiv https://doi.org/10.1101/2021.02.01.429168 (2021).
Saxena, S. & Cunningham, J. P. Towards the neural population doctrine. Curr. Opin. Neurobiol. 55, 103–111 (2019).
Fuster, J. M. & Alexander, G. E. Neuron activity related to short-term memory. Science 173, 652–654 (1971).
Constantinidis, C. et al. Persistent spiking activity underlies working memory. J. Neurosci. 38, 7020–7028 (2018).
Murray, J. D. et al. Stable population coding for working memory coexists with heterogeneous neural dynamics in prefrontal cortex. Proc. Natl Acad. Sci. USA 114, 394–399 (2017).
Rajan, K., Harvey, C. D. & Tank, D. W. Recurrent network models of sequence generation and memory. Neuron 90, 128–142 (2016).
Herrmann, M. J., Hertz, J. A. & Prügel-Bennett, A. Analysis of synfire chains. Netw. Comput. Neural Syst. 6, 403–414 (1995).
Ólafsdóttir, F., Bush, D. & Barry, C. The role of hippocampal replay in memory and planning. Curr. Biol. 28, R37–R50 (2018).
Ruff, D. A. & Cohen, M. R. Stimulus dependence of correlated variability across cortical areas. J. Neurosci. 36, 7546–7556 (2016).
Cowley, B. R. et al. Slow drift of neural activity as a signature of impulsivity in macaque visual and prefrontal cortex. Neuron 108, 551–567.e8 (2020).
Semedo, J. D., Zandvakili, A., Machens, C. K., Yu, B. M. & Kohn, A. Cortical areas interact through a aommunication subspace. Neuron 102, 249–259.e4 (2019).
Das, A. & Fiete, I. R. Systematic errors in connectivity inferred from activity in strongly recurrent networks. Nat. Neurosci. 23, 1286–1296 (2020).
White, J. G., Southgate, E., Thomson, J. N. & Brenner, S. The structure of the nervous system of the nematode Caenorhabditis elegans. Philos. Trans. R. Soc. Lond. B Biol. Sci. 314, 1–340 (1986).
Witvliet, D. et al. Connectomes across development reveal principles of brain maturation. Nature 596, 257–261 (2021).
Eichler, K. et al. The complete connectome of a learning and memory centre in an insect brain. Nature 548, 175–182 (2017).
Scheffer, L. K. et al. A connectome and analysis of the adult Drosophila central brain. eLife 9, e57443 (2020).
Seelig, J. D. & Jayaraman, V. Neural dynamics for landmark orientation and angular path integration. Nature 521, 186–191 (2015).
Kim, S. S., Rouault, H., Druckmann, S. & Jayaraman, V. Ring attractor dynamics in the Drosophila central brain. Science 356, 849–853 (2017).
Hulse, B. K. et al. A connectome of the Drosophila central complex reveals network motifs suitable for flexible navigation and context-dependent action selection. eLife https://elifesciences.org/articles/66039 (2021).
Lu, J. et al. Transforming representations of movement from body- to world-centric space. Nature (in the press).
Lyu, C., Abbott, L. F. & Maimon, G. A neuronal circuit for vector computation builds an allocentric traveling-direction signal in the Drosophila fan-shaped body. Nature (in the press).
Gardner, R. J. et al. Toroidal topology of population activity in grid cells. Preprint at bioRxiv https://doi.org/10.1101/2021.02.25.432776 (2021).
Ratcliff, R. & McKoon, G. The diffusion decision model: theory and data for two-choice decision tasks. Neural Comput. 20, 873–922 (2008).
Brunton, B. W., Botvinick, M. M. & Brody, C. D. Rats and humans can optimally accumulate evidence for decision-making. Science 340, 95–98 (2013).
Churchland, A. K. & Kiani, R. Three challenges for connecting model to mechanism in decision-making. Curr. Opin. Behav. Sci. 11, 74–80 (2016).
Meister, M. L. R., Hennig, J. A. & Huk, A. C. Signal multiplexing and single-neuron computations in lateral intraparietal area during decision-making. J. Neurosci. 33, 2254–2267 (2013).
Faisal, A. A., Selen, L. P. J. & Wolpert, D. M. Noise in the nervous system. Nat. Rev. Neurosci. 9, 292–303 (2008).
Kubo, R., Toda, M. & Hashitsume, N. Statistical Physics II: Nonequilibrium Statistical Mechanics (Springer, 1991).
Rajan, K., Abbott, L. F. & Sompolinsky, H. Stimulus-dependent suppression of chaos in recurrent neural networks. Phys. Rev. E 82, 011903 (2010).
Churchland, A. K. et al. Variance as a signature of neural computations during decision making. Neuron 69, 818–831 (2011).
Litwin-Kumar, A. & Doiron, B. Slow dynamics and high variability in balanced cortical networks with clustered connections. Nat. Neurosci. 15, 1498–1505 (2012).
Huang, C. et al. Circuit models of low-dimensional shared variability in cortical networks. Neuron 101, 337–348.e4 (2019).
Churchland, A. K. & Abbott, L. F. Conceptual and technical advances define a key moment for theoretical neuroscience. Nat. Neurosci. 19, 348–349 (2016).
Calvin, W. H. & Stevens, C. F. Synaptic noise and other sources of randomness in motoneuron interspike intervals. J. Neurophysiol. 31, 574–587 (1968).
Branco, T. & Staras, K. The probability of neurotransmitter release: variability and feedback control at single synapses. Nat. Rev. Neurosci. 10, 373–383 (2009).
Neher, E. & Sakmann, B. Single-channel currents recorded from membrane of denervated frog muscle fibres. Nature 260, 799–802 (1976).
White, J. A., Rubinstein, J. T. & Kay, A. R. Channel noise in neurons. Trends Neurosci. 23, 131–137 (2000).
Pillow, J. W. et al. Spatio-temporal correlations and visual signalling in a complete neuronal population. Nature 454, 995–999 (2008).
Goris, R. L. T., Movshon, J. A. & Simoncelli, E. P. Partitioning neuronal variability. Nat. Neurosci. 17, 858–865 (2014).
Rosenbaum, R., Smith, M. A., Kohn, A., Rubin, J. E. & Doiron, B. The spatial structure of correlated neuronal variability. Nat. Neurosci. 20, 107–114 (2017).
Darshan, R., van Vreeswijk, C. & Hansel, D. Strength of correlations in strongly recurrent neuronal networks. Phys. Rev. X 8, 031072 (2018).
Landau, I. D. & Sompolinsky, H. Coherent chaos in a recurrent neural network with structured connectivity. PLoS Comput. Biol. 14, e1006309 (2018).
Mastrogiuseppe, F. & Ostojic, S. Linking connectivity, dynamics, and computations in low-rank recurrent neural networks. Neuron 99, 609–623.e29 (2018).
Gómez-Laberge, C., Smolyanskaya, A., Nassi, J. J., Kreiman, G. & Born, R. T. Bottom-up and top-down input augment the variability of cortical neurons. Neuron 91, 540–547 (2016).
Woolley, S. C., Rajan, R., Joshua, M. & Doupe, A. J. Emergence of context-dependent variability across a basal ganglia network. Neuron 82, 208–223 (2014).
Hires, A., Gutnisky, D. A., Yu, J., O’Connor, D. H. & Svoboda, K. Low-noise encoding of active touch by layer 4 in the somatosensory cortex. eLife 4, e06619 (2015).
Meshulam, L., Gauthier, J. L., Brody, C. D., Tank, D. W. & Bialek, W. Collective behavior of place and non-place neurons in the hippocampal network. Neuron 96, 1178–1191.e4 (2017).
Kaufman, M. T., Churchland, M. M., Ryu, S. I. & Shenoy, K. V. Cortical activity in the null space: permitting preparation without movement. Nat. Neurosci. 17, 440–448 (2014).
Williamson, R. C., Doiron, B., Smith, M. A. & Yu, B. M. Bridging large-scale neuronal recordings and large-scale network models using dimensionality reduction. Curr. Opin. Neurobiol. 55, 40–47 (2019).
McGinley, M. J. et al. Waking state: rapid variations modulate neural and behavioral responses. Neuron 87, 1143–1161 (2015).
Musall, S., Urai, A. E., Sussillo, D. & Churchland, A. K. Harnessing behavioral diversity to understand neural computations for cognition. Curr. Opin. Neurobiol. 58, 229–238 (2019).
Wiltschko, A. B. et al. Mapping sub-second structure in mouse behavior. Neuron 88, 1121–1135 (2015).
Krakauer, J. W., Ghazanfar, A. A., Gomez-Marin, A., MacIver, M. A. & Poeppel, D. Neuroscience needs behavior: correcting a reductionist bias. Neuron 93, 480–490 (2017).
Juavinett, A. L., Erlich, J. C. & Churchland, A. K. Decision-making behaviors: weighing ethology, complexity, and sensorimotor compatibility. Curr. Opin. Neurobiol. 49, 42–50 (2018).
Chen, X. et al. Brain-wide organization of neuronal activity and convergent sensorimotor transformations in larval zebrafish. Neuron 100, 876–890.e5 (2018).
Pacheco, D. A., Thiberge, S. Y., Pnevmatikakis, E. & Murthy, M. Auditory activity is diverse and widespread throughout the central brain of Drosophila. Nat. Neurosci. 24, 93–104 (2021).
Ji, N. et al. A neural circuit for flexible control of persistent behavioral states. eLife 10, e62889 (2021).
Kaplan, H. S., Salazar Thula, O., Khoss, N. & Zimmer, M. Nested neuronal dynamics orchestrate a behavioral hierarchy across timescales. Neuron 105, 562–576.e9 (2020).
Hallinen, K. M. et al. Decoding locomotion from population neural activity in moving C. elegans. eLife 10, e66135 (2021).
Bargmann, C. I. & Marder, E. From the connectome to brain function. Nat. Methods 10, 483–490 (2013).
Marder, E. Neuromodulation of neuronal circuits: back to the future. Neuron 76, 1–11 (2012).
Barres, B. A. The mystery and magic of glia: a perspective on their roles in health and disease. Neuron 60, 430–440 (2008).
Taylor, S. R. et al. Molecular topography of an entire nervous system. Cell 184, 4329–4347.e23 (2021).
Dworkin, J. D. et al. The extent and drivers of gender imbalance in neuroscience reference lists. Nat. Neurosci. 23, 918–926 (2020).
Couto, J. et al. Spatially segregated responses to visuo-tactile stimuli in mouse neocortex during active sensation. Preprint at bioRxiv https://doi.org/10.1101/199364 (2019).
Bos, H., Oswald, A.-M. & Doiron, B. Untangling stability and gain modulation in cortical circuits with multiple interneuron classes. Preprint at bioRxiv https://doi.org/10.1101/2020.06.15.148114 (2020).
Schrödel, T., Prevedel, R., Aumayr, K., Zimmer, M. & Vaziri, A. Brain-wide 3D imaging of neuronal activity in Caenorhabditis elegans with sculpted light. Nat. Methods 10, 1013–1020 (2013).
Ahrens, M. B., Orger, M. B., Robson, D. N., Li, J. M. & Keller, P. J. Whole-brain functional imaging at cellular resolution using light-sheet microscopy. Nat. Methods 10, 413–420 (2013).
Yamamoto, W. & Yuste, R. Whole-body imaging of neural and muscle activity during behavior in Hydra vulgaris: effect of osmolarity on contraction bursts. eNeuro https://doi.org/10.1101/2019.12.20.883835 (2020).
Mann, K., Gallen, C. L. & Clandinin, T. R. Whole-brain calcium imaging reveals an intrinsic functional network in Drosophila. Curr. Biol. 27, 2389–2396.e4 (2017).
Aimon, S. et al. Fast near-whole–brain imaging in adult Drosophila during responses to stimuli and behavior. PLoS Biol. 17, e2006732 (2019).
Marblestone, A. H. et al. Physical principles for scalable neural recording. Front. Comput. Neurosci. 7, 134 (2013).
Kleinfeld, D. et al. Can one concurrently record electrical spikes from every neuron in a mammalian brain? Neuron 103, 1005–1015 (2019).
Stevenson, I. H. & Kording, K. P. How advances in neural recording affect data analysis. Nat. Neurosci. 14, 139–142 (2011).
Steinmetz, N. A. et al. Neuropixels 2.0: A miniaturized high-density probe for stable, long-term brain recordings. Science 372, eabf4588 (2021).
Juavinett, A. L., Bekheet, G. & Churchland, A. K. Chronically implanted Neuropixels probes enable high-yield recordings in freely moving mice. eLife 8, e47188 (2019).
Hong, G. & Lieber, C. M. Novel electrode technologies for neural recordings. Nat. Rev. Neurosci. 20, 330–345 (2019).
Nguyen, J. P. et al. Whole-brain calcium imaging with cellular resolution in freely behaving Caenorhabditis elegans. Proc. Natl Acad. Sci. USA 113, E1074–E1081 (2016).
Sofroniew, N. J. Q&A: the brain under a mesoscope: the forest and the trees. BMC Biol. 15, 82 (2017).
Harris, K. D., Quiroga, R. Q., Freeman, J. & Smith, S. L. Improving data quality in neuronal population recordings. Nat. Neurosci. 19, 1165–1174 (2016).
Trautmann, E. M. et al. Accurate estimation of neural population dynamics without spike sorting. Neuron 103, 292–308.e4 (2019).
Magland, J. et al. SpikeForest, reproducible web-facing ground-truth validation of automated neural spike sorters. eLife 9, e55167 (2020).
Buccino, A. P. et al. SpikeInterface, a unified framework for spike sorting. eLife 9, e61834 (2020).
Demas, J. et al. High-speed, cortex-wide volumetric recording of neuroactivity at cellular resolution using light beads microscopy. Nat. Methods 18, 1103–1111 (2021).
Grienberger, C. & Konnerth, A. Imaging calcium in neurons. Neuron 73, 862–885 (2012).
Ghosh, K. K. et al. Miniaturized integration of a fluorescence microscope. Nat. Methods 8, 871–878 (2011).
Aharoni, D., Khakh, B. S., Silva, A. J. & Golshani, P. All the light that we can see: a new era in miniaturized microscopy. Nat. Methods 16, 11–13 (2019).
Najafi, F. et al. Excitatory and inhibitory subnetworks are equally selective during decision-making and emerge simultaneously during learning. Neuron 105, 165–179.e8 (2020).
Huang, L. et al. Relationship between simultaneously recorded spiking activity and fluorescence signal in GCaMP6 transgenic mice. eLife 10, e51675 (2021).
Brennan, C. & Proekt, A. A quantitative model of conserved macroscopic dynamics predicts future motor commands. eLife 8, e46814 (2019).
Costa, A. C., Ahamed, T. & Stephens, G. J. Adaptive, locally linear models of complex dynamics. Proc. Natl Acad. Sci. USA 116, 1501–1510 (2019).
Linderman, S., Nichols, A., Blei, D., Zimmer, M. & Paninski, L. Hierarchical recurrent state space models reveal discrete and continuous dynamics of neural activity in C. elegans. Preprint at bioRxiv https://doi.org/10.1101/621540 (2019).
Fieseler, C., Zimmer, M. & Kutz, N. Unsupervised learning of control signals and their encodings in Caenorhabditis elegans whole-brain recordings. J. R. Soc. Interface 17, 20200459 (2020).
Venkatachalam, V. et al. Pan-neuronal imaging in roaming Caenorhabditis elegans. Proc. Natl Acad. Sci. USA 113, E1082–E1088 (2016).
Cong, L. et al. Rapid whole brain imaging of neural activity in freely behaving larval zebrafish (Danio rerio). eLife 6, e28158 (2017).
Kim, D. H. et al. Pan-neuronal calcium imaging with cellular resolution in freely swimming zebrafish. Nat. Methods 14, 1107–1114 (2017).
Symvoulidis, P. et al. NeuBtracker—imaging neurobehavioral dynamics in freely behaving fish. Nat. Methods 14, 1079–1082 (2017).
Yemini, E. et al. NeuroPAL: a multicolor atlas for whole-brain neuronal identification in C. elegans. Cell 184, 272–288.e11 (2021).
Machado, A. S., Darmohray, D. M., Fayad, J., Marques, H. G. & Carey, M. R. A quantitative framework for whole-body coordination reveals specific deficits in freely walking ataxic mice. eLife 4, e07892 (2015).
Mathis, M. W. & Mathis, A. Deep learning tools for the measurement of animal behavior in neuroscience. Curr. Opin. Neurobiol. 60, 1–11 (2020).
Pereira, T. D. et al. Fast animal pose estimation using deep neural networks. Nat. Methods 16, 117–125 (2019).
Berman, G. J., Choi, D. M., Bialek, W. & Shaevitz, J. W. Mapping the stereotyped behaviour of freely moving fruit flies. J. R. Soc. Interface 11, 20140672 (2014).
Batty, E. et al. BehaveNet: nonlinear embedding and Bayesian neural decoding of behavioral videos. in Advances in Neural Information Processing Systems 32 (eds. Wallach, H. et al.) 15680–15691 (Curran, 2019).
Calhoun, A. J., Pillow, J. W. & Murthy, M. Unsupervised identification of the internal states that shape natural behavior. Nat. Neurosci. 22, 2040–2049 (2019).
Carandini, M. & Churchland, A. K. Probing perceptual decisions in rodents. Nat. Neurosci. 16, 824–831 (2013).
The International Brain Laboratory et al. Standardized and reproducible measurement of decision-making in mice. eLife 10, e63711 (2021).
Dulac, C., O’Connell, L. A. & Wu, Z. Neural control of maternal and paternal behaviors. Science 345, 765–770 (2014).
Karigo, T. et al. Distinct hypothalamic control of same- and opposite-sex mounting behaviour in mice. Nature 589, 258–263 (2021).
Acknowledgements
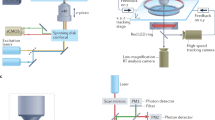
A.E.U. is supported by the German National Academy of Sciences Leopoldina and the International Brain Research Organization. B.D. is supported by National Institutes of Health (NIH) (grant no. 1U19NS107613-01, R01EB026953), Vannevar Bush faculty fellowship (no. N00014-18-1-2002) and the Simons Foundation Collaboration on the Global Brain. A.M.L. is supported by the National Institute of Neurological Disorders and Stroke of the NIH (under New Innovator award no. DP2NS116768), and Simons Foundation Award (no. SCGB 543003). A.K.C. is supported by the NIH (nos. R01EY022979 and R01EB026949) and the Simons Collaboration on the Global Brain. We thank N. Sofroniew for sharing the mesoscope image panel shown in the figure in Box 2, panel b, E. Trautman and K. Shenoy for the primate electrophysiology (Neuropixels) data in the figure in Box 2, panel c, and D. Maizels for graphic design. I. Stevenson, K. Svoboda, P. Rupprecht, A. Charles and G. Meijer suggested data points shown in Box 1, and J. Couto provided helpful comments on an earlier version of the manuscript. J. Tuthill provided insights on interpreting data from the fly connectome.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review information
Nature Neuroscience thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Urai, A.E., Doiron, B., Leifer, A.M. et al. Large-scale neural recordings call for new insights to link brain and behavior. Nat Neurosci 25, 11–19 (2022). https://doi.org/10.1038/s41593-021-00980-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41593-021-00980-9
This article is cited by
-
Random-access wide-field mesoscopy for centimetre-scale imaging of biodynamics with subcellular resolution
Nature Photonics (2024)
-
The influence of cortical activity on perception depends on behavioral state and sensory context
Nature Communications (2024)
-
Mesoscopic calcium imaging in a head-unrestrained male non-human primate using a lensless microscope
Nature Communications (2024)
-
Machine learning-based high-frequency neuronal spike reconstruction from low-frequency and low-sampling-rate recordings
Nature Communications (2024)
-
Real-time analysis of large-scale neuronal imaging enables closed-loop investigation of neural dynamics
Nature Neuroscience (2024)