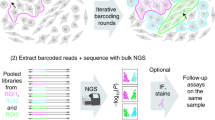
Abstract
In biological systems, spatial organization and function are interconnected. Here we present photoselective sequencing, a new method for genomic and epigenomic profiling within morphologically distinct regions. Starting with an intact biological specimen, photoselective sequencing uses targeted illumination to selectively unblock a photocaged fragment library, restricting the sequencing-based readout to microscopically identified spatial regions. We validate photoselective sequencing by measuring the chromatin accessibility profiles of fluorescently labeled cell types within the mouse brain and comparing with published data. Furthermore, by combining photoselective sequencing with a computational strategy for decomposing bulk accessibility profiles, we find that the oligodendrocyte-lineage-cell population is relatively enriched for oligodendrocyte-progenitor cells in the cortex versus the corpus callosum. Finally, we leverage photoselective sequencing at the subcellular scale to identify features of chromatin that are correlated with positioning at the nuclear periphery. These results collectively demonstrate that photoselective sequencing is a flexible and generalizable platform for exploring the interplay of spatial structures with genomic and epigenomic properties.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
All raw PSS sequencing data are available from the Sequence Read Archive under accession PRJNA938491 (cultured cell samples) and PRJNA938180 (mouse brain samples). Raw images corresponding to quantitative analyses in this study are available via Zenodo under the following: https://doi.org/10.5281/zenodo.7677794 (intensity decrease measurement), https://doi.org/10.5281/zenodo.7677827 (H3K9me3 radial intensity), https://doi.org/10.5281/zenodo.7677835 (H4K8ac radial intensity) and https://doi.org/10.5281/zenodo.7677868 (H4K20me radial intensity). scATAC-seq data (mouse hippocampus) from Sinnamon et al.23 are available through GEO (https://www.ncbi.nlm.nih.gov/geo/) under the accession GSE118987. scATAC-seq data (whole mouse brain) from Lareau et al.24 were acquired via the GEO under accession number GSE123581. Histone ChIP–seq data were obtained from the ENCODE encyclopedia44 (https://www.encodeproject.org/) under the following accession numbers: ENCFF411NQX (H3K4ac), ENCFF039LQB and ENCFF966TQK (H4K8ac), ENCFF319QES (H2AK5ac), ENCFF048DVC (H4K91ac), ENCFF088QGG (H4K20me1), ENCFF083IVC and ENCFF640TNT (H3K79me1), ENCFF179YRO (H3K9me3), ENCFF704DVN (H3K27me3), ENCFF513JSZ (H2BK15ac), ENCFF672BWZ (H3K9me1), ENCFF048DVC (H4K91ac), ENCFF091RNK (H2BK5ac), ENCFF992CZO (H3K79me2) and ENCFF058SOP (H2AK9ac). The H4K16ac data56 are available under GEO accession number GSE56307. Lamin B1 ChIP–seq data from Dou et al.37 are available under GEO accession number GSE63440. Source data are provided with this paper.
Code availability
Analysis scripts from this study are available at https://github.com/sarahmangiameli/pss-tools.
References
Zheng, H. & Xie, W. The role of 3D genome organization in development and cell differentiation. Nat. Rev. Mol. Cell Biol. 20, 535–550 (2019).
Misteli, T. Beyond the sequence: cellular organization of genome function. Cell 128, 787–800 (2007).
Schneider, R. & Grosschedl, R. Dynamics and interplay of nuclear architecture, genome organization, and gene expression. Genes Dev. 21, 3027–3043 (2007).
Misteli, T. Concepts in nuclear architecture. Bioessays 27, 477–487 (2005).
Strom, A. R. et al. Phase separation drives heterochromatin domain formation. Nature 547, 241–245 (2017).
Higgs, D. R. Enhancer–promoter interactions and transcription. Nat. Genet. 52, 470–471 (2020).
Wainwright, E. N. & Scaffidi, P. Epigenetics and cancer stem cells: unleashing, hijacking, and restricting cellular plasticity. Trends Cancer Res. 3, 372–386 (2017).
Mohammad, H. P. & Baylin, S. B. Linking cell signaling and the epigenetic machinery. Nat. Biotechnol. 28, 1033–1038 (2010).
Arzate-Mejía, R. G., Valle-García, D. & Recillas-Targa, F. Signaling epigenetics: novel insights on cell signaling and epigenetic regulation. IUBMB Life 63, 881–895 (2011).
Lovatt, D. et al. Transcriptome in vivo analysis (TIVA) of spatially defined single cells in live tissue. Nat. Methods 11, 190–196 (2014).
van der Leun, A. M. et al. Single-cell analysis of regions of interest (SCARI) using a photosensitive tag. Nat. Chem. Biol. 17, 1139–1147 (2021).
Hu, K. H. et al. ZipSeq: barcoding for real-time mapping of single cell transcriptomes. Nat. Methods 17, 833–843 (2020).
Genshaft, A. S. et al. Live cell tagging tracking and isolation for spatial transcriptomics using photoactivatable cell dyes. Nat. Commun. 12, 1–15 (2021).
Kishi, J. Y. et al. Light-Seq: light-directed in situ barcoding of biomolecules in fixed cells and tissues for spatially indexed sequencing. Nat. Methods 19, 1393–1402 (2022).
Emmert-Buck, M. R. et al. Laser capture microdissection. Science. 274, 998–1001 (1996).
Lipovsek, M. et al. Patch-seq: past, present, and future. J. Neurosci. 41, 937–946 (2021).
Tang, F. et al. mRNA-seq whole-transcriptome analysis of a single cell. Nat. Methods 6, 377–382 (2009).
Buenrostro, J. D., Wu, B., Chang, H. Y. & Greenleaf, W. J. ATAC-seq: a method for assaying chromatin accessibility genome-wide. Curr. Protoc. Mol. Biol. 109, 21.29.1–21.29.9 (2015).
Carter, B. et al. Mapping histone modifications in low cell number and single cells using antibody-guided chromatin tagmentation (ACT-seq). Nat. Commun. 10, 1–5 (2019).
Kaya-Okur, H. S. et al. CUT&Tag for efficient epigenomic profiling of small samples and single cells. Nat. Commun. 10, 1–10 (2019).
Buenrostro, J. D., Giresi, P. G., Zaba, L. C., Chang, H. Y. & Greenleaf, W. J. Transposition of native chromatin for fast and sensitive epigenomic profiling of open chromatin, DNA-binding proteins and nucleosome position. Nat. Methods 10, 1213–1218 (2013).
Chen, X. et al. ATAC-see reveals the accessible genome by (v2.29.2)-mediated imaging and sequencing. Nat. Methods 13, 1013–1020 (2016).
Sinnamon, J. R. et al. The accessible chromatin landscape of the murine hippocampus at single-cell resolution. Genome Res. 29, 857–869 (2019).
Lareau, C. A. et al. Droplet-based combinatorial indexing for massive-scale single-cell chromatin accessibility. Nat. Biotechnol. 37, 916–924 (2019).
Bravo González-Blas, C. et al. cisTopic: cis-regulatory topic modeling on single-cell ATAC-seq data. Nat. Methods 16, 397–400 (2019).
Kuhn, S., Gritti, L., Crooks, D. & Dombrowski, Y. Oligodendrocytes in development, myelin generation and beyond. Cells 8, 1424 (2019).
Bradl, M. & Lassmann, H. Oligodendrocytes: biology and pathology. Acta Neuropathol. 119, 37–53 (2010).
Rivers, L. E. et al. PDGFRA/NG2 glia generate myelinating oligodendrocytes and piriform projection neurons in adult mice. Nat. Neurosci. 11, 1392–1401 (2008).
Buchwalter, A., Kaneshiro, J. M. & Hetzer, M. W. Coaching from the sidelines: the nuclear periphery in genome regulation. Nat. Rev. Genet. 20, 39–50 (2018).
Mekhail, K. & Moazed, D. The nuclear envelope in genome organization, expression and stability. Nat. Rev. Mol. Cell Biol. 11, 317–328 (2010).
Van de Vosse, D. W., Wan, Y., Wozniak, R. W. & Aitchison, J. D. Role of the nuclear envelope in genome organization and gene expression. Wiley Interdiscip. Rev. Syst. Biol. Med. 3, 147–166 (2011).
Guelen, L. et al. Domain organization of human chromosomes revealed by mapping of nuclear lamina interactions. Nature 453, 948–951 (2008).
Pickersgill, H. et al. Characterization of the Drosophila melanogaster genome at the nuclear lamina. Nat. Genet. 38, 1005–1014 (2006).
Sadaie, M. et al. Redistribution of the Lamin B1 genomic binding profile affects rearrangement of heterochromatic domains and SAHF formation during senescence. Genes Dev. 27, 1800–1808 (2013).
Shah, P. P. et al. Lamin B1 depletion in senescent cells triggers large-scale changes in gene expression and the chromatin landscape. Genes Dev. 27, 1787–1799 (2013).
Lund, E., Oldenburg, A. R. & Collas, P. Enriched domain detector: a program for detection of wide genomic enrichment domains robust against local variations. Nucleic Acids Res. 42, e92 (2014).
Dou, Z. et al. Autophagy mediates degradation of nuclear lamina. Nature 527, 105–109 (2015).
Beliveau, B. J. et al. Single-molecule super-resolution imaging of chromosomes and in situ haplotype visualization using Oligopaint FISH probes. Nat. Commun. 6, 1–13 (2015).
Payne, A. C. et al. In situ genome sequencing resolves DNA sequence and structure in intact biological samples. Science 371, e3446 (2021).
Bolzer, A. et al. Three-dimensional maps of all chromosomes in human male fibroblast nuclei and prometaphase rosettes. PLoS Biol. 3, e157 (2005).
Martin, C. et al. Lamin B1 maintains the functional plasticity of nucleoli. J. Cell Sci. 122, 1551–1562 (2009).
Towbin, B. D. et al. Step-wise methylation of histone H3K9 positions heterochromatin at the nuclear periphery. Cell 150, 934–947 (2012).
Poleshko, A. et al. Genome-nuclear lamina interactions regulate cardiac stem cell lineage restriction. Cell 171, 573–587.e14 (2017).
Moore, J. E. et al. Expanded encyclopaedias of DNA elements in the human and mouse genomes. Nature 583, 699–710 (2020).
Corces, M. R. et al. Lineage-specific and single-cell chromatin accessibility charts human hematopoiesis and leukemia evolution. Nat. Genet. 48, 1193–1203 (2016).
Newman, A. M. et al. Determining cell type abundance and expression from bulk tissues with digital cytometry. Nat. Biotechnol. 37, 773–782 (2019).
Deng, Y. et al. Spatial profiling of chromatin accessibility in mouse and human tissues. Nature 609, 375–383 (2022).
Deng, Y. et al. Spatial-CUT&Tag: spatially resolved chromatin modification profiling at the cellular level. Science 375, 681–686 (2022).
Zhao, T. et al. Spatial genomics enables multi-modal study of clonal heterogeneity in tissues. Nature 601, 85–91 (2021).
Foster, D. S. et al. Integrated spatial multiomics reveals fibroblast fate during tissue repair. Proc. Natl Acad. Sci. USA 118, e2110025118 (2021).
Picelli, S. et al. Tn5 transposase and tagmentation procedures for massively scaled sequencing projects. Genome Res. 24, 2033–2040 (2014).
Langmead, B. & Salzberg, S. L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 9, 357–359 (2012).
Berg, D. E., Schmandt, M. A. & Lowe, J. B. Specificity of transposon Tn5 insertion. Genetics 105, 813–828 (1983).
Quinlan, A. R. & Hall, I. M. BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics 26, 841–842 (2010).
Schep, A. N., Wu, B., Buenrostro, J. D. & Greenleaf, W. J. chromVAR: inferring transcription-factor-associated accessibility from single-cell epigenomic data. Nat. Methods 14, 975–978 (2017).
Rai, T. S. et al. HIRA orchestrates a dynamic chromatin landscape in senescence and is required for suppression of neoplasia. Genes Dev. 28, 2712–2725 (2014).
Acknowledgements
We are grateful to J. Strecker for assistance with Tn5 transposase purification. We thank Z. Chiang for providing scripts for the generation of smoothed coverage traces, and for advice on computational methods. F.C. acknowledges support from NIH Early Independence Award (1DP5OD024583), the NHGRI (R01HG010647), the Burroughs Welcome Fund CASI award and the Merkin Institute. Many figure panels incorporated images generated with BioRender.com.
Author information
Authors and Affiliations
Contributions
F.C., S.M. and H.C. conceived the study. S.M., H.C. and J.A.D designed and performed experiments. S.M., A.S.E. and D.L. analyzed the data. All authors contributed to the interpretation of the results. S.M. wrote the manuscript with input from F.C. and J.D.B. All authors reviewed and edited the manuscript. F.C. and J.D.B. provided funding, technical guidance and project oversight.
Corresponding authors
Ethics declarations
Competing interests
S.M., H.C. and F.C. are listed as inventors on a patent application related to PSS. J.D.B. holds patents related to ATAC-seq and is on the scientific advisory board for Camp4, Seqwell and Celsee. F.C. is a founder of Curio Biosciences. The remaining authors declare no competing interests.
Peer review
Peer review information
Nature Methods thanks Yang Liu and the other, anonymous, reviewers for their contribution to the peer review of this work. Primary Handling Editor: Rita Strack, in collaboration with the Nature Methods team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Detailed Library structure and targeted illumination calibration.
a) Detailed PSS library structure. Central N bases represent the genomic DNA fragment. b) Calibrating the focus and registration of the targeted illumination system. Top image strip shows the photocleaved area resulting from a single stimulation point as the focal plane of the targeted illumination device is varied. Red arrow indicates correct focus. Lower left image shows poor focus and poor registration (fuzzy edges and shift) while the lower right image shows proper calibration. c) Measuring the resolution of the targeted illumination. Image strip shows individual stimulation points on a photocleavable lawn of fluorescence. The fluorescence intensity (blue line) was measured by taking the minimum pixel value of the columns in the image strip above. The minimal spot size is taken as the full width at half-prominence (yellow bars). d) Images show cells before and after the uncaging process (target cells indicated with white arrows). Magenta color represents the fluorescence intensity of the fragment library (Alexa 546). The area between the blue and yellow outlines was used to calculate median-background-pixel intensity for each image. Image contrast was chosen to highlight cellular background fluorescence. e) Images showing background subtracted images of cells before and after the uncaging process. Magenta color represents the fluorescence intensity of the fragment library (Alexa 546). Yellow outlines indicate nuclear regions.
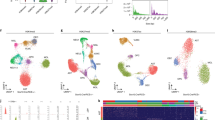
Extended Data Fig. 2 PSS in the mouse brain.
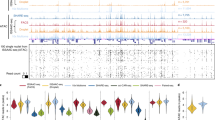
a) Schematic illustrates tissue-section trimming for mouse-brain experiments. b) Typical TSS enrichment plot for dentate gyrus granular cells (single section). c) Typical TSS enrichment plot for oligodendrocyte lineage cells (single section). d) Typical insert size distribution for dentate gyrus granule cells. e) Typical inserts size distribution for oligodendrocyte-lineage cells. f) Violin plot showing the number of unique fragments detected per selected cell or selected 100 μm2 area for all PSS ATAC-seq libraries from mouse brain samples (N = 16 tissue sections, 4 experiments). The blue line and white dot indicate the mean and median, respectively. The ends of the gray box represent the 25th and 75th percentiles, and the vertical gray line extends to the minimum and maximum data points. The violin shaped area represents the kernel density estimate of the raw data (blue dots). g) Pairwise correlation matrix (of cisTopic z-score) for individual replicates of all PSS ATAC-seq libraries (N = 16 tissue sections, 4 experiments). h) Low magnification scan of mouse hippocampal region with cortex (red) and corpus callosum (orange) regions outlined. Magenta color represents the fluorescence intensity from DRAQ5 DNA stain. i) Bar graph showing validation of decomposition algorithm using test oligodendrocyte and OPC data from an orthogonal single cell data. Truth bars represent the proportions in which the oligodendrocyte and OPC profiles were mixed. Model bars reflect the resulting fractional populations calculated by the decomposition algorithm (4 computational trials).
Extended Data Fig. 3 PSS at the nuclear periphery.
a) Violin plot showing the number of unique fragments detected per selected cell or selected 100 μm2 area for all PSS whole genome libraries (Fig. 1f and Fig. 3; N = 17 biological replicates across 2 experiments). The blue line and white dot indicate the mean and median, respectively. The ends of the gray box represent the 25th and 75th percentiles, and the vertical gray line extends to the minimum and maximum data points. The violin shaped area represents the kernel density estimate of the raw data (blue dots). b) Heatmaps showing pairwise Pearson correlation coefficients for PSS and lamin B1 ChIP-seq profiles at various bin sizes. c) Correlation between PSS nuclear periphery residuals (lamin B1 ChIP-seq signal removed) and histone ChIP-seq profiles from the ENCODE database. Dark blue boxes indicate a non-significant correlation. d) Images showing ring-like regions for calculating radial intensity profiles of histone modifications. e) Example images and radial intensity profile for H4K20me, a histone mark with a binding profile that is anticorrelated with the PSS nuclear periphery profile. f) Unimodal size distribution of sequenced fragments localized to the nuclear periphery indicates a loss of sensitivity to histone positioning.
Extended Data Fig. 4 PSS and Lamin B1 ChIP-seq enrichment across all chromosomes.
Orange and blue lines show PSS and ChIP-seq log2 enrichment profiles, respectively. PSS enrichment profiles are aggregated across N = 3 replicates from 2 experiments.
Supplementary information
Supplementary Information
Supplementary information.
Supplementary Table 1
Imaging and sequencing metrics for PSS experiments. Table gives imaging and sequencing metrics for each PSS replicate across all experiments presented in the manuscript. The table also shows which data were used to create each figure panel, and lists the accession numbers where raw sequencing data are available.
Supplementary Table 2
Complete list of indexed P7 ligation adapters. Barcode sequences are at least three hamming distances apart.
Supplementary Video 1
Movie demonstrating the photoselection process for ATAC-seq libraries HeLa cells (related to Fig. 1b). Magenta color represents the fluorescence intensity of the photocleavable PSS adapter. Nuclei outlined in blue were exposed to near-UV light during targeted illumination.
Source data
Source Data Extended Data Fig. 1
Raw image showing stimulation points on a lawn of photocleavable oligos.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mangiameli, S.M., Chen, H., Earl, A.S. et al. Photoselective sequencing: microscopically guided genomic measurements with subcellular resolution. Nat Methods 20, 686–694 (2023). https://doi.org/10.1038/s41592-023-01845-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41592-023-01845-8