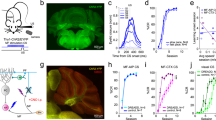
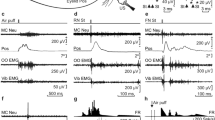
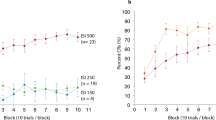
Abstract
Changes in behavioral state can profoundly influence brain function. Here we show that behavioral state modulates performance in delay eyeblink conditioning, a cerebellum-dependent form of associative learning. Increased locomotor speed in head-fixed mice drove earlier onset of learning and trial-by-trial enhancement of learned responses that were dissociable from changes in arousal and independent of sensory modality. Eyelid responses evoked by optogenetic stimulation of mossy fiber inputs to the cerebellum, but not at sites downstream, were positively modulated by ongoing locomotion. Substituting prolonged, low-intensity optogenetic mossy fiber stimulation for locomotion was sufficient to enhance conditioned responses. Our results suggest that locomotor activity modulates delay eyeblink conditioning through increased activation of the mossy fiber pathway within the cerebellum. Taken together, these results provide evidence for a novel role for behavioral state modulation in associative learning and suggest a potential mechanism through which engaging in movement can improve an individual’s ability to learn.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Vinck, M., Batista-Brito, R., Knoblich, U. & Cardin, J. A. Arousal and locomotion make distinct contributions to cortical activity patterns and visual encoding. Neuron 86, 740–754 (2015).
Niell, C. M. & Stryker, M. P. Modulation of visual responses by behavioral state in mouse visual cortex. Neuron 65, 472–479 (2010).
Bennett, C., Arroyo, S. & Hestrin, S. Subthreshold mechanisms underlying state-dependent modulation of visual responses. Neuron 80, 350–357 (2013).
Schneider, D. M., Nelson, A. & Mooney, R. A synaptic and circuit basis for corollary discharge in the auditory cortex. Nature 513, 189–194 (2014).
Zhou, M. et al. Scaling down of balanced excitation and inhibition by active behavioral states in auditory cortex. Nat. Neurosci. 17, 841–850 (2014).
Williamson, R. S., Hancock, K. E., Shinn-Cunningham, B. G. & Polley, D. B. Locomotion and task demands differentially modulate thalamic audiovisual processing during active search. Curr. Biol. 25, 1885–1891 (2015).
Ayaz, A., Saleem, A. B., Schölvinck, M. L. & Carandini, M. Locomotion controls spatial integration in mouse visual cortex. Curr. Biol. 23, 890–894 (2013).
McGinley, M. J. et al. Waking State: Rapid Variations Modulate Neural and Behavioral Responses. Neuron 87, 1143–1161 (2015).
Ozden, I., Dombeck, D. A., Hoogland, T. M., Tank, D. W. & Wang, S. S. H. Widespread state-dependent shifts in cerebellar activity in locomoting mice. PLoS One 7, e42650 (2012).
Powell, K., Mathy, A., Duguid, I. & Häusser, M. Synaptic representation of locomotion in single cerebellar granule cells. eLife 4, e07290 (2015).
Hoogland, T. M., De Gruijl, J. R., Witter, L., Canto, C. B. & De Zeeuw, C. I. Role of Synchronous Activation of Cerebellar Purkinje Cell Ensembles in Multi-joint Movement Control. Curr. Biol. 25, 1157–1165 (2015).
Ghosh, K. K. et al. Miniaturized integration of a fluorescence microscope. Nat. Methods 8, 871–878 (2011).
Medina, J. F., Nores, W. L., Ohyama, T. & Mauk, M. D. Mechanisms of cerebellar learning suggested by eyelid conditioning. Curr. Opin. Neurobiol. 10, 717–724 (2000).
Gormezano, I., Kehoe, E. J. & Marshall, B. S. Twenty years of classical conditioning research with the rabbit. Prog. Psychobiol. Physiol. Psychol. 10, 197–267 (1983).
Kim, J. J. & Thompson, R. F. Cerebellar circuits and synaptic mechanisms involved in classical eyeblink conditioning. Trends Neurosci. 20, 177–181 (1997).
De Zeeuw, C. I. & Yeo, C. H. Time and tide in cerebellar memory formation. Curr. Opin. Neurobiol. 15, 667–674 (2005).
Carey, M. R. Synaptic mechanisms of sensorimotor learning in the cerebellum. Curr. Opin. Neurobiol. 21, 609–615 (2011).
McCormick, D. A. & Thompson, R. F. Cerebellum: essential involvement in the classically conditioned eyelid response. Science 223, 296–299 (1984).
Steinmetz, J. E. Brain substrates of classical eyeblink conditioning: a highly localized but also distributed system. Behav. Brain Res. 110, 13–24 (2000).
Gentet, L. J., Avermann, M., Matyas, F., Staiger, J. F. & Petersen, C. C. H. Membrane potential dynamics of GABAergic neurons in the barrel cortex of behaving mice. Neuron 65, 422–435 (2010).
McGinley, M. J., David, S. V. & McCormick, D. A. Cortical membrane potential signature of optimal states for sensory signal detection. Neuron 87, 179–192 (2015).
Reimer, J. et al. Pupil fluctuations track fast switching of cortical states during quiet wakefulness. Neuron 84, 355–362 (2014).
Reimer, J. et al. Pupil fluctuations track rapid changes in adrenergic and cholinergic activity in cortex. Nat. Commun. 7, 13289 (2016).
Joshi, S., Li, Y., Kalwani, R. M. & Gold, J. I. Relationships between Pupil Diameter and Neuronal Activity in the Locus Coeruleus, Colliculi, and Cingulate Cortex. Neuron 89, 221–234 (2016).
Kloosterman, N. A. et al. Pupil size tracks perceptual content and surprise. Eur. J. Neurosci. 41, 1068–1078 (2015).
Steinmetz, J. E., Rosen, D. J., Chapman, P. F., Lavond, D. G. & Thompson, R. F. Classical conditioning of the rabbit eyelid response with a mossy-fiber stimulation CS: I. Pontine nuclei and middle cerebellar peduncle stimulation. Behav. Neurosci. 100, 878–887 (1986).
Hull, C. & Regehr, W. G. Identification of an inhibitory circuit that regulates cerebellar Golgi cell activity. Neuron 73, 149–158 (2012).
Osborne, L. C., Lisberger, S. G. & Bialek, W. A sensory source for motor variation. Nature 437, 412–416 (2005).
Gao, Z. et al. Excitatory Cerebellar Nucleocortical Circuit Provides Internal Amplification during Associative Conditioning. Neuron 89, 645–657 (2016).
Ishikawa, T., Shimuta, M. & Häusser, M. Multimodal sensory integration in single cerebellar granule cells in vivo. eLife 4, e12916 (2015).
Sawtell, N. B. Multimodal integration in granule cells as a basis for associative plasticity and sensory prediction in a cerebellum-like circuit. Neuron 66, 573–584 (2010).
Huang, C.-C. et al. Convergence of pontine and proprioceptive streams onto multimodal cerebellar granule cells. eLife 2, e00400 (2013).
Jörntell, H. & Ekerot, C.-F. Properties of somatosensory synaptic integration in cerebellar granule cells in vivo. J. Neurosci. 26, 11786–11797 (2006).
Koekkoek, S. K. E., Den Ouden, W. L., Perry, G., Highstein, S. M. & De Zeeuw, C. I. Monitoring kinetic and frequency-domain properties of eyelid responses in mice with magnetic distance measurement technique. J. Neurophysiol. 88, 2124–2133 (2002).
Boele, H. J., Koekkoek, S. K. E. & De Zeeuw, C. I. Cerebellar and extracerebellar involvement in mouse eyeblink conditioning: the ACDC model. Front. Cell. Neurosci. 3, 19 (2010).
Meijer, J. H. & Robbers, Y. Wheel running in the wild. Proc. Biol. Sci. 281, 20140210 (2014).
Aston-Jones, G. & Cohen, J. D. An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annu. Rev. Neurosci. 28, 403–450 (2005).
Carey, M. R. & Regehr, W. G. Noradrenergic control of associative synaptic plasticity by selective modulation of instructive signals. Neuron 62, 112–122 (2009).
Dieudonné, S. Serotonergic neuromodulation in the cerebellar cortex: cellular, synaptic, and molecular basis. Neuroscientist 7, 207–219 (2001).
Olson, L. & Fuxe, K. On the projections from the locus coeruleus noradrealine neurons: the cerebellar innervation. Brain Res. 28, 165–171 (1971).
Bloom, F. E., Hoffer, B. J. & Siggins, G. R. Studies on norepinephrine-containing afferents to Purkinje cells of art cerebellum. I. Localization of the fibers and their synapses. Brain Res. 25, 501–521 (1971).
Paukert, M. et al. Norepinephrine controls astroglial responsiveness to local circuit activity. Neuron 82, 1263–1270 (2014).
Martins, A. R. O. & Froemke, R. C. Coordinated forms of noradrenergic plasticity in the locus coeruleus and primary auditory cortex. Nat. Neurosci. 18, 1483–1492 (2015).
Ciocchi, S., Passecker, J., Malagon-Vina, H., Mikus, N. & Klausberger, T. Brain computation. Selective information routing by ventral hippocampal CA1 projection neurons. Science 348, 560–563 (2015).
Jayaprakash, N. et al. Optogenetic Interrogation of Functional Synapse Formation by Corticospinal Tract Axons in the Injured Spinal Cord. J. Neurosci. 36, 5877–5890 (2016).
Tye, K. M. et al. Amygdala circuitry mediating reversible and bidirectional control of anxiety. Nature 471, 358–362 (2011).
Bengtsson, F. & Jörntell, H. Sensory transmission in cerebellar granule cells relies on similarly coded mossy fiber inputs. Proc. Natl. Acad. Sci. USA 106, 2389–2394 (2009).
ten Brinke, M. M. et al. Evolving Models of Pavlovian Conditioning: Cerebellar Cortical Dynamics in Awake Behaving Mice. Cell Rep. 13, 1977–1988 (2015).
Chabrol, F. P., Arenz, A., Wiechert, M. T., Margrie, T. W. & DiGregorio, D. A. Synaptic diversity enables temporal coding of coincident multisensory inputs in single neurons. Nat. Neurosci. 18, 718–727 (2015).
Edgley, S. A. & Lidierth, M. The discharges of cerebellar Golgi cells during locomotion in the cat. J. Physiol. (Lond.) 392, 315–332 (1987).
Arenkiel, B. R. et al. In vivo light-induced activation of neural circuitry in transgenic mice expressing channelrhodopsin-2. Neuron 54, 205–218 (2007).
Barski, J. J., Dethleffsen, K. & Meyer, M. Cre recombinase expression in cerebellar Purkinje cells. Genesis 28, 93–98 (2000).
Madisen, L. et al. A toolbox of Cre-dependent optogenetic transgenic mice for light-induced activation and silencing. Nat. Neurosci. 15, 793–802 (2012).
Fünfschilling, U. & Reichardt, L. F. Cre-mediated recombination in rhombic lip derivatives. Genesis 33, 160–169 (2002).
Carey, M. R. et al. Presynaptic CB1 receptors regulate synaptic plasticity at cerebellar parallel fiber synapses. J. Neurophysiol. 105, 958–963 (2011).
Heiney, S. A., Kim, J., Augustine, G. J. & Medina, J. F. Precise control of movement kinematics by optogenetic inhibition of Purkinje cell activity. J. Neurosci. 34, 2321–2330 (2014).
Mostofi, A., Holtzman, T., Grout, A. S., Yeo, C. H. & Edgley, S. A. Electrophysiological localization of eyeblink-related microzones in rabbit cerebellar cortex. J. Neurosci. 30, 8920–8934 (2010).
Van Der Giessen, R. S. et al. Role of olivary electrical coupling in cerebellar motor learning. Neuron 58, 599–612 (2008).
Steinmetz, A. B. & Freeman, J. H. Localization of the cerebellar cortical zone mediating acquisition of eyeblink conditioning in rats. Neurobiol. Learn. Mem. 114, 148–154 (2014).
Yeo, C. H., Hardiman, M. J. & Glickstein, M. Classical conditioning of the nictitating membrane response of the rabbit. I. Lesions of the cerebellar nuclei. Exp. Brain Res. 60, 87–98 (1985).
Ohmae, S. & Medina, J. F. Climbing fibers encode a temporal-difference prediction error during cerebellar learning in mice. Nat. Neurosci. 18, 1798–1803 (2015).
Chettih, S. N., McDougle, S. D., Ruffolo, L. I. & Medina, J. F. Adaptive timing of motor output in the mouse: the role of movement oscillations in eyelid conditioning. Front. Integr. Neurosci. 5, 72 (2011).
Heiney, S. A., Wohl, M. P., Chettih, S. N., Ruffolo, L. I. & Medina, J. F. Cerebellar-dependent expression of motor learning during eyeblink conditioning in head-fixed mice. J. Neurosci. 34, 14845–14853 (2014).
Machado, A. S., Darmohray, D. M., Fayad, J., Marques, H. G. & Carey, M. R. A quantitative framework for whole-body coordination reveals specific deficits in freely walking ataxic mice. eLife 4, e07892 (2015).
Lee, K. H. et al. Circuit mechanisms underlying motor memory formation in the cerebellum. Neuron 86, 529–540 (2015).
Siegle, J. H., Hale, G. J., Newman, J. P. & Voigts, J. Neural ensemble communities: open-source approaches to hardware for large-scale electrophysiology. Curr. Opin. Neurobiol. 32, 53–59 (2015).
Lopes, G. et al. Bonsai: an event-based framework for processing and controlling data streams. Front. Neuroinform. 9, 7 (2015).
Van Dijck, G. et al. Probabilistic identification of cerebellar cortical neurones across species. PLoS One 8, e57669 (2013).
Acknowledgements
We thank T. Pritchett for technical assistance and P. Francisco for help on some of the auditory CS experiments. We thank G. Costa for illustrations and the Champalimaud Research Hardware Platform for technical support. We thank J. Fayad and M. Orger for advice on data analysis. We are grateful to the Carey lab and the members of the Champalimaud Neuroscience Program for helpful discussions throughout the project and in particular to H. Marques and J. Jacobs for comments on the manuscript. This work was supported by a Howard Hughes Medical Institute International Early Career Scientist Grant #55007413 (to M.R.C.), Bial Foundation Bursary #74/14 (to D.L.P.), fellowships from the Portuguese Fundação para a Ciência e a Tecnologia #BD77686/2011 (to C.A.) and #BPD109659/2015 (to D.L.P.), and European Research Council Starting Grant #640093 (to M.R.C.).
Author information
Authors and Affiliations
Contributions
C.A. and M.R.C. designed the research plan. C.A. and N.T.S. performed all experiments. C.A. and D.L.P. performed electrophysiological recordings. D.L.P. analyzed electrophysiology data. C.A. analyzed all data and prepared figures. C.A. and M.R.C. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Integrated supplementary information
Supplementary Figure 1 Rate and extent of learning depend on running speed.
A. Amplitude acquisition curve for one representative animal (gray line) fitted with a sigmoid (gray line). B. Slope of sigmoid fits of the acquisition curve for each animal. The magenta line is a linear fit (N=28, slope = 4.515, **p = 0.0017). C. Plateau of sigmoid fit for each animal. The magenta line is a linear fit (N=28, slope = 0.8, *p = 0.0376). D. As above, for fast (magenta) and slow (cyan) motorized treadmill. E. Slope of sigmoid fits of the acquisition curve for animals from each speed group superimposed on the self-paced treadmill data (grey) from (B). F. Plateau of sigmoid fit for animals from each speed group superimposed on the self-paced treadmill data (grey) from (C).
Supplementary Figure 2 Influence of arousal vs. locomotor activity on CR amplitude.
A. Relationship between pupil size and walking speed for all trials from all training sessions using a whisker (in yellow) or a tone (in red) CS. Line is average across animals; shadow indicates SEM. There was a significant positive relationship for both whisker (mixed ANOVA, n=40307 trials, N=25 animals, F(1,214.5) = 140.75, ***p = 4.82e-24) and tone (n=27204 trials, N=16 animals, F(1,130) = 127.3, ***p = 5.24e-21). B. Relationship between CR amplitude and pupil size for all CR's to a whisker or a tone CS. Line is average across animals; shadow indicates SEM. No correlation was found for either whisker (one-way ANOVA on LME, F(1,225) = 0.203, p = 0.6524) or tone (one-way ANOVA on LME, F(1,162) = 0.1736, p = 0.6775) CS. C,D. Analyzing the effects of locomotor activity separately for trials with high arousal (pupil > 0.85) and low arousal (pupil < 0.85) for a whisker CS (C, mixed ANOVA, high arousal: n=6277 trials, N=25 animals, F(1,154.9) =8.64, p < 0.01; low arousal: n=9291 trials, N=25 animals, F(1,105.8) = 7.03, **p < 0.01) and a tone CS (D, mixed ANOVA, high arousal: n=3462 trials, N=16 animals, F(1,76.6) = 22.5, ***p < 0.001; low arousal: n=6089 trials, N=16 animals, F(1,66) = 42.75, ***p < 0.001). E,F. Analyzing the effects of arousal separately for trials with higher (speed > 0.15m/s) and lower walking speed (speed < 0.15m/s) for a whisker CS (E, mixed ANOVA, high speed: n=3781 trials, N=25 animals, F(1,170.9) = 1.66, p = 0.20; low speed: n=12003 trials, N=25 animals, F(1,269.1) = 20.25, ***p < 0.001) and a tone CS (F, mixed ANOVA, high speed: n=1545 trials, N=16 animals, F(1,112.35) = 0.21, p = 0.65; low speed: n=8276 trials, N=16 animals, F(1,155.63) = 9.30, **p < 0.01). Histograms under each plot represent the number of animals in each bin for each group. G. Analyzing the effects of arousal (as measured by pupil size) for trials when the animals were still, on CR's evoked by a whisker (green) or a tone (red) CS. Line is average across animals; shadow indicates SEM. There was a significant negative relationship between pupil size and CR amplitude for both whisker (mixed ANOVA, n=9223 trials, N=25 animals, F(1,257.8) = 35.4, ***p < 0.0001) and tone (n=6930 trials, N=16 animals, F(1,191.3) = 22.4, ***p < 0.0001).
Supplementary Figure 3 Fiber optic placement for optogenetic experiments.
Representative histological samples indicating fiber placements for the optogenetic manipulations described in Figs. 4, 5, and 6. Green fluorescence indicates YFP (ChR2) expression. Dashed white circles indicate the site where the DiI coated optic fiber was implanted. Bottom two rows: Colored circles represent sites of implanted optical fibers from additional animals. A. MF-ChR2-ctx: Optical fibers were placed in an eyelid region of the cerebellar cortex of Thy1-ChR2-YFP mice expressing ChR2:YFP in a subset of mossy fibers. B. gc-ChR2-ctx: Optical fibers were placed in an eyelid region of the cerebellar cortex of Gabra6cre-ChR2-YFP mice in which ChR2:YFP was targeted to cerebellar granule cells. C. Pkj-ChR2-ctx: Optical fibers were placed in an eyelid region of the cerebellar cortex of L7cre-ChR2-YFP mice expressing ChR2:YFP in Purkinje cells. D. MF-ChR2-AIP: Optical fibers were placed in the anterior interpositus nucleus of Thy1-ChR2-YFP mice. IntA, interposed cerebellar nucleus, anterior part. Lat, lateral cerebellar nucleus. Med, medial cerebellar nucleus.
Supplementary Figure 4 Cell-type-specific ChR2-YFP expression.
Images at different magnifications from MF-ChR2-YFP, gc-ChR2-YFP and Pkj-ChR2-YFP lines demonstrating cell-type specific YFP/ChR2 expression in each line. Green fluorescence indicates YFP (ChR2) expression. A. Fluorescence images of parasagittal cerebellar sections of a Thy1-ChR2-YFP mouse expressing ChR2:YFP in a subset of mossy fibers and their terminals. Center, specific expression in MF terminals within the cerebellar cortex. Right, MF terminals labeled within the AIP. B. Fluorescence images of parasagittal cerebellar sections of a Gabra6Cre-ChR2-YFP mouse indicating ChR2:YFP expression selectively in granule cells (gc-ChR2). C. Fluorescence images of parasagittal cerebellar sections of a L7Cre-ChR2-YFP mouse expressing ChR2:YFP selectively in Purkinje cells.
Supplementary Figure 5 Photostimulation controls.
A. gc-ChR2-AIP: Fluorescence image of a coronal cerebellar slice of a Gabra6cre-ChR2-YFP mouse expressing ChR2:YFP in cerebellar granule cells. The Dil coated optical fibers were placed in the anterior interpositus nucleus (AIP). IntA, interposed cerebellar nucleus, anterior part. Lat, lateral cerebellar nucleus. Med, medial cerebellar nucleus. B. Eyelid movements in response to moderate to high intensity laser stimulation (473nm light pulses at 100Hz for 50ms) in Gabra6cre-ChR2-YFP mice expressing ChR2:YFP in cerebellar granule cells. Laser-driven responses from mice with the fiber implanted in the anterior interpositus nucleus (gray, N=5) are shown. For comparison, data from the same mouse line, but the fiber implanted in cerebellar cortex, is replotted (green) from Fig. 5E. Duration of laser stimulation is indicated by the horizontal black line. C. Eyelid responses to high intensity laser stimulation (473nm light pulses at 100Hz for 50ms) in wild-type mice (N=4, all superimposed) with an optic fiber implanted in an identified eyelid-related region of cerebellar cortex.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–5
Supplementary Video 1
Head-fixed mouse running on a motorized treadmill during an eyeblink conditioning session with a visual CS and an airpuff US.
Rights and permissions
About this article
Cite this article
Albergaria, C., Silva, N.T., Pritchett, D.L. et al. Locomotor activity modulates associative learning in mouse cerebellum. Nat Neurosci 21, 725–735 (2018). https://doi.org/10.1038/s41593-018-0129-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41593-018-0129-x
This article is cited by
-
Climbing fibers provide essential instructive signals for associative learning
Nature Neuroscience (2024)
-
Local synaptic inhibition mediates cerebellar granule cell pattern separation and enables learned sensorimotor associations
Nature Neuroscience (2024)
-
Cerebellar associative learning underlies skilled reach adaptation
Nature Neuroscience (2023)
-
Locomotion modulates olfactory learning through proprioception in C. elegans
Nature Communications (2023)
-
Synaptic mechanisms for associative learning in the cerebellar nuclei
Nature Communications (2023)