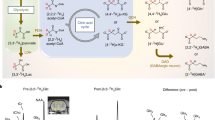
Abstract
Isotope tracing has helped to determine the metabolic activities of organs. Methods to probe metabolic heterogeneity within organs are less developed. We couple stable-isotope-labeled nutrient infusion to matrix-assisted laser desorption ionization imaging mass spectrometry (iso-imaging) to quantitate metabolic activity in mammalian tissues in a spatially resolved manner. In the kidney, we visualize gluconeogenic flux and glycolytic flux in the cortex and medulla, respectively. Tricarboxylic acid cycle substrate usage differs across kidney regions; glutamine and citrate are used preferentially in the cortex and fatty acids are used in the medulla. In the brain, we observe spatial gradations in carbon inputs to the tricarboxylic acid cycle and glutamate under a ketogenic diet. In a carbohydrate-rich diet, glucose predominates throughout but in a ketogenic diet, 3-hydroxybutyrate contributes most strongly in the hippocampus and least in the midbrain. Brain nitrogen sources also vary spatially; branched-chain amino acids contribute most in the midbrain, whereas ammonia contributes in the thalamus. Thus, iso-imaging can reveal the spatial organization of metabolic activity.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All MALDI MSI data in .mat format were deposited in FigShare at https://doi.org/10.6084/m9.figshare.15482112 (for kidneys) and https://doi.org/10.6084/m9.figshare.15505848 (for brains). Source data are provided with this paper.
Code availability
IsoScope was developed in MATLAB. MATLAB code and the users’ manual for IsoScope are available for non-commercial use in GitHub (https://github.com/xxing9703/Isoscope).
References
Hui, S. et al. Glucose feeds the TCA cycle via circulating lactate. Nature 551, 115–118 (2017).
Yankeelov, T. E., Abramson, R. G. & Quarles, C. C. Quantitative multimodality imaging in cancer research and therapy. Nat. Rev. Clin. Oncol. 11, 670–680 (2014).
Wilson, D. M. & Kurhanewicz, J. Hyperpolarized 13C MR for molecular imaging of prostate cancer. J. Nucl. Med. Publ. Soc. Nucl. Med. 55, 1567–1572 (2014).
Zhang, Z., Cheng, X., Zhao, Y. & Yang, Y. Lighting up live-cell and in vivo central carbon metabolism with genetically encoded fluorescent sensors. Annu. Rev. Anal. Chem. 13, 293–314 (2020).
Zhang, L. et al. Spectral tracing of deuterium for imaging glucose metabolism. Nat. Biomed. Eng. 3, 402–413 (2019).
Gilmore, I. S., Heiles, S. & Pieterse, C. L. Metabolic imaging at the single-cell scale: recent advances in mass spectrometry imaging. Annu. Rev. Anal. Chem. 12, 201–224 (2019).
Caprioli, R. M. Imaging mass spectrometry: molecular microscopy for the new age of biology and medicine. Proteomics 16, 1607–1612 (2016).
Dueñas, M. E., Larson, E. A. & Lee, Y. J. Toward mass spectrometry imaging in the metabolomics scale: increasing metabolic coverage through multiple on-tissue chemical modifications. Front. Plant Sci. 10, 1–11 (2019).
Niehaus, M., Soltwisch, J., Belov, M. E. & Dreisewerd, K. Transmission-mode MALDI-2 mass spectrometry imaging of cells and tissues at subcellular resolution. Nat. Methods 16, 925–931 (2019).
Jang, C., Chen, L. & Rabinowitz, J. D. Metabolomics and isotope tracing. Cell 173, 822–837 (2018).
Davidson, S. M. et al. Environment impacts the metabolic dependencies of RAS-driven non-small cell lung cancer. Cell Metab. 23, 517–528 (2016).
Hattori, K. et al. Paradoxical ATP elevation in ischemic penumbra revealed by quantitative imaging mass spectrometry. Antioxid. Redox Signal. 13, 1157–1167 (2010).
Sugiura, Y. et al. Visualization of in vivo metabolic flows reveals accelerated utilization of glucose and lactate in penumbra of ischemic heart. Sci. Rep. 6, 32361 (2016).
Wang, J. et al. MALDI-TOF MS imaging of metabolites with a N-(1-naphthyl) ethylenediamine dihydrochloride matrix and its application to colorectal cancer liver metastasis. Anal. Chem. 87, 422–430 (2015).
Wang, Z. M. et al. Specific metabolic rates of major organs and tissues across adulthood: evaluation by mechanistic model of resting energy expenditure. Am. J. Clin. Nutr. 92, 1369–1377 (2010).
Chen, Y., Fry, B. C. & Layton, A. T. Modeling glucose metabolism in the kidney. Bull. Math. Biol. 78, 1318–1336 (2016).
TeSlaa, T. et al. The source of glycolytic intermediates in mammalian tissues. Cell Metab. 33, 367–378.e5 (2021).
Hui, S. et al. Quantitative fluxomics of circulating metabolites. Cell Metab. 32, 676–688.e4 (2020).
Ross, B. D., Espinal, J. & Silva, P. Glucose metabolism in renal tubular function. Kidney Int. 29, 54–67 (1986).
Wheless, J. W. History of the ketogenic diet. Epilepsia 49 (Suppl.), 3–5 (2008).
Cooper, A.J. & Jeitner, T. M. Central role of glutamate metabolism in the maintenance of nitrogen homeostasis in normal and hyperammonemic brain.Biomolecules 6, 16 (2016).
Murashige, D. et al. Comprehensive quantification of fuel use by the failing and nonfailing human heart. Science 370, 364–368 (2020).
Jang, C. et al. Metabolite exchange between mammalian organs quantified in pigs. Cell Metab. 30, 594–606 (2019).
Marx, V. Method of the year: spatially resolved transcriptomics. Nat. Methods 18, 9–14 (2021).
Rodriques, S. G. et al. Slide-seq: a scalable technology for measuring genome-wide expression at high spatial resolution. Science 363, 1463–1467 (2019).
Agrawal, S. et al. El-MAVEN: a fast, robust, and user-friendly mass spectrometry data processing engine for metabolomics. Methods Mol. Biol. 1978, 301–321 (2019).
Ransick, A. et al. Single-cell profiling reveals sex, lineage, and regional diversity in the mouse kidney. Dev. Cell 51, 399–413 (2019).
Butler, A., Hoffman, P., Smibert, P., Papalexi, E. & Satija, R. Integrating single-cell transcriptomic data across different conditions, technologies, and species. Nat. Biotechnol. 36, 411–420 (2018).
Acknowledgements
This work was supported by the National Institutes of Health Pioneer award 1DP1DK113643 (J.D.R.), the Paul G. Allen Family Foundation grant 0034665 (J.D.R. and S.M.D.) and a Rutgers Cancer Institute of New Jersey New Investigator Award from the Cancer Center Support Grant P30 CA072720 (S.M.D.). We thank members of the Rabinowitz and Davidson Laboratories for scientific discussions and IsoScope feedback.
Author information
Authors and Affiliations
Contributions
S.M.D., J.D.R. and L.W. conceived the project. L.W., S.M.D. and J.W. developed and performed MALDI iso-imaging. L.S. and L.W. performed MS2 experiments. L.W. and S.R.J. performed LC–MS experiments. X.X. wrote the IsoScope code. L.W., X.X. and S.M.D. directed IsoScope development and performed data validation. L.W., X.Z., T.T., L.Y., M.M., X.L. and S.M.D. performed mice experiments. K.G. and S.M.D. carried out single-cell RNA-seq analysis. S.M.D., S.R.J. and K.G. performed H&E and IHC. L.W., S.M.D. and O.A.-D. interpreted histology and neuroanatomy. S.M.D., J.D.R. and L.W. wrote the manuscript. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
J.D.R. is a member of the Rutgers Cancer Institute of New Jersey and the University of Pennsylvania Diabetes Research Center; a co-founder and stockholder in Toran, Sofro and Serien Therapeutics; and advisor and stockholder in Agios Pharmaceuticals, Kadmon Pharmaceuticals, Bantam Pharmaceuticals, Colorado Research Partners, Rafael Pharmaceuticals, Barer Institute and L.E.A.F. Pharmaceuticals. S.M.D. is an associate member of Rutgers Cancer Institute of New Jersey. All other authors declare no competing interests.
Peer review
Peer review information
Nature Methods thanks Theodore Alexandrov and Martina Wallace and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Rita Strack was the primary editor on this article and managed its editorial process and peer review in collaboration with the rest of the editorial team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Iso-imaging pipeline, quantitation ions and control experiments.
a. Quantification of the circulatory turnover flux (Fcirc) at minimally perturbative infusion rates previously employed1 and somewhat higher infusion rates employed here to enable more facile measurement of downstream metabolite labeling (Supplementary Table 1). Note that the modestly higher infusion rates do not substantially perturb endogenous metabolic fluxes. b. Imaging mass spectrometry of UDP-glucose unlabeled signal intensity (M0) in control kidneys, after [U-13C]glucose infusion and after [U-13C]glycerol infusion. Note that the infusions are minimally perturbative, that is they do not alter UDP-glucose’s spatial distribution or signal intensity. c. Number of metabolites of different classes detected by MALDI–MSI using three different matrices (9-AA: 9-aminoacridine, 1,5-DAN: diaminonaphthalene, NEDC: N-(1-naphthyl) ethylenediamine) in negative ionization mode. d. Ion intensities of malate and glycerol-3-phosphate by MALDI–MSI with the indicated matrix in negative ionization mode. e. Steps used to select the quantitation metabolites for iso-imaging. 247 water soluble metabolites were readily quantified by HPLC-MS in negative mode (HPLC-MS). These were the initial candidates. Among these, 185 metabolites were observed as single LC–MS peaks without obvious isomers (‘no isomers’). Using NEDC as the matrix, 130 ions aligning with these metabolites were detected using MALDI-FTICR in negative ionization mode (MALDI-NEDC). Of these, seven metabolites showed similar isotope labeling patterns by LC–MS and MALDI (matching labeling patterns, ‘Quantitation metabolites’) from U13C-glucose infusion. Scale bars: 1 mm. n = 3 for all experiments unless otherwise indicated. Data presented as mean + /− s.d.
Extended Data Fig. 2 Identifying small molecules by stable-isotope labeling and MS/MS.
a Breakdown of metabolites grouped by unlabeled parent compound abundance. Detection limit prevents identification of certain compounds (<104) at their monoisotopic m/z and of others by their labeled compounds (<105), whose abundance falls below the detection limit. b Diagram depicting the first steps of the transsulfuration pathway starting with the reaction of [U-13C] methionine (M6) to homocysteine (M4, in two steps, not shown) and to cystathionine and labeled fraction of cystathionine (M4) from [U-13C] methionine in the pancreas by LC–MS and MALDI. c Breakdown of metabolites that can be detected and then verified by MS2. d Spectra of metabolites detected in MALDI by MS2. Experimentally measured (gray) and HMDB-adopted (red) spectra are shown on the positive and negative y axes, respectively. Fragment structures are provided for an example metabolite (N-acetyl aspartate). Molecular structures depict predicted (adopted from Metlin) neutral fragments and the corresponding m/z peaks refer to the [M-H]- ions. e. Imaging mass spectrometry of unlabeled C6H12O6 signal intensity (mixture of glucose and inositol) in murine kidney. f. LC–MS analysis showing the relative abundance of the isomers glucose and inositol in the dissected renal cortex and medulla. Scale bars: 1 mm. n = 3 for all experiments unless otherwise indicated. Data presented as mean + /- s.d.
Extended Data Fig. 3 IsoScope data input, analysis pipeline, and graphical user interface (GUI).
a. Workflow for file loading into IsoScope and options for data visualization within IsoScope. b. Graphical user interface.
Extended Data Fig. 4 Direct contributions to malate and TCA-associated amino acids in murine kidney cortex and medulla.
a The direct contribution of each nutrient tracer to malate, glutamate, and aspartate in the cortex and medulla of the murine kidney. b The direct contribution of each of the nutrient tracers to the metabolites in equilibrium with TCA cycle intermediates by LC–MS (homogenized tissue slice) compared to MALDI (whole tissue area). c Quantification of fluorescence intensity in 3 ROIs per region of the kidney in images in Fig. 3b, d. Groups were compared by one-way ANOVA. *** indicates p value <0.0001, ** p < 0.001, * p < 0.05, n.s. not significant. d Region-specific expression levels and percent of cells expressing each metabolic enzyme based on single-cell RNA-sequencing of murine kidney. n = 3 for all tracers. Data are mean + /− s.d.
Extended Data Fig. 5 Co-registration of ion images with H&E-stained brain sections.
a. Representative H&E staining (top row), ion images for glucose/inositol (m/z 179.0561) and DHA (m/z 327.2329) (middle row), and overlay of H&E with glucose/inositol and DHA (bottom row). b. LC-MS analysis shows that inositol is the dominant ion of m/z 179.0561 in the brain. c. Alternative color representation of the ions used to define brain regions: glucose/inositol (m/z 179.0561) (red) and DHA (m/z 327.2329) (cyan). d. Magnification of ion intensity of DHA and its co-registration with H&E using IsoScope. Scale bars: 1 mm.
Extended Data Fig. 6 Glutamate levels in different organs and fractional contribution of carbon-labeled substrates to glutamate in mice fed normal or ketogenic diet.
a. Relative glutamate levels in organs of the mouse. n = 2. (BAT = brown adipose tissue). b. Average carbon atom labeling of glutamine and glutamate in the brain normalized to serum after [U-13C]glutamine infusion. n = 2. c. Average carbon labeling of glutamine and glutamate in the brain normalized to serum after [U-13C]glucose infusion. n = 2. d. Iso-imaging of glutamate labeling in the murine brain from the indicated U-13C-tracers. Colors reflect the average carbon atom labeling of glutamate spatially resolved in the brain (\(L = \frac{{\mathop {\sum }\nolimits_{i = 0}^n \left( {Mi \cdot i} \right)}}{n}\) where n = 5 as glutamate has five carbons, and Mi is the ratio of glutamate’s M + i form) normalized to the average carbon atom labeling of the infused tracer in serum. e. Horizontal and coronal sections of the murine brain showing homogeneous normalized labeling of glutamate by [U-13C]glucose throughout the entire organ. f. Direct carbon contributions to glutamate in different regions of the murine brain. Calculated from data in Fig. 4c and Extended Data Fig. 5d. Scale bar: 700 µm, Data are mean + /− s.d. n = 3 for all experiments unless otherwise indicated.
Extended Data Fig. 7 Fractional contribution of nitrogen-labeled substrates to glutamate.
a. Normalized glutamate nitrogen labeling in the murine brain from the indicated 15N-tracers. Colors reflect the 15N-labeling fraction of glutamate spatially resolved in the brain normalized to labeling of the infused tracer in serum. b. 15N-labeling of the indicated tracer metabolite in the brain normalized to serum. (n=1 for 15N Ser and 15N Gly infusion) c. Magnification of internal capsule IC (Fig. 4e) and annotation using overlay of H&E and contribution to brain glutamate from circulating nitrogen sources. Scale bar: 700 µm, Data are mean + /− s.d. n = 3 for all experiments unless otherwise indicated.
Supplementary information
Supplementary Information
Supplementary Tables 1 and 2.
Supplementary Data 1
Statistical Source Data for Supplementary Fig. 1.
Supplementary Data 2
Statistical Source Data for Supplementary Fig. 4.
Supplementary Data 3
Statistical Source Data for Supplementary Fig. 6.
Source data
Source Data Fig. 1
Statistical Source Data.
Source Data Fig. 2
Statistical Source Data.
Source Data Fig. 3
Statistical Source Data.
Source Data Fig. 4
Statistical Source Data.
Source Data Fig. 5
Statistical Source Data.
Rights and permissions
About this article
Cite this article
Wang, L., Xing, X., Zeng, X. et al. Spatially resolved isotope tracing reveals tissue metabolic activity. Nat Methods 19, 223–230 (2022). https://doi.org/10.1038/s41592-021-01378-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41592-021-01378-y
This article is cited by
-
Single-cell mapping of lipid metabolites using an infrared probe in human-derived model systems
Nature Communications (2024)
-
Metabolic heterogeneity in cancer
Nature Metabolism (2024)
-
The pleiotropic functions of reactive oxygen species in cancer
Nature Cancer (2024)
-
A Novel Strategy for the Development of Functional Foods to Improve Energy Metabolism Disorders: Stable Isotope-Resolved Metabolomics
Food and Bioprocess Technology (2024)
-
Developmental basis of SHH medulloblastoma heterogeneity
Nature Communications (2024)