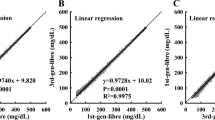
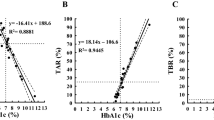
Abstract
Plasma fasting glucose (FG) levels play a pivotal role in the diagnosis of prediabetes and diabetes worldwide. Here we investigated FG values using continuous glucose monitoring (CGM) devices in nondiabetic adults aged 40–70 years. FG was measured during 59,565 morning windows of 8,315 individuals (7.16 ± 3.17 days per participant). Mean FG was 96.2 ± 12.87 mg dl−1, rising by 0.234 mg dl−1 per year with age. Intraperson, day-to-day variability expressed as FG standard deviation was 7.52 ± 4.31 mg dl−1. As there are currently no CGM-based criteria for diabetes diagnosis, we analyzed the potential implications of this variability on the classification of glycemic status based on current plasma FG-based diagnostic guidelines. Among 5,328 individuals who would have been considered to have normal FG based on the first FG measurement, 40% and 3% would have been reclassified as having glucose in the prediabetes and diabetes ranges, respectively, based on sequential measurements throughout the study. Finally, we revealed associations between mean FG and various clinical measures. Our findings suggest that careful consideration is necessary when interpreting FG as substantial intraperson variability exists and highlight the potential impact of using CGM data to refine glycemic status assessment.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The data that support the findings of this study originate from the 10K study. Reference data are available at https://github.com/ayya-keshet/CGMap. Restrictions apply to the individual-level data and they are therefore not publicly available. The data can be accessed only by request to the authors. Data in this paper is part of the Human Phenotype Project (HHP) and is accessible to researchers from universities and other research institutions at https://humanphenotypeproject.org/.
Code availability
Analyses in this study were performed using publicly available Python libraries, as detailed in Methods.
Change history
15 April 2024
A Correction to this paper has been published: https://doi.org/10.1038/s41591-024-02997-6
References
Khan, M. A. B. et al. Epidemiology of type 2 diabetes—global burden of disease and forecasted trends. J. Epidemiol. Glob. Health 10, 107–111 (2020).
Danaei, G. et al. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2.7 million participants. Lancet 378, 31–40 (2011).
American Diabetes Association. Standards of medical care in diabetes—2022 abridged for primary care providers. Clin. Diabetes 40, 10–38 (2022).
Norberg, M. et al. A combination of HbA1c, fasting glucose and BMI is effective in screening for individuals at risk of future type 2 diabetes: OGTT is not needed. J. Intern. Med. 260, 263–271 (2006).
Rasmussen, S. S. et al. Short-term reproducibility of impaired fasting glycaemia, impaired glucose tolerance and diabetes the ADDITION study, DK. Diabetes Res. Clin. Pract. 80, 146–152 (2008).
Mooy, J. M. et al. Intra-individual variation of glucose, specific insulin and proinsulin concentrations measured by two oral glucose tolerance tests in a general Caucasian population: the Hoorn study. Diabetologia 39, 298–305 (1996).
Brohall, G., Behre, C. -J., Hulthe, J., Wikstrand, J. & Fagerberg, B. Prevalence of diabetes and impaired glucose tolerance in 64-year-old Swedish women: experiences of using repeated oral glucose tolerance tests. Diabetes Care 29, 363–367 (2006).
Feskens, E. J., Bowles, C. H. & Kromhout, D. Intra- and interindividual variability of glucose tolerance in an elderly population. J. Clin. Epidemiol. 44, 947–953 (1991).
Balion, C. M. et al. Reproducibility of impaired glucose tolerance (IGT) and impaired fasting glucose (IFG) classification: a systematic review. Clin. Chem. Lab. Med. 45, 1180–1185 (2007).
Freckmann, G., Pleus, S., Grady, M., Setford, S. & Levy, B. Measures of accuracy for continuous glucose monitoring and blood glucose monitoring devices. J. Diabetes Sci. Technol. 13, 575–583 (2019).
Facchinetti, A. Continuous glucose monitoring sensors: past, present and future algorithmic challenges. Sensors 16, 2093 (2016).
Sacks, D. B. et al. Guidelines and recommendations for laboratory analysis in the diagnosis and management of diabetes mellitus. Clin. Chem. 69, 808–868 (2023).
Battelino, T. et al. Continuous glucose monitoring and metrics for clinical trials: an international consensus statement. Lancet Diabetes Endocrinol. 11, 42–57 (2023).
Keshet, A. et al. CGMap: characterizing continuous glucose monitor data in thousands of non-diabetic individuals. Cell Metab. 35, 758–769.e3 (2023).
Shilo, S. et al. 10K: a large-scale prospective longitudinal study in Israel. Eur. J. Epidemiol. 36, 1187–1194 (2021).
Shah, V. N. et al. Continuous glucose monitoring profiles in healthy nondiabetic participants: a multicenter prospective study. J. Clin. Endocrinol. Metab. 104, 4356–4364 (2019).
Jarvis, P. R. E., Cardin, J. L., Nisevich-Bede, P. M. & McCarter, J. P. Continuous glucose monitoring in a healthy population: understanding the post-prandial glycemic response in individuals without diabetes mellitus. Metab. Clin. Exp. 146, 155640 (2023).
Zeevi, D. et al. Personalized nutrition by prediction of glycemic responses. Cell 163, 1079–1094 (2015).
Alberti, K. G. M. M. & Zimmet, P. Z. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus. Provisional report of a WHO consultation. Diabet. Med. 15, 539–553 (1998).
Inzucchi, S. E. Diagnosis of diabetes. N. Engl. J. Med. 367, 542–550 (2012).
Kim, J. A. et al. Impact of visit-to-visit fasting plasma glucose variability on the development of type 2 diabetes: a nationwide population-based cohort study. Diabetes Care 41, 2610–2616 (2018).
Chang, T. et al. Highly integrated watch for noninvasive continual glucose monitoring. Microsyst. Nanoeng. 8, 25 (2022).
Hu, Y. et al. Combined use of fasting plasma glucose and glycated hemoglobin A1c in the screening of diabetes and impaired glucose tolerance. Acta Diabetol. 47, 231–236 (2010).
Gao, L. et al. Association between carotid intima-media thickness and fasting blood glucose level: a population-based cross-sectional study among low-income adults in rural China. J. Diabetes Investig. 8, 788–797 (2017).
Rodbard, D. Characterizing accuracy and precision of glucose sensors and meters. J. Diabetes Sci. Technol. 8, 980–985 (2014).
Pleus, S. et al. Rate-of-change dependence of the performance of two cgm systems during induced glucose swings. J. Diabetes Sci. Technol. 9, 801–807 (2015).
Leelarathna, L. & Wilmot, E. G. Flash forward: a review of flash glucose monitoring. Diabet. Med. 35, 472–482 (2018).
Tsoukas, M. et al. Accuracy of FreeStyle Libre in adults with type 1 diabetes: the effect of sensor age. Diabetes Technol. Ther. 22, 203–207 (2020).
Yalamanchali, S. et al. Diagnosis of obstructive sleep apnea by peripheral arterial tonometry: meta-analysis. JAMA Otolaryngol. Head. Neck Surg. 139, 1343–1350 (2013).
DRSplus TrueColor high-resolution confocal fundus camera. iCare https://www.icare-world.com/product/icare-drsplus/ (2024).
Zhou, Y. et al. Automorph: automated retinal vascular morphology quantification via a deep learning pipeline. Transl. Vis. Sci. Technol. 11, 12 (2022).
Levey, A. S. et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of diet in renal disease study group. Ann. Intern. Med. 130, 461–470 (1999).
Seabold, S. & Perktold, J. Statsmodels: econometric and statistical modeling with Python. In Proc. 9th Python in Science Conference 92–96 (SciPy, 2010).
Vallat, R. Pingouin: statistics in Python. JOSS 3, 1026 (2018).
Acknowledgements
We thank the members of the Segal group for fruitful discussions. E.S. is supported by the Crown Human Genome Center, the Larson Charitable Foundation New Scientist Fund, the Else Kröner Fresenius Foundation, the White Rose International Foundation, the Ben B. and Joyce E. Eisenberg Foundation, the Nissenbaum Family, M. Pinheiro de Andrade and V. Buchheim, M. Michels, A. Moussaieff and grants funded by the Minerva Foundation, with funding from the Federal German Ministry for Education and Research and by the European Research Council and the Israel Science Foundation. A.K. is partially supported by the Israeli Council for Higher Education via the Weizmann Data Science Research Center,
Author information
Authors and Affiliations
Contributions
S.S. conceived the project, designed and conducted the analyses, interpreted the results and wrote the paper. A.K. designed and conducted the analyses, interpreted the results and wrote the paper. H.R. conducted the analyses and interpreted the results. A.G. conducted the analyses. Y.T.-B. and Y.A. interpreted the results. E.S. directed and supervised the project.
Corresponding author
Ethics declarations
Competing interests
H.R. is an employee in Pheno.AI, Ltd, a biomedical data science company from Tel Aviv, Israel. A.K. and E.S. are paid consultants to Pheno.AI, Ltd. The other authors declare no competing interests.
Peer review
Peer review information
Nature Medicine thanks Jordi Merino and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Sonia Muliyil, in collaboration with the Nature Medicine team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Distribution of number of days analyzed per participant.
Only days with valid CGM morning windows were included.
Extended Data Fig. 2 Distribution of hours prior to and following morning time windows.
(a) The mean timing of the last meal logged prior to the morning time window (Hours). A minimum of 8 hours was predefined. (b) The mean timing of the first meal logged following the morning time window (Hours).
Extended Data Fig. 3 Exclusion process.
The full exclusion process for (a) individuals in the study and (b) valid morning windows is presented. Overall this process resulted in 8,315 individuals with 61,339 morning windows.
Extended Data Fig. 4 Distribution of fasting glucose measurements.
the distribution of mean FG values (mg/dl) of the study participants.
Extended Data Fig. 5 Progression and of fasting glucose with age by sex.
Progression of the mean FG (mg/dl) with age (years) for Females (orange) and Males (blue). Dots show the distribution of the data points. Dotted black lines show the 3rd, 10th, 50th, 90th, and 97th percentiles obtained using Lowess regression. Robust regression equation is shown in black.
Extended Data Fig. 6 Percentage of reclassification of glycemic status per followup day for individuals considered to have normal FG at baseline.
The percentage of participants, from the individuals considered to have glucose values in the normal range, that were misclassified when using only the baseline FG values as opposed to using all the FG values available in the followup data to prediabetes (yellow), suspected diabetes (orange) and diabetes (red) (see also Supplementary Table 2). Individuals who had FG below 100 mg/dl (5.6 mmol/L) and between 100–125 mg/dL (5.6–6.9 mmol/L) were classified as normal or prediabetes respectively. Individuals with only one FG measurement ≥126 mg/dL (7.0 mmol/L) were classified as suspected diabetics and individuals with more than one FG measurement ≥126 mg/dL (7.0 mmol/L) were classified as diabetics.
Extended Data Fig. 7 Cohort ethnicity.
pie chart displays the distributions of the participant’s birth country, paternal birth country, and maternal birth country (N = 5,792 participants, who contributed this data at study initiation).
Extended Data Fig. 8 Variability of Intra-person, between mornings fasting glucose following exclusion of the first and last days of CGM usage for each participant.
The distribution of the standard deviation of mean FG values (mg/dl) between different mornings of the same participant is presented.
Supplementary information
Supplementary Information
Supplementary Tables 1–5.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shilo, S., Keshet, A., Rossman, H. et al. Continuous glucose monitoring and intrapersonal variability in fasting glucose. Nat Med (2024). https://doi.org/10.1038/s41591-024-02908-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41591-024-02908-9