Abstract
Stimuli that elicit itch are detected by sensory neurons that innervate the skin. This information is processed by the spinal cord; however, the way in which this occurs is still poorly understood. Here we investigated the neuronal pathways for itch neurotransmission, particularly the contribution of the neuropeptide somatostatin. We find that in the periphery, somatostatin is exclusively expressed in Nppb+ neurons, and we demonstrate that Nppb+somatostatin+ cells function as pruriceptors. Employing chemogenetics, pharmacology and cell-specific ablation methods, we demonstrate that somatostatin potentiates itch by inhibiting inhibitory dynorphin neurons, which results in disinhibition of GRPR+ neurons. Furthermore, elimination of somatostatin from primary afferents and/or from spinal interneurons demonstrates differential involvement of the peptide released from these sources in itch and pain. Our results define the neural circuit underlying somatostatin-induced itch and characterize a contrasting antinociceptive role for the peptide.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Change history
19 April 2018
In the version of this article initially published online, the labels were switched for the right-hand pair of bars in Fig. 4e. The left one of the two should be Chloroquine + veh, the right one Chloroquine + CNO. The error has been corrected in the print, HTML and PDF versions of the article.
References
Le Pichon, C. E. & Chesler, A. T. The functional and anatomical dissection of somatosensory subpopulations using mouse genetics. Front. Neuroanat. 8, 21 (2014).
Han, L. et al. A subpopulation of nociceptors specifically linked to itch. Nat. Neurosci. 16, 174–182 (2013).
Mishra, S. K. & Hoon, M. A. The cells and circuitry for itch responses in mice. Science 340, 968–971 (2013).
Sun, Y. G. & Chen, Z. F. A gastrin-releasing peptide receptor mediates the itch sensation in the spinal cord. Nature 448, 700–703 (2007).
Sun, Y. G. et al. Cellular basis of itch sensation. Science 325, 1531–1534 (2009).
Braz, J., Solorzano, C., Wang, X. & Basbaum, A. I. Transmitting pain and itch messages: a contemporary view of the spinal cord circuits that generate gate control. Neuron 82, 522–536 (2014).
Abraira, V. E. et al. The cellular and synaptic architecture of the mechanosensory dorsal horn. Cell 168, 295–310.e19 (2017).
Cui, L. et al. Identification of early RET + deep dorsal spinal cord interneurons in gating pain. Neuron 91, 1413 (2016).
Duan, B. et al. Identification of spinal circuits transmitting and gating mechanical pain. Cell 159, 1417–1432 (2014).
Peirs, C. et al. Dorsal horn circuits for persistent mechanical pain. Neuron 87, 797–812 (2015).
Petitjean, H. et al. Dorsal horn parvalbumin neurons are gate-keepers of touch-evoked pain after nerve injury. Cell Rep. 13, 1246–1257 (2015).
Ross, S. E. et al. Loss of inhibitory interneurons in the dorsal spinal cord and elevated itch in Bhlhb5 mutant mice. Neuron 65, 886–898 (2010).
Boyle, K. A. et al. A quantitative study of neurochemically defined populations of inhibitory interneurons in the superficial dorsal horn of the mouse spinal cord. Neuroscience 363, 120–133 (2017).
Bröhl, D. et al. A transcriptional network coordinately determines transmitter and peptidergic fate in the dorsal spinal cord. Dev. Biol. 322, 381–393 (2008).
Kardon, A. P. et al. Dynorphin acts as a neuromodulator to inhibit itch in the dorsal horn of the spinal cord. Neuron 82, 573–586 (2014).
Hökfelt, T. et al. Immunohistochemical evidence for separate populations of somatostatin-containing and substance P-containing primary afferent neurons in the rat. Neuroscience 1, 131–136 (1976).
Li, C. L. et al. Somatosensory neuron types identified by high-coverage single-cell RNA-sequencing and functional heterogeneity. Cell Res. 26, 83–102 (2016).
Usoskin, D. et al. Unbiased classification of sensory neuron types by large-scale single-cell RNA sequencing. Nat. Neurosci. 18, 145–153 (2015).
Stantcheva, K. K. et al. A subpopulation of itch-sensing neurons marked by Ret and somatostatin expression. EMBO Rep. 17, 585–600 (2016).
Carlton, S. M., Du, J., Zhou, S. & Coggeshall, R. E. Tonic control of peripheral cutaneous nociceptors by somatostatin receptors. J. Neurosci. 21, 4042–4049 (2001).
Carlton, S. M. et al. Somatostatin modulates the transient receptor potential vanilloid 1 (TRPV1) ion channel. Pain 110, 616–627 (2004).
Seybold, V. S., Hylden, J. L. & Wilcox, G. L. Intrathecal substance P and somatostatin in rats: behaviors indicative of sensation. Peptides 3, 49–54 (1982).
Wiesenfeld-Hallin, Z. Intrathecal somatostatin modulates spinal sensory and reflex mechanisms: behavioral and electrophysiological studies in the rat. Neurosci. Lett. 62, 69–74 (1985).
Chapman, V. & Dickenson, A. H. The effects of sandostatin and somatostatin on nociceptive transmission in the dorsal horn of the rat spinal cord. Neuropeptides 23, 147–152 (1992).
Gutierrez-Mecinas, M., Furuta, T., Watanabe, M. & Todd, A. J. A quantitative study of neurochemically defined excitatory interneuron populations in laminae I-III of the mouse spinal cord. Mol. Pain 12, 1744806916629065 (2016).
Christensen, A. J. et al. In vivo interrogation of spinal mechanosensory circuits. Cell Rep 17, 1699–1710 (2016).
Hökfelt, T. Neuropeptides in perspective: the last ten years. Neuron 7, 867–879 (1991).
Mishra, S. K., Holzman, S. & Hoon, M. A. A nociceptive signaling role for neuromedin B. J. Neurosci. 32, 8686–8695 (2012).
Nguyen, M. Q., Wu, Y., Bonilla, L. S., von Buchholtz, L. J. & Ryba, N. J. P. Diversity amongst trigeminal neurons revealed by high throughput single cell sequencing. PLoS One 12, e0185543 (2017).
Armbruster, B. N., Li, X., Pausch, M. H., Herlitze, S. & Roth, B. L. Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proc. Natl. Acad. Sci. USA 104, 5163–5168 (2007).
Foster, E. et al. Targeted ablation, silencing, and activation establish glycinergic dorsal horn neurons as key components of a spinal gate for pain and itch. Neuron 85, 1289–1304 (2015).
Polgár, E., Durrieux, C., Hughes, D. I. & Todd, A. J. A quantitative study of inhibitory interneurons in laminae I-III of the mouse spinal dorsal horn. PLoS One 8, e78309 (2013).
Lu, Y. et al. A feed-forward spinal cord glycinergic neural circuit gates mechanical allodynia. J. Clin. Invest. 123, 4050–4062 (2013).
Li, L. et al. The functional organization of cutaneous low-threshold mechanosensory neurons. Cell 147, 1615–1627 (2011).
Bell, A. M., Gutierrez-Mecinas, M., Polgár, E. & Todd, A. J. Spinal neurons that contain gastrin-releasing peptide seldom express Fos or phosphorylate extracellular signal-regulated kinases in response to intradermal chloroquine. Mol. Pain 12, 1744806916649602 (2016).
Todd, A. J. et al. The expression of vesicular glutamate transporters VGLUT1 and VGLUT2 in neurochemically defined axonal populations in the rat spinal cord with emphasis on the dorsal horn. Eur. J. Neurosci. 17, 13–27 (2003).
Zeyda, T., Diehl, N., Paylor, R., Brennan, M. B. & Hochgeschwender, U. Impairment in motor learning of somatostatin null mutant mice. Brain Res. 906, 107–114 (2001).
Yasaka, T., Tiong, S. Y., Hughes, D. I., Riddell, J. S. & Todd, A. J. Populations of inhibitory and excitatory interneurons in lamina II of the adult rat spinal dorsal horn revealed by a combined electrophysiological and anatomical approach. Pain 151, 475–488 (2010).
Chrubasik, J. et al. Somatostatin, a potent analgesic. Lancet 2, 1208–1209 (1984).
Taurà, P. et al. Epidural somatostatin as an analgesic in upper abdominal surgery: a double-blind study. Pain 59, 135–140 (1994).
Bencivinni, I., Ferrini, F., Salio, C., Beltramo, M. & Merighi, A. The somatostatin analogue octreotide inhibits capsaicin-mediated activation of nociceptive primary afferent fibres in spinal cord lamina II (substantia gelatinosa). Eur. J. Pain 15, 591–599 (2011).
Akiyama, T., Iodi Carstens, M. & Carstens, E. Transmitters and pathways mediating inhibition of spinal itch-signaling neurons by scratching and other counterstimuli. PLoS One 6, e22665 (2011).
Cevikbas, F. et al. Synergistic antipruritic effects of gamma aminobutyric acid A and B agonists in a mouse model of atopic dermatitis. J. Allergy Clin. Immunol. 140, 454–464.e2 (2017).
Simmons, D. R., Spike, R. C. & Todd, A. J. Galanin is contained in GABAergic neurons in the rat spinal dorsal horn. Neurosci. Lett. 187, 119–122 (1995).
Gutierrez-Mecinas, M., Watanabe, M. & Todd, A. J. Expression of gastrin-releasing peptide by excitatory interneurons in the mouse superficial dorsal horn. Mol. Pain 10, 79 (2014).
Sun, S. et al. Leaky gate model: intensity-dependent coding of pain and itch in the spinal cord. Neuron 93, 840–853.e5 (2017).
Solorzano, C. et al. Primary afferent and spinal cord expression of gastrin-releasing peptide: message, protein, and antibody concerns. J. Neurosci. 35, 648–657 (2015).
Chiu, I. M. et al. Transcriptional profiling at whole population and single cell levels reveals somatosensory neuron molecular diversity. eLife 3, e04660 (2014).
Bautista, D. M., Wilson, S. R. & Hoon, M. A. Why we scratch an itch: the molecules, cells and circuits of itch. Nat. Neurosci. 17, 175–182 (2014).
Taniguchi, H. et al. A resource of Cre driver lines for genetic targeting of GABAergic neurons in cerebral cortex. Neuron 71, 995–1013 (2011).
Madisen, L. et al. A toolbox of Cre-dependent optogenetic transgenic mice for light-induced activation and silencing. Nat. Neurosci. 15, 793–802 (2012).
Madisen, L. et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat. Neurosci. 13, 133–140 (2010).
Krashes, M. J. et al. An excitatory paraventricular nucleus to AgRP neuron circuit that drives hunger. Nature 507, 238–242 (2014).
Vasyutina, E. et al. RBP-J (Rbpsuh) is essential to maintain muscle progenitor cells and to generate satellite cells. Proc. Natl. Acad. Sci. USA 104, 4443–4448 (2007).
Mishra, S. K., Tisel, S. M., Orestes, P., Bhangoo, S. K. & Hoon, M. A. TRPV1-lineage neurons are required for thermal sensation. EMBO J. 30, 582–593 (2011).
Danielian, P. S., Muccino, D., Rowitch, D. H., Michael, S. K. & McMahon, A. P. Modification of gene activity in mouse embryos in utero by a tamoxifen-inducible form of Cre recombinase. Curr. Biol. 8, 1323–1326 (1998).
Chaplan, S. R., Bach, F. W., Pogrel, J. W., Chung, J. M. & Yaksh, T. L. Quantitative assessment of tactile allodynia in the rat paw. J. Neurosci. Methods 53, 55–63 (1994).
Roberson, D. P. et al. Activity-dependent silencing reveals functionally distinct itch-generating sensory neurons. Nat. Neurosci. 16, 910–918 (2013).
Hylden, J. L. & Wilcox, G. L. Intrathecal morphine in mice: a new technique. Eur. J. Pharmacol. 67, 313–316 (1980).
Roth, B. L. DREADDs for neuroscientists. Neuron 89, 683–694 (2016).
Gutierrez-Mecinas, M. et al. Preprotachykinin A is expressed by a distinct population of excitatory neurons in the mouse superficial spinal dorsal horn including cells that respond to noxious and pruritic stimuli. Pain 158, 440–456 (2017).
Dressler, G. R. & Douglass, E. C. Pax-2 is a DNA-binding protein expressed in embryonic kidney and Wilms tumor. Proc. Natl. Acad. Sci. USA 89, 1179–1183 (1992).
Lee, T., Kaneko, T., Taki, K. & Mizuno, N. Preprodynorphin-, preproenkephalin-, and preprotachykinin-expressing neurons in the rat neostriatum: an analysis by immunocytochemistry and retrograde tracing. J. Comp. Neurol. 386, 229–244 (1997).
Mullen, R. J., Buck, C. R. & Smith, A. M. NeuN, a neuronal specific nuclear protein in vertebrates. Development 116, 201–211 (1992).
Proudlock, F., Spike, R. C. & Todd, A. J. Immunocytochemical study of somatostatin, neurotensin, GABA, and glycine in rat spinal dorsal horn. J. Comp. Neurol. 327, 289–297 (1993).
Acknowledgements
We thank the NIDCR Gene targeting facility for help generating chimeric Sstf/f mice. We also thank C. Birchmeier, Max Delbrück Center, Berlin; M. Krashes, NIH, Bethesda; and Q. Ma, Dana-Faber Cancer Institute, Boston, for generously providing mice. We are very grateful to T. Furuta, Kyoto University, Kyoto, for the gift of PPD antibody, to A. Bell and D. Hughes for comments on the manuscript, to X. Gu for help in some of the experiments and to R. Kerr and C. Watt for expert technical help. This work was supported by the intramural research program of the National Institute of Dental and Craniofacial Research (NIDCR)-National Institutes of Health (MAH) and grants from the Medical Research Council (MR/L003430/1), the Biotechnology and Biological Sciences Research Council (grant N006119), the Wellcome Trust (102645) (A.J.T.), the Swiss National Science Foundation (156393) (H.U.Z.) and the Natural Science Foundation of China (31671247) (J.H.). The Sstf/f mice used in this study were generated from ES cells obtained from the National Center for Research Resources (NCRR)-NIH-supported KOMP repository and engineered by the Welcome Trust Sanger Institute and the Mouse Biology Program.
Author information
Authors and Affiliations
Contributions
J.H., E.P., J.S.R., A.J.T. and M.A.H. designed the experiments. J.H., E.P., S.K.M., H.J.S., P.-Y. T., M.K., N.I., K.A.B. and A.C.D. performed experiments. H.W. and H.U.Z. provided assistance and M.W. provided reagents. A.J.T. and M.A.H. wrote and edited the paper, with comments from all other authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Integrated supplementary information
Supplementary Figure 1 ISH analysis of the expression of Nmb, Gal, Tac1, Calca, Nppb and Sst in the DRG.
Triple label in situ hybridization was used to assess the numbers of neurons expressing genes for the neuropeptides: A, neuromedin B (Nmb) and galanin (Gal); B, tachykinin 1 (Tac1, substance P) and calcitonin gene related peptide (Calca); C, natriuretic polypeptide b (Nppb) and somatostatin (Sst) relative to the pan-neuronal gene β3-tubulin (Tubb3). The fractions of neuropeptide expressing neurons were determined relative to Tubb3-positive cells, D. Data are means ±SEM (n=3 animals, 3 sections per mouse). Mean cell counts for each labeling experiment were, Nmb 855, Gal 215, and Tubb3 1400; Tac1 457, Calca 476, and Tubb3 1006; Nppb 92, Sst 89, and Tubb3 1059.
Supplementary Figure 2 Sstcre neurons innervate the skin and can be reliably activated using optogenetics.
A, double label ISH reveals that in SstCre;Ai9 mice expression of tdTomato (tdT, magenta) occurs largely in Nppb-neurons (green). White arrows indicate Nppb-expressing neurons not expressing tdTomato, and the grey arrow indicates a tdTomato-positive neurons that does not express Nppb. Similar results were obtained from 3 mice. B, Somatostain-neurons innervate superficial layers of the skin. A section through the ear of a Sstcre;Ai9 mouse reveals tdTomato fibers (red) that are close to the surface of the skin (image is superimposed on a DIC-image to highlight the location of fibers); also see1,2 for evidence of cutaneous segregation of somatostatin-expressing afferents. C, Neurons from Sstcre;Ai32 mice can be optogenetically activated with a variety of frequencies of light stimulation. Cultured DRG-neurons from Sstcre;Ai32 were stimulated at 1, 5, 20, and 40 Hz and electrophysiological responses recorded under current (left panel), or voltage clamp (right panel) conditions. n=11/11 neurons responding, all neurons exhibited non-saturating responses up to 20Hz. 1. Molander, C., Ygge, J. & Dalsgaard, C.J. Substance P-, somatostatin- and calcitonin gene-related peptide-like immunoreactivity and fluoride resistant acid phosphatase-activity in relation to retrogradely labeled cutaneous, muscular and visceral primary sensory neurons in the rat. Neurosci Lett 74, 37-42 (1987). 2. O’Brien, C., Woolf, C.J., Fitzgerald, M., Lindsay, R.M. & Molander, C. Differences in the chemical expression of rat primary afferent neurons which innervate skin, muscle or joint. Neuroscience 32, 493-502 (1989).
Supplementary Figure 3 Chemogenetic activation and transmitter phenotype in mCherry-labeled neurons.
A. Immunostaining for Pax2 was used to define inhibitory cells among the mCherry-labelled neurons in the PdynCre mice and a mean of 97 (range 82-120) cells were analysed in sections from 5 mice. For the nNOSCreERT2 mice, Sst2a immunoreactivity was used to identify inhibitory mCherry+ neurons (mean 86 cells, range 72-120), because both Pax2 and nNOS antibodies were raised in rabbit. The proportion of mCherry neurons that were Pax2-immunoreactive in PdynCre mice was 72.7% (62.9-90.2%), while the proportion of mCherry neurons that were Sst2a-immunoreactive in the nNOSCreERT2 mice was 57% (45-67.1%). Similar results were obtained in 5 mice of each genotype. B. In both PdynCre and nNOSCreERT2 mice the great majority of mCherry neurons were Fos+ after CNO treatment, irrespective of their neurotransmitter phenotype (95.9% [188/196] and 92.1% [93/101], respectively, for inhibitory and excitatory mCherry cells in PdynCre mice and 98.2% [108/110] and 86.1% [142/165], respectively, for inhibitory and excitatory mCherry cells in nNOSCreERT2 mice). Data were pooled from 3 CNO-treated mice of each genotype and absolute cell numbers are shown in square brackets. Very few Fos+ cells were seen in vehicle-treated mice: 1.3% [2/154] and 5.7% [2/35], respectively for inhibitory and excitatory mCherry cells in PdynCre mice and 0% [0/63] and 1.1% [1/93], respectively, for inhibitory and excitatory mCherry cells in nNOSCreERT2 mice. Data were pooled from 2 vehicle-treated mice of each genotype and absolute cell numbers are shown in square brackets. Similarly, the majority of Fos+ cells were also mCherry-positive in both mouse lines in the CNO-treated mice: 84.6% [281/332] and 63.5 [251/395], respectively for the PdynCre and nNOSCreERT2 lines. Few Fos cells were seen in the vehicle-treated mice, and some of these were mCherry-positive: 19% [4/21] and 3% [1/33], respectively for the PdynCre and nNOSCreERT2 lines. Experiments were performed on 5 mice of each genotype, which had received intraspinal injections of AAV2.flex.hM3Dq-mCherry into the L3 and L5 spinal segments. For each genotype, three of the mice were treated with CNO and two with vehicle two hours before perfusion fixation. Graphs show individual percentages for each of these mice.
Supplementary Figure 4 Laminar distribution of mCherry expression and lack of expression in dorsal root ganglia in PdynCre and nNOSCreERT2 mice that had received intraspinal injections of AAV2.flex.hM3Dq-mCherry.
A-D show transverse sections through the rostral part of the L3 segment of a PdynCre mouse (A,B) and through the caudal part of L3 of a nNOSCreERT2 mouse (C,D). In both genotypes, mCherry-positive cells form a dense band in the superficial dorsal horn, and there are scattered neurons in deeper laminae of the dorsal horn, consistent with the distribution of dynorphin- and nNOS-expressing neurons. Images to the right (B, D) show mCherry-immunoreactivity superimposed on a dark-field image, which indicates the location of the gray matter. Similar results were obtained from 5 animals of each genotype. E-J show scans of L4 dorsal root ganglia from the right side (ipsilateral to the intraspinal injection) of PdynCre (E-G) and nNOSCreERT2 (H-J) mice. In each case, immunostaining for mCherry is shown on the left, a dark-field image in the middle and a merged image on the right. No mCherry-positive cells were observed in the DRGs in either strain. Scale bar = 200 μm. Similar results were obtained from 4 nNOSCreERT2 animals and from 2 PdynCre animals. The time spent licking the calf in response to intradermal injection of chloroquine (100 μg) was reduced following chemogenetic activation (CNO) in PdynCre mice (K), whereas there was no effect on responses in nNOSCreERT2 animals (L). Significant differences were assessed using two-sided unpaired Student’s t-tests (t21 = 2.82, *p = 0.0103; ns not significant, t23 = 1.2, p = 0.2422). Data represent means ± SEM (n=11, 12, 12, and 13 animals, for PdynCre treated with CNO and vehicle and for nNOSCreERT2 treated with CNO and vehicle, respectively).
Supplementary Figure 5 Dynorphin excitatory neurons are chemogenetically activated; these include vertical cells, and there is a spatial segregation of inhibitory and excitatory preprodynorphin cells in glabrous and hairy skin territories in the dorsal horn.
A shows part of a transverse section of spinal cord taken from a PdynCre mouse that had been injected with AAV2-flex-hM3Dq-mCherry and treated with CNO 2 hours prior to perfusion fixation. A neuron in the outer part of lamina II, which is marked with an asterisk (*), expresses hM3Dq-mCherry (mCh, red), and is also immunoreactive for Fos (green), showing that it was chemogenetically activated. This is a projection of 6 confocal optical sections at 2 μm z-spacing, to show details of the dendritic tree. Arrowheads indicate prominent ventrally-directed dendrites, which are characteristic of vertical cells (a type of excitatory interneuron). The images on the right show the same cell in a projected image from only 2 optical sections. The nucleus of this cell is negative for Pax2 (blue) confirming that this is an excitatory neuron (see Figure S3 for numbers). Scale = 20 μm. Similar results were obtained from 3 animals. We identified 17 mCherry-positive lamina II neurons with ventral dendrites that entered lamina III in the sections analysed (n = 5 mice) and found that 16 of these (94%) were Pax2-negative (i.e. excitatory neurons). B. Excitatory dynorphin cells are largely restricted to regions of the superficial dorsal horn that are innervated from glabrous skin. Plots of inhibitory (Pax2-positive, blue circles) and excitatory (Pax2-negative, red circles) neurons that were immunoreactive for preprodynorphin (PPD) in three segments from the cervical enlargement: C7, C8 and T1 (data pooled from 2 mice) are shown. For C7 and C8, the border between regions innervated by hairy and glabrous skin, determined by immunostaining for VGLUT3 (not shown), is indicated by a dashed line. The regions lateral and medial to this line are innervated from hairy and glabrous skin, respectively. In the T1 segment, the VGLUT3 plexus extended right across the mediolateral extent of the dorsal horn, indicating that the whole region was innervated from hairy skin. C shows quantification of the of inhibitory and excitatory PdynCre neurons in the lumbar (L3, L4, L5) and cervical (C7, C8, T1) enlargements. All cells illustrated in the plates in panel A and in Fig 5 are included, and these have been divided into those in hairy and glabrous skin territories. Note that the proportions of excitatory PPD cells differ significantly between hairy skin territory (11.1% for lumbar, 10.6% for cervical) and glabrous skin territory (52.9% for lumbar, 62.8% for cervical). These differences were highly significant (Fisher exact probability test, two-sided, p = 7.5 × 10−11 for cervical and 5.4 × 10−9 for lumbar). Lumbar and cervical enlargements therefore display similar differential distribution of excitatory and inhibitory PdynCre neurons in the medial and lateral dorsal spinal cord. Data for the lumbar and cervical enlargements were obtained from 3 and 2 animals, respectively.
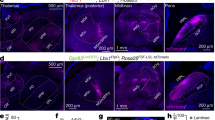
Supplementary Figure 6 Sstf/f;Trpv1cre and Sstf/f;Lbx1cre mice do not exhibit itch phenotypes.
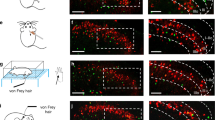
A, Sstf/f; Trpv1cre, and B, Sstf/f; Lbx1cre mice do not display significant differences in their scratch responses to intra-dermal injection of a variety of compounds, compared to littermate controls. Data are means ±SEM (n= 7, 8, 6, 6, 6, 6, 8, 8, 6, 6, 5, 6, 6, 6, 8, 8, 8, 9, 6, and 7); no significant (ns) difference in response were observed between genotypes assessed using two-sided unpaired Student t-tests (t13 = 0.5919, t10 = 0.3334, t10 = 1.703, t14 = 0.7476, t10 = 0.011, t9 = 1.565, t10 = 0.3265, t14 = 0.7429, t15 = 0.2209, t11 = 1.273, *p = 0.5641, 0.7457, 0.1194, 0.4671, 0.9913, 0.1520, 0.7508, 0.4698, 0.8282, and 0.2292). C, testing with von Frey filaments revealed significantly lower thresholds for reflex paw withdrawal responses for Sstf/f;Trpv1cre, Sstf/f;Lbx1cre and Sstf/f;Wnt1cre mice compared to control littermates, differences assessed using two-sided unpaired Student t-tests (t10 = 2.607, 2.507, 1.581, *p = 0.0262, 0.0311, and ns p = 0.1449). Data are means ±SEM (n= 6 animals).
Supplementary Figure 7 Response of dynorphin cells to somatostatin. Whole-cell patch-clamp recordings were made from eight eGFP+ neurons in spinal cord slices from PdynCre mice that had received intraspinal injections of AAV.flex.eGFP.
A.An example trace showing membrane hyperpolarization in response to bath-applied somatostatin (2 μM) in a dynorphin cell. All 8 cells tested showed clear hyperpolarization within a few minutes of the start of somatostatin application. B. Changes in the membrane potential of individual cells (circle, n = 8) are plotted, and the mean values (gray bar ± SD) are shown in control conditions (−58.0 ± 5.2 mV) and in the presence of somatostatin (−67.4 ± 6.5 mV). Somatostatin caused statistically significant hyperpolarization (t7 = 3.935, p = 0.0056, two-sided paired t-test, n = 8) in the dynorphin cells. C. Subthreshold currents measured in response to a voltage step protocol (−90 to −50 mV, 5 mV increments, 500 ms duration) while the cell was held at −60 mV under control conditions and in the presence of somatostatin. A reduction in the input resistance and an outward shift of the measured current during somatostatin application are apparent in this example trace. D. A plot of the values for each cell (circles, n = 8) before and during somatostatin application shows that all of the neurons have their input resistance reduced during somatostatin application. The mean values (gray bar ± SD) are shown for control (858.7 ± 380.7 MΩ) and somatostatin-treated conditions (516.9 ± 288.7 MΩ). A two-sided paired t-test indicates that this effect is significant (t7 = 6.243, p = 0.00043). E. The current-voltage (I-V) relationship was plotted using the voltage step protocol (as in C) in control conditions and in the presence of somatostatin. The I-V relationship in control conditions was then subtracted from that in the presence of somatostatin, which demonstrates the I-V relationship for the current responsible for the somatostatin-induced hyperpolarization. This current appears to reverse at −80.6 mV, which suggests that activation of somatostatin receptors was coupled to a downstream effect of opening G-protein coupled inwardly rectifying potassium (GIRK) channels. This intracellular coupling mechanism was previously suggested for another population of sst2a-expressing inhibitory interneurons in the mouse superficial dorsal horn3. Data were obtained from 8 cells and are shown as mean ± standard deviation. F. Patterns of action potential firing in control conditions (top) and during somatostatin application (bottom). Action potentials were evoked by injecting square current pulses (1 s). Of the 8 cells tested, 5 initially exhibited a tonic firing pattern while 3 cells showed an initial bursting pattern. However, after somatostatin application, only 1 cell continued to show tonic firing and the remaining 7 cells exhibited an initial bursting pattern. Note that the hyperpolarizing effect of somatostatin was counteracted by adding bias currents to the recorded cell to restore the membrane potential to around −60 mV. This conversion in firing patterns after somatostatin application indicates that not only the resting membrane potential, but also the ability of the cell to generate action potentials, was inhibited by somatostatin. G-I. Confocal optical section through the cell body of one of the recorded neurons, showing expression of GFP (green) and avidin-rhodamine (magenta), which was used to detect Neurobiotin. The recorded cell is GFP+. J. The morphology of the cell seen in a projection of 55 optical sections at 1 μm z-separation. Scale bars I = 20 μm, J = 50 μm. 3.Iwagaki, N., Garzillo, F., Polgar, E., Riddell, J.S. & Todd, A.J. Neurochemical characterisation of lamina II inhibitory interneurons that express GFP in the PrP-GFP mouse. Mol Pain 9, 56 (2013).
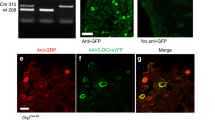
Supplementary Figure 8 Contacts from boutons that contain somatostatin and VGLUT2 onto dynorphin cells that express the Sst2a receptor.
A-C confocal image of part of a sagittal section from a PdynCre mouse that had received an intraspinal injection of AAV.flex.eGFP. eGFP is shown in green and sst2a receptor in magenta. Nine eGFP+ cells are present in this field, and 8 of these (marked with asterisks) are sst2a -immunoreactive, while 1 (arrowhead) lacks the receptor. Between 70 and 79 (mean 74.3) eGFP+ cells were identified in sections from the L3 segment of 3 mice, and 78.2% (68.9-78.5%) of these were sst 2a-immunoreactive. This is consistent with our finding that 90% of lamina I-II preprodynorphin neurons in hairy skin territory are inhibitory, and that 91% of inhibitory PPD cells are sst 2a-immunoreactive4. The box indicates the cell body of the neuron that is illustrated in D-I. D-I Contacts from boutons that co-express somatostatin (SST, red) and VGLUT2 (blue) onto the cell body (D-F) and dendrite (G-I) of a eGFP+ cell that was immunoreactive for sst 2a (magenta in D). Contacts are indicated with arrows. J. Plot of the contacts from SST/VGLUT2 boutons onto the cell bodies (yellow circles) or dendrites (blue circles) of 5 of the cells that were analysed. The top left cell is the one in illustrated in A (surrounded by box) and D-I. Confocal images in A-C are projected from 4 optical sections at 1 μm z-spacing. Those in D-F and G-I are projected from 3 and 5 (respectively) optical sections at 0.5 μm z-spacing. Scale bars: A-C = 50 μm, D-I = 5 μm, J = 50 μm. 4. Boyle, K.A. et al. A quantitative study of neurochemically defined populations of inhibitory interneurons in the superficial dorsal horn of the mouse spinal cord. Neuroscience 363, 120-133 (2017).
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–8
Rights and permissions
About this article
Cite this article
Huang, J., Polgár, E., Solinski, H.J. et al. Circuit dissection of the role of somatostatin in itch and pain. Nat Neurosci 21, 707–716 (2018). https://doi.org/10.1038/s41593-018-0119-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41593-018-0119-z
This article is cited by
-
Transmembrane protein 184B (TMEM184B) promotes expression of synaptic gene networks in the mouse hippocampus
BMC Genomics (2023)
-
Calretinin-expressing islet cells are a source of pre- and post-synaptic inhibition of non-peptidergic nociceptor input to the mouse spinal cord
Scientific Reports (2023)
-
Neuronal pentraxin 2 is required for facilitating excitatory synaptic inputs onto spinal neurons involved in pruriceptive transmission in a model of chronic itch
Nature Communications (2022)
-
PIEZO1 transduces mechanical itch in mice
Nature (2022)
-
Neuron-specific spinal cord translatomes reveal a neuropeptide code for mouse dorsal horn excitatory neurons
Scientific Reports (2021)