Abstract
Despite a gradual decline in smoking rates over time, exposure to secondhand smoke (SHS) continues to cause harm to nonsmokers, who are disproportionately children and women living in low- and middle-income countries. We comprehensively reviewed the literature published by July 2022 concerning the adverse impacts of SHS exposure on nine health outcomes. Following, we quantified each exposure–response association accounting for various sources of uncertainty and evaluated the strength of the evidence supporting our analyses using the Burden of Proof Risk Function methodology. We found all nine health outcomes to be associated with SHS exposure. We conservatively estimated that SHS increases the risk of ischemic heart disease, stroke, type 2 diabetes and lung cancer by at least around 8%, 5%, 1% and 1%, respectively, with the evidence supporting these harmful associations rated as weak (two stars). The evidence supporting the harmful associations between SHS and otitis media, asthma, lower respiratory infections, breast cancer and chronic obstructive pulmonary disease was weaker (one star). Despite the weak underlying evidence for these associations, our results reinforce the harmful effects of SHS on health and the need to prioritize advancing efforts to reduce active and passive smoking through a combination of public health policies and education initiatives.
Similar content being viewed by others
Main
Tobacco use is one of the leading risk factors for disease burden and mortality worldwide, contributing to 229.8 million (95% uncertainty interval: 213.1–246.4 million) disability-adjusted life years and 8.7 million (8.1–9.3 million) deaths in 2019 (ref. 1). Secondhand smoke (SHS) exposure, alternatively referred to as passive or involuntary smoking, is a major tobacco-related public health concern for nonsmokers. Despite a gradual decline in smoking rates over the past half-century2, it is estimated that approximately 37% of the global population is still exposed to the smoke emitted from the burning end of tobacco products or exhaled from smokers, with higher rates of exposure among women and children compared to men, and evident racial and economic disparities3,4. This is concerning as tobacco smoke is composed of thousands of chemicals and compounds, including many carcinogens, which when inhaled damage the human body and lead to disease and death5.
The 2019 Global Burden of Diseases, Injuries, and Risk Factors Study (GBD) estimated that 1.3 million (1.0–1.6) deaths were attributable to SHS globally in 2019, with the largest burden concentrated in low- and middle-income countries6. These patterns have made SHS a priority for tobacco control efforts, especially after the adoption of the World Health Organization’s Framework Convention on Tobacco Control, a global treaty aimed at implementing evidence-based measures to reduce both active and passive smoking7. Therefore, providing an updated summary of the exposure–response relationship between SHS and multiple adverse health outcomes, as well as innovatively quantifying the strength of the evidence supporting these relationships, is essential to continue to inform tobacco control policy, research funders and clinical recommendations and guide individual decisions related to smoking practices.
Over time, advances in understanding the harms of SHS have raised awareness of the importance of protecting nonsmokers from tobacco smoke. Smoke-free initiatives, in particular, have changed attitudes and social norms toward SHS exposure and have been a key contributor to the decline of smoking prevalence8. Nevertheless, as world populations grow, the number of smokers continues to rise, increasing the number of nonsmokers at risk of SHS exposure9.
Over the past decades, the body of evidence concerning the relationship between SHS and health has greatly evolved with the outline of plausible biological mechanisms and in-depth consideration of the available evidence, moving from the first reported association with lung cancer in the 1986 Surgeon Generals’ report10 to the inference of causal relationships between SHS and a range of diseases affecting and adverse health outcomes for adults and children, including cardiovascular diseases, some respiratory illnesses, middle ear disease, low birth weight and sudden infant death syndrome11,12. Additionally, previous research, including meta-analyses, found suggestive evidence of an association between SHS exposure and breast cancer13,14,15. Despite these findings, substantial heterogeneity is detected across and within SHS risk–outcome assessments in terms of quantity and quality of studies and reported strength of associations. Variation across studies in the definitions of risk exposure used is also observed, with some reporting the risk associated with SHS exposure in specific settings16 or from specific sources (that is, maternal, paternal)17. Furthermore, given the limited availability of studies that assess exposure to tobacco smoke on the basis of environmental and biological samples, and the lack of a standard measure of SHS exposure, the units and dose categories reported across studies vary widely. Together, these inconsistencies can limit the comparability and consolidation of evidence concerning the health effects of SHS.
In this context, in this Article, we aimed to quantify the exposure–response associations between SHS and nine health outcomes—lung and breast cancer, ischemic heart disease (IHD), stroke, chronic obstructive pulmonary disease (COPD), lower respiratory infections, asthma, type 2 diabetes and otitis media—as well as the strength of the available evidence, using an objective, comprehensive and comparative framework. The Burden of Proof Risk Function (BPRF) derives a conservative estimate of the smallest harmful effects of SHS exposure on given health outcomes that are consistent with the available evidence and to summarize the strength of risk–outcome associations and their underlying evidence into a star-rating measure, ranging from one star (weak evidence of an association) to five stars (consistent evidence of a strong association), to aid the interpretation and comparability of results18. The main findings and policy implications of this work are summarized in Table 1.
Results
Overview
Following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines19, we systematically searched the literature for studies reporting associations between SHS exposure and each of the nine health outcomes of interest. Definitions of each of the outcomes are reported in Supplementary Table 1. In total, we reviewed 7,109 unique records published between 1 January 1970 and 31 July 2022 identified in PubMed and Web of Science. Through citation searching, 1,972 additional records were identified for screening. Following our predefined inclusion and exclusion criteria (Methods), 410 publications reporting relative risks (RRs) associated with SHS measured as a dichotomous exposure remained for inclusion in our analyses. The data extraction template is presented in Supplementary Table 2, and the review workflow is detailed for each health outcome in the PRISMA flow diagrams (Supplementary Figs. 1–9). The majority of the studies used a case–control design (n = 235), followed by prospective cohort (n = 156), nested case–control (n = 10), retrospective cohort (n = 5), case–cohort (n = 3) and case-crossover (n = 1) designs. The BPRF analyses for asthma (n = 125)20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144 and lung cancer (n = 104)145,146,147,148,149,150,151,152,153,154,155,156,157,158,159,160,161,162,163,164,165,166,167,168,169,170,171,172,173,174,175,176,177,178,179,180,181,182,183,184,185,186,187,188,189,190,191,192,193,194,195,196,197,198,199,200,201,202,203,204,205,206,207,208,209,210,211,212,213,214,215,216,217,218,219,220,221,222,223,224,225,226,227,228,229,230,231,232,233,234,235,236,237,238,239,240,241,242,243,244,245,246,247,248 reported in the present study were based on evidence from the highest number of studies, while COPD (n = 21)48,177,208,225,236,249,250,251,252,253,254,255,256,257,258,259,260,261,262,263,264 and type 2 diabetes (n = 9)265,266,267,268,269,270,271,272,273 analyses were based on the lowest number of studies. The included studies represent 623 observations from over 178 locations (Supplementary Table 3). Pooled RR estimates for each SHS risk–outcome relationship are provided in Table 2, along with key analytic parameters and characteristics. Forest plots depicting each risk–outcome association are presented in the Extended Data file (Extended Data Figs. 1–9), and all included effect sizes by study are reported in Supplementary Tables 4–12.
Cardiovascular diseases
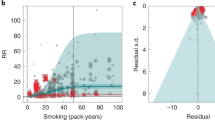
We identified 37 studies (59 observations)177,207,208,215,225,236,252,262,274,275,276,277,278,279,280,281,282,283,284,285,286,287,288,289,290,291,292,293,294,295,296,297,298,299,300,301,302 quantifying the relationship between SHS exposure and IHD and 20 studies (26 observations)176,207,208,225,236,252,262,278,296,297,303,304,305,306,307,308,309,310,311,312 assessing the relationship between SHS and stroke (Table 2 and Supplementary Tables 4 and 5). Our conservative analysis of the effect of SHS on IHD yielded an estimated RR of 1.26 (1.05–1.52) (Table 2, Fig. 1a and Extended Data Fig. 1), inclusive of between-study heterogeneity (gamma). We estimated the BPRF—which corresponds to the fifth quantile of RR closest to null and represents the lowest estimate of harmful SHS risk consistent with available evidence—to be 1.08, suggesting that SHS exposure increases an individual’s risk of IHD by a conservative minimum of 8%. In the BPRF framework, this translates to a risk–outcome score (ROS) of 0.04, which distinguishes the SHS–IHD relationship as a two-star risk–outcome pair, which can be interpreted as weak evidence of an association based on the available data (Table 2). Covariates accounting for cases where exposure to SHS was measured at baseline only (rather than multiple times during follow-up) and use of nonprospective cohort design were found to be statistically significant and were adjusted for within our final model (Table 2).
a,b, These modified funnel plots show the residuals of the reported mean RR relative to 0, the null value, on the x axis and the residuals of the standard error, as estimated from both the reported standard error and gamma, relative to 0 on the y axis, for IHD (a) and stroke (b). The light-blue vertical interval corresponds to the 95% uncertainty interval incorporating between-study heterogeneity; the dark-blue vertical interval corresponds to the 95% uncertainty interval (UI) without between-study heterogeneity; the dots are each included observation; the red Xs are outliered observations; the gray dotted line reflects the null log(RR); the blue line is the mean log(RR) for SHS and the outcome of interest; and the red line is the Burden of Proof function at the fifth quantile for these harmful risk–outcome associations.
Similarly, a weak but statistically significant relationship was found between SHS exposure and the risk of stroke. The estimated RR and uncertainty inclusive of between-study heterogeneity was 1.16 (1.03–1.32) (Table 2, Fig. 1b and Extended Data Fig. 2). Based on our conservative interpretation of the data, we estimated a BPRF of 1.05, indicating that exposure to tobacco smoke was associated with at least a 5% higher risk of stroke. This corresponds to a ROS of 0.02 and a two-star rating, consistent with weak evidence. In the final model, we adjusted for potential selection bias (based on percentage follow-up for longitudinal study designs and percentages of cases and controls for which exposure data could be ascertained for case–control designs) and for studies based on self-reported outcomes, as these covariates were found to be statistically significant by our bias covariate algorithm (Table 2).
The two-star rating for IHD was consistent with sensitivity analyses in which we restricted the models to studies with a prospective cohort design (Supplementary Table 13), subset to observations of never smokers only (Supplementary Table 14), and applied both these restrictions at the same time (Supplementary Table 15). When restricted to prospective cohort data for never smokers only, the association between SHS and stroke was downgraded to one star (ROS −0.001) (Extended Data Fig. 10). We did not detect publication bias, as identified by Egger’s regression test, in the primary analysis or in any of the sensitivity analyses for the cardiovascular outcomes (Table 2 and Supplementary Tables 13–15).
Cancer
The conservative BPRF analysis indicated that passive smoking was weakly associated with an increased risk of lung cancer, based on a BPRF of 1.00 and a corresponding ROS of 0.001 (Table 2), which translates to a two-star rating at the lower threshold of the two-star range and suggests that SHS exposure was associated with at least around 1% higher risk of lung cancer. When between-study heterogeneity and other sources of uncertainty were accounted for, the estimated RR was 1.37 (0.94–1.99) (Table 2, Fig. 2a and Extended Data Fig. 3). The bias covariate algorithm selected observations that did not originally control for smoking to be adjusted in the final model (Table 2). In a sensitivity analysis in which we restricted the data to prospective cohort studies, the strength of the association was even lower (BPRF 0.95, ROS −0.03), downgrading the relationship to a one-star rating (Extended Data Fig. 10 and Supplementary Table 13).
a,b, These modified funnel plots show the residuals of the reported mean RR relative to 0, the null value, on the x axis and the residuals of the standard error, as estimated from both the reported standard error and gamma, relative to 0 on the y axis, for lung cancer (a) and breast cancer (b). The light-blue vertical interval corresponds to the 95% uncertainty interval incorporating between-study heterogeneity; the dark-blue vertical interval corresponds to the 95% uncertainty interval (UI) without between-study heterogeneity; the dots are each included observation; the red Xs are outliered observations; the gray dotted line reflects the null log(RR); the blue line is the mean log(RR) for SHS and the outcome of interest; the red line is the Burden of Proof function at the fifth quantile for these harmful risk–outcome associations.
Our conservative BPRF analysis also found weak evidence of a harmful association between exposure to tobacco smoke and risk of breast cancer (BPRF 0.81, ROS −0.11, one-star rating; Table 2). The meta-analysis, which is supported by 51 unique studies170,220,313,314,315,316,317,318,319,320,321,322,323,324,325,326,327,328,329,330,331,332,333,334,335,336,337,338,339,340,341,342,343,344,345,346,347,348,349,350,351,352,353,354,355,356,357,358,359,360,361 and 79 observations (Supplementary Table 7), yielded an RR of 1.22 (0.75–1.98), inclusive of between-study heterogeneity (Table 2, Fig. 2b and Extended Data Fig. 4). In our model, observations that did not control for smoking and those from study designs other than prospective cohorts were adjusted since these covariates were found to be significant by our algorithm (Table 2). In further sensitivity analyses, the one-star relationship was still observed when we restricted to observations from never smokers only (Extended Data Fig. 1 and Supplementary Table 14). However, when restricting to prospective cohort studies, we found no statistically significant evidence of an association between exposure to SHS and the risk of breast cancer in our fixed-effect model without between-study heterogeneity; that is, the estimated RR and associated uncertainty without gamma includes the null. These risk–outcome pairs are automatically assigned a zero-star rating, and the BPRF and ROS are not computed (Extended Data Fig. 10 and Supplementary Table 13).
Based on Egger’s regression test, no significant evidence of publication bias was found for the main lung cancer and breast cancer models or the exploratory models (Table 2 and Supplementary Tables 13–15). Visual inspection of the funnel plots supported this finding (Fig. 2).
Respiratory conditions
We evaluated the association between exposure to SHS and three respiratory conditions: asthma, lower respiratory infections and COPD. Based on the conservative BPRF framework, the evidence supporting each of these relationships was weak (one-star rating), when between-study heterogeneity and other sources of bias were taken into account. Across these outcomes, no significant publication bias was detected in the primary models (Table 2) or in the sensitivity analyses (Supplementary Tables 13–16). For SHS and asthma, a risk–outcome pair not yet included in the GBD, the estimated RR incorporating between-study heterogeneity into the uncertainty was 1.21 (0.88–1.66) (Table 2, Fig. 3a and Extended Data Fig. 5). Data points associated with a self-reported diagnosis and those restricted to children (age ≤16 years) were adjusted for in our main model, as the corresponding bias covariates were found to be statistically significant (Table 2). The BPRF and ROS were 0.93 and −0.04, respectively, which equates to a one-star risk classification. When restricting to prospective cohort studies, a two-star rating for the relationship between SHS and asthma was observed (Extended Data Fig. 10 and Supplementary Tables 13).
These modified funnel plots show the residuals of the reported mean RR relative to 0, the null value, on the x axis and the residuals of the standard error, as estimated from both the reported standard error and gamma, relative to 0 on the y axis, for asthma (a), lower respiratory infections (b) and COPD (c). The light-blue vertical interval corresponds to the 95% uncertainty interval incorporating between-study heterogeneity; the dark-blue vertical interval corresponds to the 95% uncertainty interval (UI) without between-study heterogeneity; the dots are each included observation; the red Xs are outliered observations; the gray dotted line reflects the null log(RR); the blue line is the mean log(RR) for SHS and the outcome of interest; the red line is the Burden of Proof function at the fifth quantile for these harmful risk–outcome associations.
The meta-analysis of the risk of lower respiratory infections associated with SHS exposure included 50 studies53,64,91,134,362,363,364,365,366,367,368,369,370,371,372,373,374,375,376,377,378,379,380,381,382,383,384,385,386,387,388,389,390,391,392,393,394,395,396,397,398,399,400,401,402,403,404,405,406,407 and 66 observations (Supplementary Table 9) and yielded an RR and uncertainty interval inclusive of between-study heterogeneity of 1.34 (0.81–2.19) (Table 2, Fig. 3b and Extended Data Fig. 6). The BPRF (0.88) and corresponding ROS (−0.06) translated into a one-star rating, consistent with weak evidence of an association between passive smoking and increased risk of lower respiratory infections. The covariate selection algorithm flagged studies performed among populations that were not generalizable and those that used exposure definitions other than current SHS (for example, ever exposure to SHS) to be adjusted in our final model (Table 2). The strength of association as measured in the BPRF framework was not sensitive to any additional restrictions we applied to the input data, meaning that the one-star rating was still observed when we subset the data to prospective cohorts, never-smoking samples and a combination of the two (Extended Data Fig. 10 and Supplementary Tables 13–15).
Similar to the results for asthma and lower respiratory infections, the ROS for COPD was also negative (−0.14), equating to a one-star rating, indicating weak evidence of an association between SHS exposure and the risk of COPD. When accounting for between-study heterogeneity, the RR was 1.44 (0.67–3.12) (Table 2, Fig. 3c and Extended Data Fig. 7). Covariates representing studies that did not control for smoking and those with potential selection bias were found to be significant in our primary model and were adjusted for accordingly (Table 2). When including observations from seven prospective cohorts only, we found no statistically significant evidence of an association between SHS exposure and COPD when not including between-study heterogeneity (RR 1.21 (0.93–1.57, without gamma)). This was similar to the result we found when subsetting the data to never-smoking populations (RR 1.15 (0.95–1.40, without gamma)). The one-star association was observed, however, in a sensitivity analysis in which we applied both data restrictions simultaneously (Extended Data Fig. 10 and Supplementary Tables 13–15).
Other health outcomes
Our conservative Burden of Proof assessment found evidence of weak harmful effects between SHS exposure and risk of type 2 diabetes, with an RR of 1.16 (0.98–1.37) when accounting for between-study heterogeneity (Table 2, Fig. 4a and Extended Data Fig. 8). The BPRF value was 1.01 with a corresponding ROS of 0.005, which suggests that passive smoking is associated with at least a 1% higher risk of type 2 diabetes, translating to a two-star risk. The two-star relationship remained consistent in our sensitivity analysis in which we subset the input data to observations of never smokers only (Extended Data Fig. 10 and Supplementary Table 14). Restricting the data to prospective cohort studies resulted in a downgrade in star rating to a one-star risk (Extended Data Fig. 10 and Supplementary Table 13). Moreover, the automated covariate selection did not find any significant bias covariates for inclusion in the main or alternative final models (Table 2 and Supplementary Tables 13–15). No publication bias was found in the type 2 diabetes models.
a,b, These modified funnel plots show the residuals of the reported mean RR relative to 0, the null value, on the x axis and the residuals of the standard error, as estimated from both the reported standard error and gamma, relative to 0 on the y axis, for type 2 diabetes (a) and otitis media (b). The light-blue vertical interval corresponds to the 95% uncertainty interval incorporating between-study heterogeneity; the dark-blue vertical interval corresponds to the 95% uncertainty interval (UI) without between-study heterogeneity; the dots are each included observation; the red Xs are outliered observations; the gray dotted line reflects the null log(RR); the blue line is the mean log(RR) for SHS and the outcome of interest; the red line is the Burden of Proof function at the fifth quantile for these harmful risk–outcome associations.
For otitis media, our meta-analysis of 24 studies132,385,408,409,410,411,412,413,414,415,416,417,418,419,420,421,422,423,424,425,426,427,428,429 and 32 observations (Supplementary Table 12) yielded an RR of 1.12 (0.92–1.36) when accounting for between-study heterogeneity (Table 2, Fig. 4b and Extended Data Fig. 9). The corresponding BPRF was 0.95, which equates to a ROS of −0.03 and a one-star rating (weak evidence of association). Bias covariates that captured nonprospective cohort studies and studies in which the outcome of interest was self-reported (rather than diagnosed by a doctor) were detected as significant and adjusted for within our final model (Table 2). All studies included in our otitis media model were conducted in never-smoker populations (or classified as such given the age of the studied population (Methods and Supplementary Information Section 2.2)); however, when restricting our analysis to prospective cohort studies, the ROS was slightly higher, elevating the risk–outcome relationship to a two-star rating, with no bias covariates found statistically significant (Extended Data Fig. 10 and Supplementary Table 13). We found no publication bias in our primary model, but a statistically significant evidence of publication bias was found in our prospective cohort sensitivity analysis.
Discussion
In this study, we applied the Burden of Proof framework to quantify the relationship between exposure to SHS and nine health outcomes and to assess the strength of the evidence underlying these associations430. As suggested by our estimates not accounting for between-study heterogeneity, we found evidence that passive smoking is associated with statistically significant increases in the risk of all nine health outcomes. When taking the BPRF to conservatively interpret the available data by accounting for between-study heterogeneity and other sources of bias, the evidence suggests that being exposed to SHS increased the risk of IHD, stroke and type 2 diabetes by a minimum of 8%, 5% and 1%, respectively, corresponding to two-star associations with SHS. The two-star rating was also found for the relationship with lung cancer, for which SHS was found to increase the risk by a minimum of around 1%. The available evidence of associations between SHS and otitis media, asthma, lower respiratory infections, breast cancer and COPD are weaker and these risk–outcome pairs were classified as one-star associations.
As long known, being exposed to SHS is irrefutably harmful to human health and our findings are broadly in support of tobacco control measures aimed at protecting nonsmokers from tobacco smoke. Overall, we found SHS to have small to moderate quantitative impacts on health—mean effect sizes range from 1.12 for otitis media to 1.44 for COPD—which is in line with previous assessments13,431,432,433,434,435,436,437,438,439,440,441 and anticipated on the basis of mechanistic processes leading to diseases5. The modest strength of the association coupled with heterogeneity present in the underlying data across all nine risk–outcome pairs analyzed resulted in a body of evidence rated as weak under the proposed BPRF rating system (one and two stars), despite the relatively large number of studies included for some of the outcomes.
Nonetheless, even under our conservative interpretation of the available data using the BPRF approach, a particular area of considerable increased risk is cardiovascular health. This finding is consistent with the conclusions drawn by other studies in regard to both IHD and stroke431,442,443,444,445. In previous dose–response analyses, the harmful effects of SHS on cardiovascular diseases have been found even at low doses of exposure446,447,448. This is of particular concern as IHD and stroke are the two major causes of premature death and loss of healthy life worldwide449. Similarly, our findings also suggest that the risk of lung cancer and type 2 diabetes are also elevated for those exposed to SHS. Lung cancer was the fifth leading cause of death globally in 2019 and type 2 diabetes was the eighth leading cause, highlighting the potential benefit that could be achieved for these causes and overall disease burden by further reducing active and passive smoking449.
For otitis media, asthma, lower respiratory infections, breast cancer and COPD, the evidence supporting an association with passive smoking is even weaker, with a one-star rating. In the BPRF framework, one-star associations denote risk–outcome pairs for which it would not be surprising if the inclusion of additional data, when available, modifies our findings. Although we found evidence suggesting an association between SHS exposure and these other investigated health outcomes, the associations did not achieve statistical significance when using the BPRF approach to capture uncertainty that accounts for between-study heterogeneity. These findings highlight that the lack of consistent findings across studies is a major factor underlying the weak ROSs assigned to these exposure–outcome associations. The substantial inconsistency across studies with different designs and degrees of selection and information bias is not unusual for a risk factor with weak strength of associations, such as SHS exposure. In particular, we found insufficient evidence to support an association with SHS when restricting to prospective cohort studies (breast cancer) and never smokers (COPD), even when not incorporating between-study heterogeneity in our estimates of uncertainty. Indeed, authors have drawn markedly different conclusions about the presence and magnitude of association between passive exposure to tobacco smoke and breast cancer, especially when accounting for age group and menopausal status11,12,346,350,450. Because breast cancer is the most frequent type of cancer in women and accounts for substantial morbidity and mortality, research should continue to examine its association with exposure to SHS451.
Our study contributes to previous iterations of the GBD by not only increasing the number of studies informing each of the existing SHS–outcome associations but by assessing the relationship between passive smoking and asthma, a risk–outcome pair not yet incorporated into the GBD but deemed eligible for further consideration. Similar to our findings, population-specific meta-analyses found positive associations between passive exposure to tobacco smoke and both an overall increase in asthma risk within the Asian population452 and the occurrence of childhood asthma453. Expanding the evidence base around SHS and other health outcomes is a means to more accurately capture the full breadth of disease burden attributable to this risk.
Furthermore, the BPRF framework employed in this study addresses many of the limitations of existing meta-analytical approaches18. Given the high degree of inconsistency observed across results in the SHS literature, using the BPRF to capture the unexplained sources of variation between studies is particularly relevant for our study. Moreover, the translation of our conservative findings surrounding the health effects of SHS into a star rating simplifies the communication and interpretation of the available evidence. However, viewed in isolation, neither the calculated effect sizes nor the BPRF or star ratings imply causality or lack thereof. These are some of the components to be considered when defining health policy and research funding priorities. The high prevalence of exposure to SHS in a scenario with an increasing number of smokers and the harmful associations with conditions of global relevance warrant policy focus even with weak evidence supporting the analyses when compared to other less prevalent risks associated with rare or less severe outcomes and strong supporting evidence.
In spite of the observed variability in the SHS data, which accounts in part for the ROS and star-rating results we obtained, our study reaffirms that exposure to SHS is a harmful risk factor of great public health importance. As outlined by the World Health Organization, smoke-free policies in combination with strategies promoting active smoking cessation and noninitiation are among the most effective tobacco control interventions to reduce passive smoking and protect health454. Studies of the effects of smoke-free laws found that hospital admission and mortality rates for cardiovascular and respiratory conditions decreased after the implementation of smoking bans455,456,457,458,459. However, comprehensive smoke-free legislation (that is, covering all indoor public places) is in place in only 67 countries, protecting less than 25% of the world’s population7. Therefore, faster-paced implementation and adequate enforcement of this type of policy can play an important role in minimizing the burden of smoking-attributable diseases and deaths among nonsmokers. Moreover, private homes remain a major source of SHS exposure, particularly for women and children3,460, and our findings can help reinforce awareness of the adverse consequences of SHS exposure and promote adoption of voluntary restrictions in homes461.
When interpreting this study’s results, a number of limitations need to be taken into consideration, most of which are associated with the limitations of the available data, which in turn may have led to an underestimation of the RRs in our findings. First, we used studies in which exposure to SHS was self-reported, either directly or measured by proxy (that is, living with a smoking parent or spouse), and this can result in misclassification of exposed and nonexposed participants. Second, the information collected by surveys frequently asks about current exposure; this means that we lack information on cumulative exposure to SHS and formerly exposed individuals could have been misclassified as unexposed. Third, to account for the lack of a standardized way of capturing exposure to SHS in existing studies, we classify exposure to SHS as dichotomous (exposed or unexposed); however, this may oversimplify the risk profile associated with SHS by not accounting for differences in intensity or frequency of exposure. Fourth, our results draw upon data that rely on a range of exposure definitions. For example, the underlying studies capture information about exposure to SHS at either home or work and, in the absence of these, at any location more broadly. Previous studies have found different effect sizes for SHS exposure at home and at work442,443,462, a factor that was not investigated in our analysis. However, a covariate was created to assess if data points associated with exposure at any location were significantly different from those associated with exposure at work or home, which is the SHS definition adopted by the GBD. Because we use the GBD exposure definition, we also do not include data for exposure in public settings, which are largely limited. In the included studies, those not exposed at work or home may be exposed to SHS at other settings, and this bias, similar to our first limitation above, will tend to underestimate the true RR. Finally, despite the inclusion of asthma, a new health outcome to be considered for inclusion in the GBD, the outcomes assessed here do not necessarily reflect the harms associated with SHS in full. Future efforts could synthesize the available evidence concerning the relationship between SHS and other health outcomes for which some evidence of an association exist, for example, maternal outcomes and low birth weight463.
In conclusion, our study, which examines the relationship between SHS exposure and nine health outcomes using the BPRF framework developed by Zheng and colleagues430, reaffirms that SHS should be an area of priority for policymakers, physicians and public health advocates for strengthening tobacco-control measures, especially in locations with high smoking and SHS prevalence. Due to heterogeneity and uncertainty in the data, small effect sizes, small numbers of studies or a combination of these reasons, the existing strength of evidence on the health effects of SHS was considered weak, especially for the relationship with otitis media, asthma, lower respiratory infections, breast cancer and COPD. Even when applying a conservative interpretation of the evidence, our results suggest that exposure to SHS increases the risk to nonsmokers for cardiovascular outcomes, lung cancer and type 2 diabetes. Prospective cohort studies with greater consistency in case definitions, more precise measurement of exposures and larger samples can result in less inconsistent data, and thus more targeted recommendations.
Methods
Overview
In this study, we employed the BPRF methodology developed by Zheng and colleagues430 to conservatively estimate the association between SHS exposure and nine health outcomes and assess the strength of the evidence supporting each of these associations. We define SHS as the current exposure, among nonsmokers, to smoke from any combustible tobacco product at home or at work, the same definition used in the GBD studies. BPRF methods have already been employed to assess the health effects associated with smoking464, high systolic blood pressure465 and consumption of unprocessed red meat466 and vegetables467. Specifically, the BPRF framework uses a meta-regression–Bayesian, regularized, trimmed (MR-BRT) tool to estimate pooled RRs, along with uncertainty intervals, accounting for systematic bias, within-study correlation and unexplained between-study heterogeneity. Briefly, we followed the six analytical steps included in the BPRF meta-analytical approach, namely: (1) conducting a systematic review and extracting data from identified studies reporting on the association between SHS exposure and the outcomes of interest; (2) estimating a pooled RR that compares the risk of being exposed to SHS relative to those not exposed to SHS; (3) testing and adjusting for systematic sources of bias within input sources; (4) quantifying unexplained between-study heterogeneity while adjusting for within-study correlation and the number of studies; (5) evaluating publication and reporting bias; and (6) estimating the BPRF to generate a conservative estimate of the risk associated with SHS exposure and to compute a corresponding ROS. The BPRF is defined as the 5th (if harmful) or 95th (if protective) quantile estimate of the risk closest to the null estimate, with the 5th quantile reflecting the smallest harmful effect of a risk exposure on a given health outcome that is consistent with the available evidence. The ROS, which is the signed value of the log RR, reflects the effect size and strength of evidence for each risk–outcome association estimated. ROSs are translated into a star-rating scale from 1 to 5 to aid the interpretation of the results. We describe each of these steps below, and further details are available elsewhere430.
Similar to previous studies using BPRF methods464,465,466,467, the RRs, BPRFs and ROSs estimated in this study are not specific to or disaggregated by certain populations, meaning that we did not estimate RRs separately by geography, sex or age group. However, the assessment of the association between SHS and breast cancer relied on studies that were conducted in female-only populations. For asthma, we conducted a children-specific sensitivity analysis that is described along other sensitivity analyses below.
The present study complies with the PRISMA guidelines19 (Supplementary Tables 17 and 18 and Supplementary Figs. 1–9) and Guidelines for Accurate and Transparent Health Estimates Reporting (GATHER) recommendations (Supplementary Table 19)468. As a component of the GBD, the present analysis was approved by the University of Washington institutional review board committee (study no. 9060).
Health outcomes of interest
We selected outcomes on the basis of the availability of epidemiological evidence on their potential relationship with SHS. Eight out of the nine outcomes of interest—lung and breast cancer, IHD, stroke, COPD, lower respiratory infections, type 2 diabetes and otitis media—constitute SHS risk–outcome pairs considered in previous iterations of the GBD and were initially selected using the World Cancer Research Fund criteria for convincing or probable evidence as detailed in Murray et al.1. Through review of published meta-analyses and systematic reviews and consultations with key external experts, we identified asthma as an additional health outcome of interest to SHS researchers and one for which sufficient literature was available to enable BPRF analytic methods; we therefore included it in our analysis. Reference and alternative definitions of each of the outcomes are listed in Supplementary Table 1.
Systematic review
We conducted separate systematic reviews to identify peer-reviewed literature reporting relative measures of association quantifying the relationship between SHS exposure and each health outcome of interest. We searched PubMed and Web of Science for studies published between 1 January 1970 and 31 July 2022. Furthermore, we reviewed the citation lists of the systematic reviews and meta-analyses captured in our searches to identify additional pertinent studies.
Briefly, after deduplicating the search results, each study’s title and abstract were manually screened by a single reviewer for inclusion eligibility. Subsequently, the full text was retrieved and screened, and data were extracted from those studies that passed our inclusion criteria of being published in English; being a case–control, cohort, case-cohort or case-crossover study conducted in participant groups likely to be generalizable; using suitable exposure and outcome definitions; and reporting both a relative measure of association (that is, RR, odds ratio or hazard ratio) and some measure of uncertainty (for example, sample size, standard error or confidence intervals). In terms of outcome definitions, studies using either a reference or an alternative health outcome definition met our inclusion criteria (Supplementary Table 1). As for SHS exposure, we included studies with varied SHS definitions, including proxies, but restricted to those reporting dichotomous current or ever exposure (that is, yes/no exposure). We excluded studies reporting only former exposure to SHS and those only assessing exposure in specific public settings. To better match our SHS definition, we also excluded studies and observations reporting health risk for current smokers. Finally, for all outcomes but otitis media, lower respiratory infections and asthma, we excluded studies that exclusively assessed childhood exposure to SHS to best account for the exposure temporality reflected in the SHS definition in GBD. In the case that multiple studies provided estimates from the same cohort, we included only the study with the largest sample or follow-up period so as not to duplicate data. The search strings used in each database, detailed inclusion and exclusion criteria, and outcome-specific PRISMA flow diagrams are available in Supplementary Figs. 1–9.
Data from eligible publications were manually extracted into a template designed to capture information about study and sample characteristics, exposure and outcome definitions, ascertainment methods, effect size and corresponding uncertainty reported for each model/population, and covariates included in the statistical analyses. We also assessed each study for risk of potential bias following the Grading of Recommendations, Assessment, Development and Evaluations (GRADE) approach and recorded the information in the extraction template469. As part of the exposure definition review, we cataloged multiple aspects of SHS exposure linked to each reported effect size, including the location of exposure (home or/and work combined; home; work; or any/unspecified location), the source of exposure (family; parental; maternal; paternal; spouse; or any/unspecified source), the timing of exposure (current or ever), and the smoking status of the exposed population (nonsmoker; never smoker; former smoker; or any/unspecified). Those studies performed only among children aged 15 years or less with an original ‘unspecified’ smoking status were reassigned to ‘adjusted never smokers’ and treated as ‘never smokers’ and ‘controlled for smoking’ in our analyses. In the GBD, we assume no smoking prevalence for ages under 10 years; given the small prevalence for ages 10–15 and since most of the identified childhood studies included those past age 10, we believe this classification best reflects the smoking status of the studied population in these cases. All extracted data underwent manual quality assurance by the research team to verify accuracy. For a full list of extracted variables, with corresponding definitions, see Supplementary Table 2.
Estimating pooled RRs for each risk–outcome pair
We selected the effect sizes to be used in our meta-analytic approach within each included study and health outcome based on a prioritization cascade. All included effect sizes are reported in Supplementary Tables 4–12. Starting with the exposure definition, we chose the data points that closest matched the GBD risk definition in terms of the smoking status of the exposed population, followed by the location of exposure, the source of exposure, and the temporality. Thus, data points for nonsmokers currently exposed to SHS at home or work combined were prioritized over the other ones. In the absence of this exact definition, we prioritized the inclusion of effect sizes for each/any of the components of the GBD risk definition (that is, never smoker; former smoker; home; work) over those associated with a broader definition (that is, any/unspecified location or smoking status). Due to data sparsity, ‘ever exposure’ definitions were accepted for inclusion if results for ‘current exposure’ were not available. We did not include observations referring to exposure in specific settings other than home or work (for example, public settings or public transportation) or exposure among current smokers. Bias covariates were created to capture the impact of using alternate exposure definitions.
After this first selection stage, we proceeded with identifying the least granular analyses to be used in our models. For example, within each study and outcome, sex- and age-specific results were dropped in favor of aggregated data points, and results associated with the entire study population were retained over those for subgroup analyses when possible. We also favored observations reporting the risk of incidence and mortality combined over those that estimated each outcome type separately in cases where both were available. Moreover, for stroke, we dropped observations for subtypes (ischemic and hemorrhagic stroke) in favor of those for overall stroke due to data availability restrictions and to allow for best comparability across studies. In our last data selection step, the most-adjusted remainder data points within each study outcome were selected for inclusion in our analyses. This selection process is described in more detail in Supplementary Information.
To reduce the influence on our model of multiple observations coming from the same study, we adjusted the standard errors of effect sizes reported for multiple non-mutually exclusive exposure groups in each study by a factor matching the number of repeated measurements within each age–sex–smoking status group (Supplementary Information Section 2.2).
Finally, we used the MR-BRT tool to conduct each risk–outcome meta-regression analysis with the log-space RR of the outcome modeled as the dependent variable and exposure to SHS as the dichotomous independent variable (exposed to SHS versus not exposed to SHS). These analyses generated a single estimate of pooled RR of the given health outcome occurring for those exposed to SHS relative to unexposed counterparts. Following the BPRF methodology, we applied a 10% likelihood-based data-trimming algorithm to detect and remove outliers that may otherwise over-influence the model. This approach is suggested for all analyses with more than ten data points; therefore, it was implemented across all of our primary risk–outcome assessments and most of our sensitivity analyses470.
Testing and adjusting for biases across study designs and characteristics
Following the GRADE approach, we used the extracted data related to specific study characteristics to create binary covariates that captured potential sources of systematic bias within our input datasets. These covariates reflected the risk of bias associated with study design (prospective cohorts versus others), representativeness of the study population, exposure measurement (measured at baseline only versus multiple times during follow-up), outcome assessment method (self-report versus medical records), degree of control for confounding, and potential for selection bias (based on percentage follow-up for longitudinal study designs and percentages of cases and controls for which exposure data could be ascertained for case–control designs). Additionally, given SHS-specific characteristics, we created covariates to indicate whether a study controlled for smoking, regardless of other confounders, and whether the definition of SHS matched the one in GBD in terms of the location of exposure (home or work exposure versus broader definitions). A covariate reflecting studies performed among females only was also created. For the stroke models, we created two bias covariates to account for possible differences between studies reporting subtype-specific effect size only and those reporting stroke as an aggregated outcome; for asthma we created a specific covariate to indicate if a study was performed among children only (≤16 years old). Detailed information about each of the bias covariates is provided in Supplementary Information Section 5 (Supplementary Table 20). We systematically tested for the effect of bias covariates using a selection algorithm, which uses a step-wise Lasso strategy to identify statistically significant covariates at a threshold of 0.05, and adjusted for the selected bias covariates in the final model used to generate the RR estimates. Covariates were eligible for testing if there was a minimum of two data points in the model associated with each covariate value. If multiple covariates had the same distribution of values within a model, we randomly selected one of the covariates to be tested.
Quantifying remaining between-study heterogeneity
After adjusting for study-level bias covariates, we used a linear mixed-effects model to capture the remaining unexplained between-study heterogeneity, in which we included a study-level random slope (gamma) and a study-level random intercept for within-study correlation. We derived the uncertainty of gamma using the inverse Fisher information matrix, which is sensitive to the number of studies, study design and reported uncertainty. The draws of gamma are used to derive the conservative uncertainty interval estimate for our RR (with gamma), estimated from both the uncertainty surrounding the mean effect and the 95th quantile of between-study heterogeneity. The RR without gamma, as reported in Table 2, is reported with an uncertainty derived without fully accounting for between-study heterogeneity and reflects the RR estimates that are typically reported in traditional meta-analyses, while that with gamma better reflects the degree of consistency across the underlying studies. In this study, the RR metric of primary interest was the pooled RR with 95% uncertainty intervals that are inclusive (using gamma) of the effect of between-study heterogeneity. The estimated gamma for each risk–outcome primary assessment is presented in Supplementary Table 21.
Evaluating publication and reporting bias
To assess the presence of publication or reporting bias, we visually inspected the funnel plots (Figs. 1–4) produced for each risk–outcome evaluation, which show the residuals of the reported mean RR against the residuals of the standard error from each individual study. Visual inspection of the plots was accompanied by Egger’s regression tests to test for significant correlation between the standard error and the reported effect size. We did not find evidence of publication or reporting bias across any of the risk–outcome pairs in our primary models. We found publication bias for otitis media in one of our sensitivity analyses. We flagged the potential publication bias but did not correct for it in the model.
Estimating the BPRF
In our final step, we estimated the BPRF, which reflects the most conservative estimate of the association between exposure to SHS and the selected health outcomes that is consistent with the available evidence. For dichotomous harmful risk factors, the BPRF corresponds to the fifth quantile of RR closest to null, derived from the RR model inclusive of between-study heterogeneity. For each risk–outcome pair, the BPRF can be used to compute measures of increased or decreased risk of developing the health outcome due to exposure to the risk factor. BPRF values can be converted into ROSs, defined as the signed value of the average log RR of the BPRF. Large positive ROSs correspond to strong and consistent evidence of an association, while small positive ROSs and negative ROSs reflect weak evidence for an association, based on the available data. To facilitate the interpretation and comparison of the ROS results, the BPRF framework translates the ROS into star rating categories ranging from one to five (one star, ≤0.0 ROS; two stars, >0.0–0.14 ROS; three stars, >0.14–0.41 ROS; four stars, >0.41–0.62 ROS; five stars, >0.62 ROS). A one-star rating indicates weak evidence of association, while a five-star rating indicates very strong evidence. Zero-star risk–outcome pairs are not based on ROSs values but are defined as pairs for which there is no evidence of a statistically significant association between the risk and the health outcome when not accounting for between-study heterogeneity (that is, the 95% uncertainty interval without gamma crosses the null). Risk–outcome pairs receiving a one- through five-star rating are eligible for inclusion in the GBD.
Model validation
The validity of the BPRF approach to meta-analyze data extracted across studies has been extensively and rigorously evaluated by Zheng and colleagues430. For the present study, we conducted three main sensitivity analyses to examine the robustness of our primary findings to our data input in which we kept most of the model parameters consistent but (1) restricted our analysis to studies with a prospective cohort design; (2) subset our input data to never-smoking samples only; and (3) applied both these restrictions in conjunction. For asthma, specifically, we ran an additional model in which we restrict the data to those studies performed among children only (≤16 years old). The only modification in our model parameters was related to the implementation of the 10% data trimming, which is dependent on the number of observations available for each outcome model (that is, data are trimmed only if ten observations or more are included). We present the detailed results of these sensitivity analyses in Supplementary Tables 13–16.
Statistical analysis and reproducibility
Analyses were carried out using R version 4.0.5 and Python version 3.10.9.
This investigation relied on existing published data. No statistical method was used to predetermine sample size. For each health outcome, we included all studies that met our inclusion criteria. This study did not engage in primary data collection, randomization or blinding. Therefore, data exclusions were not relevant to the present study, and, as such, no data were excluded from the analyses. We have made our data and code available to foster reproducibility.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The findings from this study are supported by data extracted from published literature. We cite all studies included in our analyses in our manuscript. Studies’ characteristics are presented in Supplementary Table 3, and data points included in each analysis are available in Supplementary Tables 4–12. Details on data sources can also be found on the Burden of Proof visualization tool (https://vizhub.healthdata.org/burden-of-proof/).
Code availability
All code used for these analyses is publicly available online (https://github.com/ihmeuw-msca/burden-of-proof/).
Change history
30 January 2024
A Correction to this paper has been published: https://doi.org/10.1038/s41591-024-02832-y
References
Murray, C. J. L. et al. Global burden of 87 risk factors in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet 396, 1223–1249 (2020).
Dai, X., Gakidou, E. & Lopez, A. D. Evolution of the global smoking epidemic over the past half century: strengthening the evidence base for policy action. Tob. Control 31, 129–137 (2022).
Mbulo, L. et al. Secondhand smoke exposure at home among one billion children in 21 countries: findings from the Global Adult Tobacco Survey (GATS). Tob. Control 25, e95–e100 (2016).
Gakidou, E. et al. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 390, 1345–1422 (2017).
How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease: A Report of the Surgeon General (US Department of Health and Human Services, 2010).
Zhai, C. et al. Global, regional, and national deaths, disability-adjusted life years, years lived with disability, and years of life lost for the global disease burden attributable to second-hand smoke, 1990–2019: a systematic analysis for the Global Burden of Disease Study. Sci. Total Environ. 862, 160677 (2023).
WHO Report on the Global Tobacco Epidemic 2021: Addressing New and Emerging Products. (World Health Organization, 2021).
Flor, L. S., Reitsma, M. B., Gupta, V., Ng, M. & Gakidou, E. The effects of tobacco control policies on global smoking prevalence. Nat. Med. 27, 239–243 (2021).
Reitsma, M. B. et al. Spatial, temporal, and demographic patterns in prevalence of smoking tobacco use and attributable disease burden in 204 countries and territories, 1990–2019: a systematic analysis from the Global Burden of Disease Study 2019. Lancet 397, 2337–2360 (2021).
The health consequences of involuntary smoking: a report of the Surgeon General, 1986. US Department of Health and Human Services https://stacks.cdc.gov/view/cdc/20799 (1986).
The health consequences of smoking: 50 years of progress. a report of the Surgeon General. US Department of Health and Human Services https://www.cdc.gov/tobacco/ (2014).
Tobacco Smoke and Involuntary Smoking (International Agency for Research on Cancer, 2004).
Kim, A.-S., Ko, H.-J., Kwon, J.-H. & Lee, J.-M. Exposure to secondhand smoke and risk of cancer in never smokers: a meta-analysis of epidemiologic studies. Int. J. Environ. Res. Public Health 15, 1981 (2018).
Macacu, A., Autier, P., Boniol, M. & Boyle, P. Active and passive smoking and risk of breast cancer: a meta-analysis. Breast Cancer Res. Treat. 154, 213–224 (2015).
Carreras, G. et al. Burden of disease attributable to second-hand smoke exposure: a systematic review. Prev. Med. 129, 105833 (2019).
Stayner, L. et al. Lung cancer risk and workplace exposure to environmental tobacco smoke. Am. J. Public Health 97, 545–551 (2007).
Tabuchi, T. et al. Maternal and paternal indoor or outdoor smoking and the risk of asthma in their children: a nationwide prospective birth cohort study. Drug Alcohol Depend. 147, 103–108 (2015).
Aravkin, A. Y. et al. Reply to: concerns about the Burden of Proof studies. Nat. Med. 29, 826–827 (2023).
Page, M. J. et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372, n71 (2021).
Polk, S. et al. A prospective study of Fel d1 and Der p1 exposure in infancy and childhood wheezing. Am. J. Respir. Crit. Care Med. 170, 273–278 (2004).
Wang, J. et al. A prospective study on the role of smoking, environmental tobacco smoke, indoor painting and living in old or new buildings on asthma, rhinitis and respiratory symptoms. Environ. Res 192, 110269 (2021).
Coogan, P. F. et al. Active and passive smoking and the incidence of asthma in the Black Women’s Health Study. Am. J. Respir. Crit. Care Med. 191, 168–176 (2015).
Thorn, J., Brisman, J. & Torén, K. Adult-onset asthma is associated with self-reported mold or environmental tobacco smoke exposures in the home. Allergy 56, 287–292 (2001).
Flodin, U., Jönsson, P., Ziegler, J. & Axelson, O. An epidemiologic study of bronchial asthma and smoking. Epidemiology 6, 503–505 (1995).
van Beijsterveldt, T. C. E. M. & Boomsma, D. I. An exploration of gene–environment interaction and asthma in a large sample of 5-year-old Dutch twins. Twin Res. Hum. Genet. 11, 143–149 (2008).
Huang, P.-C. et al. Are phthalate exposure related to oxidative stress in children and adolescents with asthma? A cumulative risk assessment approach. Antioxidants 11, 1315 (2022).
Chan, T.-C., Hu, T.-H., Chu, Y.-H. & Hwang, J.-S. Assessing effects of personal behaviors and environmental exposure on asthma episodes: a diary-based approach. BMC Pulm. Med. 19, 231 (2019).
Rennie, D. C. et al. Assessment of endotoxin levels in the home and current asthma and wheeze in school-age children. Indoor Air 18, 447–453 (2008).
Oddy, W. H. et al. Association between breast feeding and asthma in 6 year old children: findings of a prospective birth cohort study. BMJ 319, 815–819 (1999).
Taveras, E. M. et al. Association of birth weight with asthma-related outcomes at age 2 years. Pediatr. Pulmonol. 41, 643–648 (2006).
Tadaki, H. et al. Association of cord blood cytokine levels with wheezy infants in the first year of life. Pediatr. Allergy Immunol. 20, 227–233 (2009).
Aversa, Z. et al. Association of infant antibiotic exposure with childhood health outcomes. Mayo Clin. Proc. 96, 66–77 (2021).
Vázquez Nava, F. et al. Associations between family history of allergy, exposure to tobacco smoke, active smoking, obesity, and asthma in adolescents. Arch. Bronconeumol. 42, 621–626 (2006).
Schroer, K. T. et al. Associations between multiple environmental exposures and Glutathione S-Transferase P1 on persistent wheezing in a birth cohort. J. Pediatr. 154, 401–408, 408.e1 (2009).
Melsom, T. et al. Asthma and indoor environment in Nepal. Thorax 56, 477–481 (2001).
Norbäck, D. et al. Asthma and rhinitis among Chinese children—indoor and outdoor air pollution and indicators of socioeconomic status (SES). Environ. Int. 115, 1–8 (2018).
Mumcuoglu, K. Y. et al. Asthma in Gaza refugee camp children and its relationship with house dust mites. Ann. Allergy 72, 163–166 (1994).
Lawson, J. A., Janssen, I., Bruner, M. W., Hossain, A. & Pickett, W. Asthma incidence and risk factors in a national longitudinal sample of adolescent Canadians: a prospective cohort study. BMC Pulm. Med. 14, 51 (2014).
Pokharel, P. K., Pokharel, P., Bhatta, N. K., Pandey, R. M. & Erkki, K. Asthma symptomatics school children of Sonapur. Kathmandu Univ. Med. J. 5, 484–487 (2007).
Goksör, E., Amark, M., Alm, B., Gustafsson, P. M. & Wennergren, G. Asthma symptoms in early childhood—what happens then? Acta Paediatr. 95, 471–478 (2006).
Toizumi, M. et al. Asthma, rhinoconjunctivitis, eczema, and the association with perinatal anthropometric factors in Vietnamese children. Sci. Rep. 9, 2655 (2019).
Hedman, L., Bjerg, A., Sundberg, S., Forsberg, B. & Rönmark, E. Both environmental tobacco smoke and personal smoking is related to asthma and wheeze in teenagers. Thorax 66, 20–25 (2011).
Boker, F., Alzahrani, A., Alsaeed, A., Alzhrani, M. & Albar, R. Cesarean section and development of childhood bronchial asthma: is there a risk? Open Access Maced. J. Med. Sci. 7, 347–351 (2019).
Surdu, S., Montoya, L. D., Tarbell, A. & Carpenter, D. O. Childhood asthma and indoor allergens in native americans in New York. Environ. Health 5, 22 (2006).
Ehrlich, R. et al. Childhood asthma and passive smoking. Urinary cotinine as a biomarker of exposure. Am. Rev. Respir. Dis. 145, 594–599 (1992).
Yang, C. Y., Lin, M. C. & Hwang, K. C. Childhood asthma and the indoor environment in a subtropical area. Chest 114, 393–397 (1998).
Zheng, T. et al. Childhood asthma in Beijing, China: a population-based case–control study. Am. J. Epidemiol. 156, 977–983 (2002).
David, G. L., Koh, W.-P., Lee, H.-P., Yu, M. C. & London, S. J. Childhood exposure to environmental tobacco smoke and chronic respiratory symptoms in non-smoking adults: the Singapore Chinese Health Study. Thorax 60, 1052–1058 (2005).
Galobardes, B. et al. Childhood wheezing, asthma, allergy, atopy, and lung function: different socioeconomic patterns for different phenotypes. Am. J. Epidemiol. 182, 763–774 (2015).
Guo, S.-L., Liu, F., Ren, C.-J., Xing, C.-H. & Wang, Y.-J. Correlations of LTα and NQO1 gene polymorphisms with childhood asthma. Eur. Rev. Med. Pharm. Sci. 23, 7557–7562 (2019).
Carlsten, C., Dimich-Ward, H., DyBuncio, A., Becker, A. B. & Chan-Yeung, M. Cotinine versus questionnaire: early-life environmental tobacco smoke exposure and incident asthma. BMC Pediatr. 12, 187 (2012).
Patrick, D. M. et al. Decreasing antibiotic use, the gut microbiota, and asthma incidence in children: evidence from population-based and prospective cohort studies. Lancet Respir. Med. 8, 1094–1105 (2020).
Charoenca, N. et al. Determining the burden of secondhand smoke exposure on the respiratory health of Thai children. Tob. Induc. Dis. 11, 7 (2013).
Arif, A. A. & Racine, E. F. Does longer duration of breastfeeding prevent childhood asthma in low-income families? J. Asthma 54, 600–605 (2017).
Usemann, J. et al. Dynamics of respiratory symptoms during infancy and associations with wheezing at school age. ERJ Open Res. 4, 00037–02018 (2018).
Sherman, C. B., Tosteson, T. D., Tager, I. B., Speizer, F. E. & Weiss, S. T. Early childhood predictors of asthma. Am. J. Epidemiol. 132, 83–95 (1990).
Milner, J. D., Stein, D. M., McCarter, R. & Moon, R. Y. Early infant multivitamin supplementation is associated with increased risk for food allergy and asthma. Pediatrics 114, 27–32 (2004).
Midodzi, W. K., Rowe, B. H., Majaesic, C. M., Saunders, L. D. & Senthilselvan, A. Early life factors associated with incidence of physician-diagnosed asthma in preschool children: results from the Canadian Early Childhood Development cohort study. J. Asthma 47, 7–13 (2010).
Slob, E. M. A. et al. Early-life antibiotic use and risk of asthma and eczema: results of a discordant twin study. Eur. Respir. J. 55, 1902021 (2020).
Sun, W., Svendsen, E. R., Karmaus, W. J. J., Kuehr, J. & Forster, J. Early-life antibiotic use is associated with wheezing among children with high atopic risk: a prospective European study. J. Asthma 52, 647–652 (2015).
Grabenhenrich, L. B. et al. Early-life determinants of asthma from birth to age 20 years: a German birth cohort study. J. Allergy Clin. Immunol. 133, 979–988 (2014).
Chen, Y.-C., Tsai, C.-H. & Lee, Y. L. Early-life indoor environmental exposures increase the risk of childhood asthma. Int. J. Hyg. Environ. Health 215, 19–25 (2011).
von Kobyletzki, L. B. et al. Eczema in early childhood is strongly associated with the development of asthma and rhinitis in a prospective cohort. BMC Dermatol 12, 11 (2012).
Håberg, S. E., Stigum, H., Nystad, W. & Nafstad, P. Effects of pre- and postnatal exposure to parental smoking on early childhood respiratory health. Am. J. Epidemiol. 166, 679–686 (2007).
Boneberger, A. et al. Environmental risk factors in the first year of life and childhood asthma in the Central South of Chile. J. Asthma 48, 464–469 (2011).
Jaakkola, M. S., Piipari, R., Jaakkola, N. & Jaakkola, J. J. K. Environmental tobacco smoke and adult-onset asthma: a population-based incident case–control study. Am. J. Public Health 93, 2055–2060 (2003).
Jaakkola, J. J., Nafstad, P. & Magnus, P. Environmental tobacco smoke, parental atopy, and childhood asthma. Environ. Health Perspect. 109, 579–582 (2001).
Litonjua, A. A., Carey, V. J., Burge, H. A., Weiss, S. T. & Gold, D. R. Exposure to cockroach allergen in the home is associated with incident doctor-diagnosed asthma and recurrent wheezing. J. Allergy Clin. Immunol. 107, 41–47 (2001).
Ratageri, V. H., Kabra, S. K., Dwivedi, S. N. & Seth, V. Factors associated with severe asthma. Indian Pediatr. 37, 1072–1082 (2000).
El-Sharif, N., Abdeen, Z., Barghuthy, F. & Nemery, B. Familial and environmental determinants for wheezing and asthma in a case–control study of school children in Palestine. Clin. Exp. Allergy 33, 176–186 (2003).
Polosa, R., Al-Delaimy, W. K., Russo, C., Piccillo, G. & Sarvà, M. Greater risk of incident asthma cases in adults with allergic rhinitis and effect of allergen immunotherapy: a retrospective cohort study. Respir. Res 6, 153 (2005).
Pattemore, P. K. et al. Hair nicotine at 15 months old, tobacco exposure and wheeze or asthma from 15 months to 6 years old. Pediatr. Pulmonol. 53, 443–451 (2018).
Leen, M. G., O’Connor, T., Kelleher, C., Mitchell, E. B. & Loftus, B. G. Home environment and childhood asthma. Ir. Med. J. 87, 142–144 (1994).
Strachan, D. P., Butland, B. K. & Anderson, H. R. Incidence and prognosis of asthma and wheezing illness from early childhood to age 33 in a national British cohort. BMJ 312, 1195–1199 (1996).
Morfín-Maciel, B., Barragán-Meijueiro, M., de, L. M. & Nava-Ocampo, A. A. Individual and family household smoking habits as risk factors for wheezing among adolescents. Prev. Med. 43, 98–100 (2006).
Azizi, B. H., Zulkifli, H. I. & Kasim, S. Indoor air pollution and asthma in hospitalized children in a tropical environment. J. Asthma 32, 413–418 (1995).
Mommers, M. et al. Indoor environment and respiratory symptoms in children living in the Dutch–German borderland. Int. J. Hyg. Environ. Health 208, 373–381 (2005).
Yang, C. Y., Tien, Y. C., Hsieh, H. J., Kao, W. Y. & Lin, M. C. Indoor environmental risk factors and childhood asthma: a case–control study in a subtropical area. Pediatr. Pulmonol. 26, 120–124 (1998).
McConnell, R. et al. Indoor risk factors for asthma in a prospective study of adolescents. Epidemiology 13, 288–295 (2002).
van der Valk, R. J. P. et al. Interaction of a 17q12 variant with both fetal and infant smoke exposure in the development of childhood asthma-like symptoms. Allergy 67, 767–774 (2012).
Jedrychowski, W. et al. Length at birth and effect of prenatal and postnatal factors on early wheezing phenotypes. Kraków epidemiologic cohort study. Int. J. Occup. Med. Environ. Health 21, 111–119 (2008).
Hunt, A., Crawford, J. A., Rosenbaum, P. F. & Abraham, J. L. Levels of household particulate matter and environmental tobacco smoke exposure in the first year of life for a cohort at risk for asthma in urban Syracuse, NY. Environ. Int. 37, 1196–1205 (2011).
Milanzi, E. B. et al. Lifetime secondhand smoke exposure and childhood and adolescent asthma: findings from the PIAMA cohort. Environ. Health 16, 14 (2017).
Sears, M. R. et al. Long-term relation between breastfeeding and development of atopy and asthma in children and young adults: a longitudinal study. Lancet 360, 901–907 (2002).
Kanoh, M. et al. Longitudinal study of parental smoking habits and development of asthma in early childhood. Prev. Med. 54, 94–96 (2012).
Lilljeqvist, A.-C., Faleide, A. O. & Watten, R. G. Low birthweight, environmental tobacco smoke, and air pollution: risk factors for childhood asthma? Pediatr. Asthma Allergy Immunol. 11, 95–102 (1997).
Wada, T. et al. Maternal exposure to smoking and infant’s wheeze and asthma: Japan Environment and Children’s Study. Allergol. Int. 70, 445–451 (2021).
Tanaka, K. et al. Maternal smoking and environmental tobacco smoke exposure and the risk of allergic diseases in Japanese infants: the Osaka Maternal and Child Health Study. J. Asthma 45, 833–838 (2008).
Thacher, J. D. et al. Maternal smoking during pregnancy and early childhood and development of asthma and rhinoconjunctivitis—a MeDALL Project. Environ. Health Perspect. 126, 047005 (2018).
Neuman, Å. et al. Maternal smoking in pregnancy and asthma in preschool children: a pooled analysis of eight birth cohorts. Am. J. Respir. Crit. Care Med. 186, 1037–1043 (2012).
Frassanito, A. et al. Modifiable environmental factors predispose term infants to bronchiolitis but bronchiolitis itself predisposes to respiratory sequelae. Pediatr. Pulmonol. 57, 640–647 (2022).
Hwang, B.-F., Liu, I.-P. & Huang, T.-P. Molds, parental atopy and pediatric incident asthma. Indoor Air 21, 472–478 (2011).
Li, X., Sundquist, J., Calling, S., Zöller, B. & Sundquist, K. Mothers, places and risk of hospitalization for childhood asthma: a nationwide study from Sweden: epidemiology of allergic disease. Clin. Exp. Allergy 43, 652–658 (2013).
Izuhara, Y. et al. Mouth breathing, another risk factor for asthma: the Nagahama Study. Allergy 71, 1031–1036 (2016).
Carr, T. F., Stern, D. A., Halonen, M., Wright, A. L. & Martinez, F. D. Non-atopic rhinitis at age 6 is associated with subsequent development of asthma. Clin. Exp. Allergy 49, 35–43 (2019).
Klinnert, M. D. et al. Onset and persistence of childhood asthma: predictors from infancy. Pediatrics 108, E69 (2001).
O’Connell, E. J. & Logan, G. B. Parental smoking in childhood asthma. Ann. Allergy 32, 142–145 (1974).
Clark, S. J., Warner, J. O. & Dean, T. P. Passive smoking amongst asthmatic children. Questionnaire or objective assessment? Clin. Exp. Allergy 24, 276–280 (1994).
Willers, S., Svenonius, E. & Skarping, G. Passive smoking and childhood asthma. Urinary cotinine levels in children with asthma and in referents. Allergy 46, 330–334 (1991).
Kim, A. et al. Perinatal factors and the development of childhood asthma. Ann. Allergy Asthma Immunol. 120, 292–299 (2018).
Hagendorens, M. M. et al. Perinatal risk factors for sensitization, atopic dermatitis and wheezing during the first year of life (PIPO study). Clin. Exp. Allergy 35, 733–740 (2005).
Carrasco, P. et al. Pre and postnatal exposure to mercury and respiratory health in preschool children from the Spanish INMA Birth Cohort Study. Sci. Total Environ. 782, 146654 (2021).
Thacher, J. D. et al. Pre- and postnatal exposure to parental smoking and allergic disease through adolescence. Pediatrics 134, 428–434 (2014).
van der Werff, S. D. et al. Prediction of asthma by common risk factors: a follow-up study in Cuban schoolchildren. J. Investig. Allergol. Clin. Immunol. 23, 415–420 (2013).
Balemans, W. A. F. et al. Prediction of asthma in young adults using childhood characteristics: development of a prediction rule. J. Clin. Epidemiol. 59, 1207–1212 (2006).
Rosa, M. J. et al. Prenatal exposure to polycyclic aromatic hydrocarbons, environmental tobacco smoke and asthma. Respir. Med. 105, 869–876 (2011).
Selby, A. et al. Prevalence estimates and risk factors for early childhood wheeze across Europe: the EuroPrevall birth cohort. Thorax 73, 1049–1061 (2018).
Elder, D. E., Hagan, R., Evans, S. F., Benninger, H. R. & French, N. P. Recurrent wheezing in very preterm infants. Arch. Dis. Child Fetal Neonatal Ed. 74, F165–F171 (1996).
Takemura, Y. et al. Relation between breastfeeding and the prevalence of asthma: the Tokorozawa Childhood Asthma and Pollinosis Study. Am. J. Epidemiol. 154, 115–119 (2001).
Kurukulaaratchy, R. J., Matthews, S. & Arshad, S. H. Relationship between childhood atopy and wheeze: what mediates wheezing in atopic phenotypes? Ann. Allergy Asthma Immunol. 97, 84–91 (2006).
Al-Qerem, W. A., Ling, J., Pullen, R. & McGarry, K. Reported prevalence of allergy and asthma in children from urban and rural Egypt. Air Qual. Atmos. Health 9, 613–620 (2016).
Lemanske, R. F. et al. Rhinovirus illnesses during infancy predict subsequent childhood wheezing. J. Allergy Clin. Immunol. 116, 571–577 (2005).
Pokharel, P. K., Kabra, S. K., Kapoor, S. K. & Pandey, R. M. Risk factors associated with bronchial asthma in school going children of rural Haryana. Indian J. Pediatr. 68, 103–106 (2001).
Majeed, R., Rajar, U. D. M., Shaikh, N., Majeed, F. & Arain, A. A. Risk factors associated with childhood asthma. J. Coll. Physicians Surg. Pak. 18, 299–302 (2008).
Al-Kubaisy, W., Ali, S. H. & Al-Thamiri, D. Risk factors for asthma among primary school children in Baghdad, Iraq. Saudi Med. J. 26, 460–466 (2005).
Mpairwe, H. et al. Risk factors for asthma among schoolchildren who participated in a case–control study in urban Uganda. eLife 8, e49496 (2019).
Zejda, J. E. & Kowalska, M. Risk factors for asthma in school children—results of a seven-year follow-up. Cent. Eur. J. Public Health 11, 149–154 (2003).
Ehrlich, R. I. et al. Risk factors for childhood asthma and wheezing. Importance of maternal and household smoking. Am. J. Respir. Crit. Care Med. 154, 681–688 (1996).
Bozicević, I. & Oresković, S. Risk factors in asthmatic patients in Croatia. Coll. Antropol. 24, 325–334 (2000).
Kamran, A., Hanif, S. & Murtaza, G. Risk factors of childhood asthma in children attending Lyari General Hospital. J. Pak. Med. Assoc. 65, 647–650 (2015).
Palvo, F. et al. Risk factors of childhood asthma in Sao Jose do Rio Preto, Sao Paulo, Brazil. J. Trop. Pediatr. 54, 253–257 (2008).
Karunasekera, K. A., Jayasinghe, J. A. & Alwis, L. W. Risk factors of childhood asthma: a Sri Lankan study. J. Trop. Pediatr. 47, 142–145 (2001).
Flexeder, C. et al. Second-hand smoke exposure in adulthood and lower respiratory health during 20 year follow up in the European Community Respiratory Health Survey. Respir. Res. 20, 33 (2019).
Tanaka, K., Miyake, Y., Furukawa, S. & Arakawa, M. Secondhand smoke exposure and risk of wheeze in early childhood: a prospective pregnancy birth cohort study. Tob. Induc. Dis. 15, 30 (2017).
McKeever, T. M. et al. Siblings, multiple births, and the incidence of allergic disease: a birth cohort study using the West Midlands general practice research database. Thorax 56, 758–762 (2001).
Hadnadjev, M. & Ilić, M. Smoking and asthma in children. Med. Glas. (Zenica) 8, 266–272 (2011).
Genuneit, J. et al. Smoking and the incidence of asthma during adolescence: results of a large cohort study in Germany. Thorax 61, 572–578 (2006).
Horwood, L. J., Fergusson, D. M. & Shannon, F. T. Social and familial factors in the development of early childhood asthma. Pediatrics 75, 859–868 (1985).
Bergmann, R. L., Edenharter, G., Bergmann, K. E., Lau, S. & Wahn, U. Socioeconomic status is a risk factor for allergy in parents but not in their children. Clin. Exp. Allergy 30, 1740–1745 (2000).
Fagbule, D. & Ekanem, E. E. Some environmental risk factors for childhood asthma: a case–control study. Ann. Trop. Paediatr. 14, 15–19 (1994).
Kumar, P. H. & Devgan, A. The association of breastfeeding with childhood asthma: a case–control study from India. Cureus 13, e19810 (2021).
Daigler, G. E., Markello, S. J. & Cummings, K. M. The effect of indoor air pollutants on otitis media and asthma in children. Laryngoscope 101, 293–296 (1991).
Butland, B. K., Strachan, D. P. & Anderson, H. R. The home environment and asthma symptoms in childhood: two population based case–control studies 13 years apart. Thorax 52, 618–624 (1997).
Marbury, M. C., Maldonado, G. & Waller, L. The indoor air and children’s health study: methods and incidence rates. Epidemiology 7, 166–174 (1996).
Nguyen, T. et al. The National Asthma Survey–New York State: association of the home environment with current asthma status. Public Health Rep. 125, 877–887 (2010).
Bener, A., Ehlayel, M. & Sabbah, A. The pattern and genetics of pediatric extrinsic asthma risk factors in polluted environment. Eur. Ann. Allergy Clin. Immunol. 39, 58–63 (2007).
Ponsonby, A. L. et al. The relation between infant indoor environment and subsequent asthma. Epidemiology 11, 128–135 (2000).
Muñoz, B. et al. The relationship among IL-13, GSTP1, and CYP1A1 polymorphisms and environmental tobacco smoke in a population of children with asthma in Northern Mexico. Environ. Toxicol. Pharm. 33, 226–232 (2012).
Snodgrass, A. M. et al. Tobacco smoke exposure and respiratory morbidity in young children. Tob. Control 25, e75–e82 (2016).
den Dekker, H. T. et al. Tobacco smoke exposure, airway resistance, and asthma in school-age children: the Generation R Study. Chest 148, 607–617 (2015).
Murray, C. S. et al. Tobacco smoke exposure, wheeze, and atopy. Pediatr. Pulmonol. 37, 492–498 (2004).
Khozime, A., Mirsadraee, M. & Borji, H. Toxocara sero-prevalence and its relationship with allergic asthma in asthmatic patients in north-eastern Iran. J. Helminthol. 93, 677–680 (2019).
Fernando, D. et al. Toxocara seropositivity in Sri Lankan children with asthma. Pediatr. Int. 51, 241–245 (2009).
Youssef, M. M. et al. Urinary bisphenol A concentrations in relation to asthma in a sample of Egyptian children. Hum. Exp. Toxicol. 37, 1180–1186 (2018).
Villeneuve, P. J. et al. A case–control study of long-term exposure to ambient volatile organic compounds and lung cancer in Toronto, Ontario, Canada. Am. J. Epidemiol. 179, 443–451 (2014).
Zhong, L., Goldberg, M. S., Gao, Y. T. & Jin, F. A case–control study of lung cancer and environmental tobacco smoke among nonsmoking women living in Shanghai, China. Cancer Causes Control 10, 607–616 (1999).
Sasco, A. J. et al. A case–control study of lung cancer in Casablanca, Morocco. Cancer Causes Control 13, 609–616 (2002).
Wang, S. Y. et al. A comparative study of the risk factors for lung cancer in Guangdong, China. Lung Cancer 14, S99–S105 (1996).
Robles, A. I. et al. A DRD1 polymorphism predisposes to lung cancer among those exposed to secondhand smoke during childhood. Cancer Prev. Res. 7, 1210–1218 (2014).
Wang, A. et al. Active and passive smoking in relation to lung cancer incidence in the Women’s Health Initiative Observational Study prospective cohort. Ann. Oncol. 26, 221–230 (2015).
Zatloukal, P., Kubík, A., Pauk, N., Tomásek, L. & Petruzelka, L. Adenocarcinoma of the lung among women: risk associated with smoking, prior lung disease, diet and menstrual and pregnancy history. Lung Cancer 41, 283–293 (2003).
Torres-Durán, M. et al. Alpha-1 antitrypsin deficiency and lung cancer risk: a case–control study in never-smokers. J. Thorac. Oncol. 10, 1279–1284 (2015).
Tubío-Pérez, R. A. et al. Alpha-1 antitrypsin deficiency and risk of lung cancer in never-smokers: a multicentre case–control study. BMC Cancer 22, 81 (2022).
Ferreccio, C. et al. Arsenic, tobacco smoke, and occupation: associations of multiple agents with lung and bladder cancer. Epidemiology 24, 898–905 (2013).
Kabat, G. C. Aspects of the epidemiology of lung cancer in smokers and nonsmokers in the United States. Lung Cancer 15, 1–20 (1996).
Miller, D. P. et al. Association between self-reported environmental tobacco smoke exposure and lung cancer: modification by GSTP1 polymorphism. Int. J. Cancer 104, 758–763 (2003).
Zhuang, J. et al. Association between smoking and environmental tobacco smoke with lung cancer risk: a case–control study in the Fujian Chinese population. J. Public Health 30, 2047–2057 (2022).
Sobue, T. Association of indoor air pollution and lifestyle with lung cancer in Osaka, Japan. Int. J. Epidemiol. 19, S62–S66 (1990).
He, F., Chang, S.-C., Wallar, G. M., Zhang, Z.-F. & Cai, L. Association of XRCC3 and XRCC4 gene polymorphisms, family history of cancer and tobacco smoking with non-small-cell lung cancer in a Chinese population: a case–control study. J. Hum. Genet 58, 679–685 (2013).
Hirayama, T. Cancer mortality in nonsmoking women with smoking husbands based on a large-scale cohort study in Japan. Prev. Med. 13, 680–690 (1984).
Wang, F. L., Love, E. J., Liu, N. & Dai, X. D. Childhood and adolescent passive smoking and the risk of female lung cancer. Int. J. Epidemiol. 23, 223–230 (1994).
Seki, T. et al. Cigarette smoking and lung cancer risk according to histologic type in Japanese men and women. Cancer Sci. 104, 1515–1522 (2013).
Xu, X. et al. Combined and interaction effect of Chlamydia pneumoniae infection and smoking on lung cancer: a case–control study in Southeast China. BMC Cancer 20, 903 (2020).
Gallegos-Arreola, M. P. et al. CYP1A1 *2B and *4 polymorphisms are associated with lung cancer susceptibility in Mexican patients. Int. J. Biol. Markers 23, 24–30 (2008).
Wenzlaff, A. S. et al. CYP1A1 and CYP1B1 polymorphisms and risk of lung cancer among never smokers: a population-based study. Carcinogenesis 26, 2207–2212 (2005).
Ng, D. P. K., Tan, K.-W., Zhao, B. & Seow, A. CYP1A1 polymorphisms and risk of lung cancer in non-smoking Chinese women: influence of environmental tobacco smoke exposure and GSTM1/T1 genetic variation. Cancer Causes Control 16, 399–405 (2005).
Yoon, K.-A. et al. CYP1B1, CYP1A1, MPO, and GSTP1 polymorphisms and lung cancer risk in never-smoking Korean women. Lung Cancer 60, 40–46 (2008).
Gorlova, O. Y. et al. DNA repair capacity and lung cancer risk in never smokers. Cancer Epidemiol. Biomark. Prev. 17, 1322–1328 (2008).
Li, K. & Yu, S. Economic status, smoking, occupational exposure to rubber, and lung cancer: a case-cohort study. J. Environ. Sci. Health C 20, 21–28 (2002).
Jee, S. H., Ohrr, H. & Kim, I. S. Effects of husbands’ smoking on the incidence of lung cancer in Korean women. Int. J. Epidemiol. 28, 824–828 (1999).
Li, J. et al. Environmental tobacco smoke and cancer risk, a prospective cohort study in a Chinese population. Environ. Res. 191, 110015 (2020).
Jöckel, K. H., Pohlabeln, H., Ahrens, W. & Krauss, M. Environmental tobacco smoke and lung cancer. Epidemiology 9, 672–675 (1998).
Fontham, E. T. et al. Environmental tobacco smoke and lung cancer in nonsmoking women. A multicenter study. JAMA 271, 1752–1759 (1994).
Cardenas, V. M. et al. Environmental tobacco smoke and lung cancer mortality in the American Cancer Society’s Cancer Prevention Study. II. Cancer Causes Control 8, 57–64 (1997).
Stockwell, H. G. et al. Environmental tobacco smoke and lung cancer risk in nonsmoking women. J. Natl Cancer Inst. 84, 1417–1422 (1992).
Wen, W. et al. Environmental tobacco smoke and mortality in Chinese women who have never smoked: prospective cohort study. BMJ 333, 376 (2006).
Enstrom, J. E. & Kabat, G. C. Environmental tobacco smoke and tobacco related mortality in a prospective study of Californians, 1960–98. BMJ 326, 1057 (2003).
Mbeje, N. P., Ginindza, T. & Jafta, N. Epidemiological study of risk factors for lung cancer in KwaZulu-Natal, South Africa. Int. J. Environ. Res. Public Health 19, 6752 (2022).
Al-Zoughool, M. et al. Exposure to environmental tobacco smoke (ETS) and risk of lung cancer in Montreal: a case–control study. Environ. Health 12, 112 (2013).
Du, Y. et al. Exposure to environmental tobacco smoke and female lung cancer. Indoor Air 5, 231–236 (1995).
Boffetta, P. et al. Exposure to environmental tobacco smoke and risk of adenocarcinoma of the lung. Int. J. Cancer 83, 635–639 (1999).
Zaridze, D., Maximovitch, D., Zemlyanaya, G., Aitakov, Z. N. & Boffetta, P. Exposure to environmental tobacco smoke and risk of lung cancer in non-smoking women from Moscow, Russia. Int. J. Cancer 75, 335–338 (1998).
Chen, S.-C., Wong, R.-H., Shiu, L.-J., Chiou, M.-C. & Lee, H. Exposure to mosquito coil smoke may be a risk factor for lung cancer in Taiwan. J. Epidemiol. 18, 19–25 (2008).
Cassidy, A., Myles, J. P., Duffy, S. W., Liloglou, T. & Field, J. K. Family history and risk of lung cancer: age-at-diagnosis in cases and first-degree relatives. Br. J. Cancer 95, 1288–1290 (2006).
Yang, S.-Y. et al. Fanconi anemia genes in lung adenocarcinoma—a pathway-wide study on cancer susceptibility. J. Biomed. Sci. 23, 23 (2016).
Yin, Z. et al. Genetic polymorphisms of TERT and CLPTM1L, cooking oil fume exposure, and risk of lung cancer: a case–control study in a Chinese non-smoking female population. Med. Oncol. 31, 114 (2014).
Han, L. et al. Genetic predisposition to lung adenocarcinoma among never-smoking Chinese with different epidermal growth factor receptor mutation status. Lung Cancer 114, 79–89 (2017).
Raspanti, G. A. et al. Household air pollution and lung cancer risk among never-smokers in Nepal. Environ. Res. 147, 141–145 (2016).
Jin, Z.-Y. et al. Household ventilation may reduce effects of indoor air pollutants for prevention of lung cancer: a case–control study in a Chinese population. PLoS ONE 9, e102685 (2014).
Abdel-Rahman, O. Incidence and mortality of lung cancer among never smokers in relationship to secondhand smoking: findings from the PLCO trial. Clin. Lung Cancer 21, 415–420.e2 (2020).
Behera, D. & Balamugesh, T. Indoor air pollution as a risk factor for lung cancer in women. J. Assoc. Physicians India 53, 190–192 (2005).
Galeone, C. et al. Indoor air pollution from solid fuel use, chronic lung diseases and lung cancer in Harbin, Northeast China. Eur. J. Cancer Prev. 17, 473–478 (2008).
Sloan, C. D. et al. Indoor and outdoor air pollution and lung cancer in New Hampshire and Vermont. Toxicol. Environ. Chem. 94, https://doi.org/10.1080/02772248.2012.659930 (2012).
Garfinkel, L., Auerbach, O. & Joubert, L. Involuntary smoking and lung cancer: a case–control study. J. Natl Cancer Inst. 75, 463–469 (1985).
Lee, C. H. et al. Lifetime environmental exposure to tobacco smoke and primary lung cancer of non-smoking Taiwanese women. Int. J. Epidemiol. 29, 224–231 (2000).
Johnson, K. C., Hu, J. & Mao, Y., Canadian Cancer Registries Epidemiology Research Group. Lifetime residential and workplace exposure to environmental tobacco smoke and lung cancer in never-smoking women, Canada 1994–97. Int. J. Cancer 93, 902–906 (2001).
Gao, Y. T. et al. Lung cancer among Chinese women. Int. J. Cancer 40, 604–609 (1987).
Janerich, D. T. et al. Lung cancer and exposure to tobacco smoke in the household. N. Engl. J. Med. 323, 632–636 (1990).
Pirie, K. et al. Lung cancer in never smokers in the UK Million Women Study. Int. J. Cancer 139, 347–354 (2016).
Liang, D. et al. Lung cancer in never-smokers: a multicenter case–control study in north China. Front. Oncol. 9, 1354 (2019).
Kabat, G. C. & Wynder, E. L. Lung cancer in nonsmokers. Cancer 53, 1214–1221 (1984).
Wang, T. J., Zhou, B. S. & Shi, J. P. Lung cancer in nonsmoking Chinese women: a case–control study. Lung Cancer 14, S93–S98 (1996).
Rylander, R. & Axelsson, G. Lung cancer risks in relation to vegetable and fruit consumption and smoking. Int. J. Cancer 118, 739–743 (2006).
Humble, C. G., Samet, J. M. & Pathak, D. R. Marriage to a smoker and lung cancer risk. Am. J. Public Health 77, 598–602 (1987).
Koo, L. C., Ho, J. H., Saw, D. & Ho, C. Y. Measurements of passive smoking and estimates of lung cancer risk among non-smoking Chinese females. Int. J. Cancer 39, 162–169 (1987).
Weiss, J. M. et al. Menstrual and reproductive factors in association with lung cancer in female lifetime nonsmokers. Am. J. Epidemiol. 168, 1319–1325 (2008).
Hill, S. E., Blakely, T., Kawachi, I. & Woodward, A. Mortality among lifelong nonsmokers exposed to secondhand smoke at home: cohort data and sensitivity analyses. Am. J. Epidemiol. 165, 530–540 (2007).
McGhee, S. M. et al. Mortality associated with passive smoking in Hong Kong. BMJ 330, 287–288 (2005).
Boffetta, P. et al. Multicenter case–control study of exposure to environmental tobacco smoke and lung cancer in Europe. J. Natl Cancer Inst. 90, 1440–1450 (1998).
Davis, A. et al. No association between global DNA methylation in peripheral blood and lung cancer risk in nonsmoking women: results from a multicenter study in Eastern and Central Europe. Eur. J. Cancer Prev. 27, 1–5 (2018).
Veglia, F. et al. Occupational exposures, environmental tobacco smoke, and lung cancer. Epidemiology 18, 769–775 (2007).
Masjedi, M. R. et al. Opium could be considered an independent risk factor for lung cancer: a case–control study. Respiration 85, 112–118 (2013).
Consonni, D. et al. Outdoor particulate matter (PM10) exposure and lung cancer risk in the EAGLE study. PLoS ONE 13, e0203539 (2018).
Ren, Y.-W. et al. P53 Arg72Pro and MDM2 SNP309 polymorphisms cooperate to increase lung adenocarcinoma risk in Chinese female non-smokers: a case control study. Asian Pac. J. Cancer Prev. 14, 5415–5420 (2013).
Hole, D. J., Gillis, C. R., Chopra, C. & Hawthorne, V. M. Passive smoking and cardiorespiratory health in a general population in the west of Scotland. BMJ 299, 423–427 (1989).
Kalandidi, A. et al. Passive smoking and diet in the etiology of lung cancer among non-smokers. Cancer Causes Control 1, 15–21 (1990).
Rapiti, E., Jindal, S. K., Gupta, D. & Boffetta, P. Passive smoking and lung cancer in Chandigarh, India. Lung Cancer 23, 183–189 (1999).
Kurahashi, N. et al. Passive smoking and lung cancer in Japanese non-smoking women: a prospective study. Int. J. Cancer 122, 653–657 (2008).
Brownson, R. C., Alavanja, M. C., Hock, E. T. & Loy, T. S. Passive smoking and lung cancer in nonsmoking women. Am. J. Public Health 82, 1525–1530 (1992).
Nishino, Y. et al. Passive smoking at home and cancer risk: a population-based prospective study in Japanese nonsmoking women. Cancer Causes Control 12, 797–802 (2001).
Sun, Y.-Q. et al. Passive smoking in relation to lung cancer incidence and histologic types in Norwegian adults: the HUNT study. Eur. Respir. J. 50, 1700824 (2017).
Speizer, F. E., Colditz, G. A., Hunter, D. J., Rosner, B. & Hennekens, C. Prospective study of smoking, antioxidant intake, and lung cancer in middle-aged women (USA). Cancer Causes Control 10, 475–482 (1999).
Kabat, G. C., Stellman, S. D. & Wynder, E. L. Relation between exposure to environmental tobacco smoke and lung cancer in lifetime nonsmokers. Am. J. Epidemiol. 142, 141–148 (1995).
Shen, X. B., Wang, G. X. & Zhou, B. S. Relation of exposure to environmental tobacco smoke and pulmonary adenocarcinoma in non-smoking women: a case control study in Nanjing. Oncol. Rep. 5, 1221–1223 (1998).
Lee, P. N., Chamberlain, J. & Alderson, M. R. Relationship of passive smoking to risk of lung cancer and other smoking-associated diseases. Br. J. Cancer 54, 97–105 (1986).
Schwartz, A. G. et al. Reproductive factors, hormone use, estrogen receptor expression and risk of non small-cell lung cancer in women. J. Clin. Oncol. 25, 5785–5792 (2007).
Bräuner, E. V. et al. Residential radon and lung cancer incidence in a Danish cohort. Environ. Res. 118, 130–136 (2012).
Chan-Yeung, M. et al. Risk factors associated with lung cancer in Hong Kong. Lung Cancer 40, 131–140 (2003).
Minichilli, F. et al. Risk Factors for Lung Cancer in the Province of Lecce: results from the PROTOS case–control study in Salento (Southern Italy). Int. J. Environ. Res Public Health 19, 8775 (2022).
Ger, L. P., Hsu, W. L., Chen, K. T. & Chen, C. J. Risk factors of lung cancer by histological category in Taiwan. Anticancer Res. 13, 1491–1500 (1993).
Yang, L. et al. Risk factors shared by COPD and lung cancer and mediation effect of COPD: two center case–control studies. Cancer Causes Control 26, 11–24 (2015).
Liang, H. et al. Risk of lung cancer following nonmalignant respiratory conditions among nonsmoking women living in Shenyang, Northeast China. J. Women’s Health 18, 1989–1995 (2009).
Franco-Marina, F., Villalba Caloca, J. & Corcho-Berdugo, A., Grupo Interinstitucional de Cáncer Pulmonar. Role of active and passive smoking on lung cancer etiology in Mexico City. Salud Publica Mex. 48, S75–S82 (2006).
Phukan, R. K. et al. Role of household exposure, dietary habits and glutathione S-Transferases M1, T1 polymorphisms in susceptibility to lung cancer among women in Mizoram India. Asian Pac. J. Cancer Prev. 15, 3253–3260 (2014).
Asomaning, K. et al. Second hand smoke, age of exposure and lung cancer risk. Lung Cancer 61, 13–20 (2008).
He, Y. et al. Secondhand smoke exposure predicted COPD and other tobacco-related mortality in a 17-year cohort study in China. Chest 142, 909–918 (2012).
Wu, A. H., Henderson, B. E., Pike, M. C. & Yu, M. C. Smoking and other risk factors for lung cancer in women. J. Natl Cancer Inst. 74, 747–751 (1985).
Liu, Z. Y., He, X. Z. & Chapman, R. S. Smoking and other risk factors for lung cancer in Xuanwei, China. Int. J. Epidemiol. 20, 26–31 (1991).
Svensson, C., Pershagen, G. & Klominek, J. Smoking and passive smoking in relation to lung cancer in women. Acta Oncol. 28, 623–629 (1989).
Lam, T. H. et al. Smoking, passive smoking and histological types in lung cancer in Hong Kong Chinese women. Br. J. Cancer 56, 673–678 (1987).
Cheng, E. S. et al. Solid fuel, secondhand smoke, and lung cancer mortality: a prospective cohort of 323,794 chinese never-smokers. Am. J. Respir. Crit. Care Med. 206, 1153–1162 (2022).
Hansen, M. S., Licaj, I., Braaten, T., Lund, E. & Gram, I. T. The fraction of lung cancer attributable to smoking in the Norwegian Women and Cancer (NOWAC) Study. Br. J. Cancer 124, 658–662 (2021).
Dalager, N. A. et al. The relation of passive smoking to lung cancer. Cancer Res. 46, 4808–4811 (1986).
He, F. et al. The relationship of lung cancer with menstrual and reproductive factors may be influenced by passive smoking, cooking oil fumes, and tea intake: a case–control study in Chinese women. Medicine 96, e8816 (2017).
Wang, X.-R., Chiu, Y.-L., Qiu, H., Au, J. S. K. & Yu, I. T.-S. The roles of smoking and cooking emissions in lung cancer risk among Chinese women in Hong Kong. Ann. Oncol. 20, 746–751 (2009).
Spitz, M. R. et al. Variants in inflammation genes are implicated in risk of lung cancer in never smokers exposed to second-hand smoke. Cancer Discov. 1, 420–429 (2011).
Lan, Q. et al. Variation in lung cancer risk by smoky coal subtype in Xuanwei, China. Int. J. Cancer 123, 2164–2169 (2008).
Hernández-Garduño, E., Brauer, M., Pérez-Neria, J. & Vedal, S. Wood smoke exposure and lung adenocarcinoma in non-smoking Mexican women. Int. J. Tuberc. Lung Dis. 8, 377–383 (2004).
Huang, H.-C. et al. Association between chronic obstructive pulmonary disease and PM2.5 in Taiwanese nonsmokers. Int. J. Hyg. Environ. Health 222, 884–888 (2019).
Johannessen, A., Bakke, P. S., Hardie, J. A. & Eagan, T. M. L. Association of exposure to environmental tobacco smoke in childhood with chronic obstructive pulmonary disease and respiratory symptoms in adults. Respirology 17, 499–505 (2012).
Xu, F. et al. Better understanding the influence of cigarette smoking and indoor air pollution on chronic obstructive pulmonary disease: a case–control study in Mainland China. Respirology 12, 891–897 (2007).
Sandler, D. P., Comstock, G. W., Helsing, K. J. & Shore, D. L. Deaths from all causes in non-smokers who lived with smokers. Am. J. Public Health 79, 163–167 (1989).
Chan-Yeung, M. et al. Determinants of chronic obstructive pulmonary disease in Chinese patients in Hong Kong. Int. J. Tuberc. Lung Dis. 11, 502–507 (2007).
Vineis, P. et al. Environmental tobacco smoke and risk of respiratory cancer and chronic obstructive pulmonary disease in former smokers and never smokers in the EPIC prospective study. BMJ 330, 277 (2005).
Salameh, P. et al. Exposure to outdoor air pollution and chronic bronchitis in adults: a case–control study. Int. J. Occup. Environ. Med. 3, 165–177 (2012).
Pahwa, P. et al. Incidence and longitudinal changes in prevalence of chronic bronchitis in farm and non-farm rural residents of Saskatchewan. J. Occup. Environ. Med. 61, 347–356 (2019).
Behrendt, C. E. Mild and moderate-to-severe COPD in nonsmokers: distinct demographic profiles. Chest 128, 1239–1244 (2005).
Gerbase, M. W. et al. Respiratory effects of environmental tobacco exposure are enhanced by bronchial hyperreactivity. Am. J. Respir. Crit. Care Med. 174, 1125–1131 (2006).
Salama, B. M., Abukanna, A. & Hegazy, A. Risk factors associated with chronic obstructive pulmonary disease in Arar, Saudi Arabia: a case–control study. Med. Sci. 24, 2487–2493 (2020).
Chen, Y. et al. Risk factors for small airway obstruction among Chinese island residents: a case–control study. PLoS ONE 8, e68556 (2013).
Wu, C.-F. et al. Second-hand smoke and chronic bronchitis in Taiwanese women: a health-care based study. BMC Public Health 10, 44 (2010).
Diver, W. R., Jacobs, E. J. & Gapstur, S. M. Secondhand smoke exposure in childhood and adulthood in relation to adult mortality among never smokers. Am. J. Prev. Med. 55, 345–352 (2018).
Ding, Y. et al. The analyses of risk factors for COPD in the Li ethnic group in Hainan, People’s Republic of China. Int. J. Chron. Obstruct. Pulmon. Dis. 10, 2593–2600 (2015).
Dennis, R. J., Maldonado, D., Norman, S., Baena, E. & Martinez, G. Woodsmoke exposure and risk for obstructive airways disease among women. Chest 109, 115–119 (1996).
Hayashino, Y. et al. A prospective study of passive smoking and risk of diabetes in a cohort of workers: the High-Risk and Population Strategy for Occupational Health Promotion (HIPOP-OHP) study. Diabetes Care 31, 732–734 (2008).
Huang, C. et al. Association between environmental tobacco smoke exposure and risk of type 2 diabetes mellitus in Chinese female never smokers: a population-based cohort study. J. Diabetes 12, 339–346 (2020).
Zhang, L., Curhan, G. C., Hu, F. B., Rimm, E. B. & Forman, J. P. Association between passive and active smoking and incident type 2 diabetes in women. Diabetes Care 34, 892–897 (2011).
Kim, B. J., Kim, J. H., Kang, J. G., Kim, B. S. & Kang, J. H. Association between secondhand smoke exposure and diabetes mellitus in 131 724 Korean never smokers using self-reported questionnaires and cotinine levels: gender differences. J. Diabetes 13, 43–53 (2021).
Kowall, B. et al. Association of passive and active smoking with incident type 2 diabetes mellitus in the elderly population: the KORA S4/F4 cohort study. Eur. J. Epidemiol. 25, 393–402 (2010).
Zeng, H. et al. Interaction of PTPRD (rs17584499) polymorphism with passive smoking in Chinese women with susceptibility to type 2 diabetes. Int. J. Diabetes Dev. Ctries 43, 304–308 (2023).
Oba, S. et al. Passive smoking and type 2 diabetes among never-smoking women: the Japan Public Health Center-based Prospective Study. J. Diabetes Investig. 11, 1352–1358 (2020).
Rias, Y. A. et al. Secondhand smoke correlates with elevated neutrophil–lymphocyte ratio and has a synergistic effect with physical inactivity on increasing susceptibility to type 2 diabetes mellitus: a community-based case control study. Int. J. Environ. Res. Public Health 17, 5696 (2020).
Jiang, L. et al. Secondhand smoke, obesity, and risk of type II diabetes among California teachers. Ann. Epidemiol. 32, 35–42 (2019).
Kawachi, I. et al. A prospective study of passive smoking and coronary heart disease. Circulation 95, 2374–2379 (1997).
Pitsavos, C. et al. Association between exposure to environmental tobacco smoke and the development of acute coronary syndromes: the CARDIO2000 case–control study. Tob. Control 11, 220–225 (2002).
Awawdi, K., Steiner, H., Green, M. S. & Zelber-Sagi, S. Association between second-hand smoking and acute coronary heart disease among Arab women with multiple risk factors. Eur. J. Public Health 26, 141–145 (2016).
Ciruzzi, M. et al. Case–control study of passive smoking at home and risk of acute myocardial infarction. Argentine FRICAS Investigators. Factores de Riesgo Coronario en América del Sur. J. Am. Coll. Cardiol. 31, 797–803 (1998).
Kastorini, C.-M. et al. Comparative analysis of cardiovascular disease risk factors influencing nonfatal acute coronary syndrome and ischemic stroke. Am. J. Cardiol. 112, 349–354 (2013).
McElduff, P. et al. Coronary events and exposure to environmental tobacco smoke: a case–control study from Australia and New Zealand. Tob. Control 7, 41–46 (1998).
Clark, M. L., Butler, L. M., Koh, W.-P., Wang, R. & Yuan, J.-M. Dietary fiber intake modifies the association between secondhand smoke exposure and coronary heart disease mortality among Chinese non-smokers in Singapore. Nutrition 29, 1304–1309 (2013).
Spencer, C. A., Jamrozik, K. & Lambert, L. Do simple prudent health behaviours protect men from myocardial infarction? Int. J. Epidemiol. 28, 846–852 (1999).
Ding, D. et al. Effect of household passive smoking exposure on the risk of ischaemic heart disease in never-smoke female patients in Hong Kong. Tob. Control 18, 354–357 (2009).
Svendsen, K. H., Kuller, L. H., Martin, M. J. & Ockene, J. K. Effects of passive smoking in the Multiple Risk Factor Intervention Trial. Am. J. Epidemiol. 126, 783–795 (1987).
Garland, C., Barrett-Connor, E., Suarez, L., Criqui, M. H. & Wingard, D. L. Effects of passive smoking on ischemic heart disease mortality of nonsmokers. A prospective study. Am. J. Epidemiol. 121, 645–650 (1985).
Steenland, K., Thun, M., Lally, C. & Heath, C. Environmental tobacco smoke and coronary heart disease in the American Cancer Society CPS-II cohort. Circulation 94, 622–628 (1996).
Rosenlund, M. et al. Environmental tobacco smoke and myocardial infarction among never-smokers in the Stockholm Heart Epidemiology Program (SHEEP). Epidemiology 12, 558–564 (2001).
Rashid, N. A., Nawi, A. M. & Khadijah, S. Exploratory analysis of traditional risk factors of ischemic heart disease (IHD) among predominantly Malay Malaysian women. BMC Public Health 19, 545 (2019).
Muscat, J. E. & Wynder, E. L. Exposure to environmental tobacco smoke and the risk of heart attack. Int. J. Epidemiol. 24, 715–719 (1995).
Sadeghi, M. et al. Impact of secondhand smoke exposure in former smokers on their subsequent risk of coronary heart disease: evidence from the population-based cohort of the Tehran Lipid and Glucose Study. Epidemiol. Health 42, e2020009 (2020).
Malinauskiene, V. et al. Outdoor and indoor air pollution and myocardial infarction among women in Kaunas, Lithuania: a case–control study. Pol. J. Environ. Stud. 20, 969–976 (2011).
Sulo, G., Burazeri, G., Dehghan, A. & Kark, J. D. Partner’s smoking status and acute coronary syndrome: population-based case–control study in Tirana, Albania. Croat. Med. J. 49, 751–756 (2008).
La Vecchia, C., D’Avanzo, B., Franzosi, M. G. & Tognoni, G. Passive smoking and the risk of acute myocardial infarction GISSI-EFRIM investigations. Lancet 341, 505–506 (1993).
Janghorbani, M. & Sadeghigolmakani, N. Passive smoking and the risk of coronary heart disease among married non-smoking women. Med. J. Islam. Repub. Iran 11, 203–208 (1997).
Dobson, A. J., Alexander, H. M., Heller, R. F. & Lloyd, D. M. Passive smoking and the risk of heart attack or coronary death. Med. J. Aust. 154, 793–797 (1991).
He, Y. et al. Passive smoking at work as a risk factor for coronary heart disease in Chinese women who have never smoked. BMJ 308, 380–384 (1994).
Gallo, V. et al. Second-hand smoke, cotinine levels, and risk of circulatory mortality in a large cohort study of never-smokers. Epidemiology 21, 207–214 (2010).
Kobayashi, Y. et al. Secondhand smoke and the risk of incident cardiovascular disease among never-smoking women. Prev. Med. 162, 107145 (2022).
Notara, V. et al. Smoking determines the 10-year (2004–2014) prognosis in patients with acute coronary syndrome: the GREECS observational study. Tob. Induc. Dis. 13, 38 (2015).
Rossi, M., Negri, E., La Vecchia, C. & Campos, H. Smoking habits and the risk of non-fatal acute myocardial infarction in Costa Rica. Eur. J. Cardiovasc. Prev. Rehabil. 18, 467–474 (2011).
Fatmi, Z. et al. Solid fuel use is a major risk factor for acute coronary syndromes among rural women: a matched case control study. Public Health 128, 77–82 (2014).
Attard, R. et al. The impact of passive and active smoking on inflammation, lipid profile and the risk of myocardial infarction. Open Heart 4, e000620 (2017).
Nishtar, S. et al. Waist-hip ratio and low HDL predict the risk of coronary artery disease in Pakistanis. Curr. Med. Res. Opin. 20, 55–62 (2004).
Anderson, C. S. et al. Active and passive smoking and the risk of subarachnoid hemorrhage: an international population-based case–control study. Stroke 35, 633–637 (2004).
Qureshi, A. I., Suri, M. F. K., Kirmani, J. F. & Divani, A. A. Cigarette smoking among spouses: another risk factor for stroke in women. Stroke 36, e74–e76 (2005).
You, R. X., Thrift, A. G., McNeil, J. J., Davis, S. M. & Donnan, G. A. Ischemic stroke risk and passive exposure to spouses’ cigarette smoking. Melbourne Stroke Risk Factor Study (MERFS) Group. Am. J. Public Health 89, 572–575 (1999).
Hou, L. et al. Passive smoking and stroke in men and women: a national population-based case–control study in China. Sci. Rep. 7, 45542 (2017).
Bonita, R., Duncan, J., Truelsen, T., Jackson, R. T. & Beaglehole, R. Passive smoking as well as active smoking increases the risk of acute stroke. Tob. Control 8, 156–160 (1999).
Yamada, S. et al. Risk factors for fatal subarachnoid hemorrhage: the Japan Collaborative Cohort Study. Stroke 34, 2781–2787 (2003).
Malek, A. M., Cushman, M., Lackland, D. T., Howard, G. & McClure, L. A. Secondhand smoke exposure and stroke: The Reasons for Geographic and Racial Differences in Stroke (REGARDS) Study. Am. J. Prev. Med. 49, e89–e97 (2015).
Glymour, M. M., Defries, T. B., Kawachi, I. & Avendano, M. Spousal smoking and incidence of first stroke: the Health and Retirement Study. Am. J. Prev. Med. 35, 245–248 (2008).
Nishino, Y. et al. Stroke mortality associated with environmental tobacco smoke among never-smoking Japanese women: a prospective cohort study. Prev. Med. 67, 41–45 (2014).
Poulsen, A. H. et al. Urinary cadmium and stroke—a case–cohort study in Danish never-smokers. Environ. Res. 200, 111394 (2021).
Hirose, K. et al. A large-scale, hospital-based case–control study of risk factors of breast cancer according to menopausal status. Jpn J. Cancer Res. 86, 146–154 (1995).
Lash, T. L. & Aschengrau, A. A null association between active or passive cigarette smoking and breast cancer risk. Breast Cancer Res. Treat. 75, 181–184 (2002).
Woo, C. et al. A prospective study of passive cigarette smoke exposure and breast cancer. Am. J. Epidemiol. 151, 11 (2000).
Dossus, L. et al. Active and passive cigarette smoking and breast cancer risk: results from the EPIC cohort. Int. J. Cancer 134, 1871–1888 (2014).
Lash, T. L. & Aschengrau, A. Active and passive cigarette smoking and the occurrence of breast cancer. Am. J. Epidemiol. 149, 5–12 (1999).
Hanaoka, T. et al. Active and passive smoking and breast cancer risk in middle-aged Japanese women. Int. J. Cancer 114, 317–322 (2005).
Roddam, A. W. et al. Active and passive smoking and the risk of breast cancer in women aged 36–45 years: a population based case–control study in the UK. Br. J. Cancer 97, 434–439 (2007).
Gao, C.-M. et al. Active and passive smoking, and alcohol drinking and breast cancer risk in Chinese women. Asian Pac. J. Cancer Prev. 14, 993–996 (2013).
Lin, Y. et al. Active smoking, passive smoking, and breast cancer risk: findings from the Japan Collaborative Cohort Study for Evaluation of Cancer Risk. J. Epidemiol. 18, 77–83 (2008).
Smith, S. J., Deacon, J. M. & Chilvers, C. E. Alcohol, smoking, passive smoking and caffeine in relation to breast cancer risk in young women. UK National Case-Control Study Group. Br. J. Cancer 70, 112–119 (1994).
El-Sheikh, N. et al. Assessment of human papillomavirus infection and risk factors in Egyptian women with breast cancer. Breast Cancer 15, 1178223421996279 (2021).
Strumylaite, L. et al. Association between lifetime exposure to passive smoking and risk of breast cancer subtypes defined by hormone receptor status among non-smoking Caucasian women. PLoS ONE 12, e0171198 (2017).
Luo, J. et al. Association of active and passive smoking with risk of breast cancer among postmenopausal women: a prospective cohort study. BMJ 342, d1016 (2011).
Hu, M. et al. Bleomycin-induced mutagen sensitivity, passive smoking, and risk of breast cancer in Chinese women: a case–control study. Cancer Causes Control 24, 629–636 (2013).
Chaveepojnkamjorn, W., Thotong, R., Sativipawee, P. & Pitikultang, S. Body mass index and breast cancer risk among Thai premenopausal women: a case–control study. Asian Pac. J. Cancer Prev. 18, 3097–3101 (2017).
White, A. J., D’Aloisio, A. A., Nichols, H. B., DeRoo, L. A. & Sandler, D. P. Breast cancer and exposure to tobacco smoke during potential windows of susceptibility. Cancer Causes Control 28, 667–675 (2017).
Marzouk, D. A. et al. Breast cancer and hormonal intake among Egyptian females. Eur. J. Oncol. 14, 37–52 (2009).
Rollison, D. E., Brownson, R. C., Hathcock, H. L. & Newschaffer, C. J. Case–control study of tobacco smoke exposure and breast cancer risk in Delaware. BMC Cancer 8, 157 (2008).
Hsieh, Y.-C. et al. CHRNA9 polymorphisms and smoking exposure synergize to increase the risk of breast cancer in Taiwan. Carcinogenesis 35, 2520–2525 (2014).
Nishino, Y. et al. Cigarette smoking and breast cancer risk in relation to joint estrogen and progesterone receptor status: a case–control study in Japan. Springerplus 3, 65 (2014).
Ilic, M., Vlajinac, H. & Marinkovic, J. Cigarette smoking and breast cancer: a case–control study in Serbia. Asian Pac. J. Cancer Prev. 14, 6643–6647 (2014).
Xue, F., Willett, W. C., Rosner, B. A., Hankinson, S. E. & Michels, K. B. Cigarette smoking and the incidence of breast cancer. Arch. Intern. Med. 171, 125–133 (2011).
Millikan, R. C. et al. Cigarette smoking, N-acetyltransferases 1 and 2, and breast cancer risk. Cancer Epidemiol. Biomark. Prev. 7, 371–378 (1998).
Fararouei, M. et al. Dietary habits and physical activity are associated with the risk of breast cancer among young Iranian women: a case–control study on 1010 premenopausal women. Clin. Breast Cancer 19, e127–e134 (2019).
Tang, L.-Y. et al. Effects of passive smoking on breast cancer risk in pre/post-menopausal women as modified by polymorphisms of PARP1 and ESR1. Gene 524, 84–89 (2013).
Fu, X. J. et al. Environmental and DNA repair risk factors for breast cancer in South China. Int. J. Hyg. Environ. Health 218, 313–318 (2015).
Yassin, S., Younis, M., Abuzerr, S., Darwish, M. & Mustafa, A. A. Extrinsic risk factors for women breast cancer in Gaza Strip, Palestine: associations and interactions in a case–control study. Adv. Breast Cancer Res. 8, 11–30 (2019).
Dianatinasab, M., Fararouei, M., Mohammadianpanah, M., Zare-Bandamiri, M. & Rezaianzadeh, A. Hair coloring, stress, and smoking increase the risk of breast cancer: a case–control study. Clin. Breast Cancer 17, 650–659 (2017).
Ahern, T. P., Lash, T. L., Egan, K. M. & Baron, J. A. Lifetime tobacco smoke exposure and breast cancer incidence. Cancer Causes Control 20, 1837–1844 (2009).
Zhao, Y., Shi, Z. & Liu, L. Matched case–control study for detecting risk factors of breast cancer in women living in Chengdu. Zhonghua Liu Xing Bing Xue Za Zhi 20, 91–94 (1999).
Alberg, A. J. et al. N-acetyltransferase 2 (NAT2) genotypes, cigarette smoking, and the risk of breast cancer. Cancer Detect. Prev. 28, 187–193 (2004).
Johnson, K. C., Hu, J. & Mao, Y., Canadian Cancer Registries Epidemiology Research Group. Passive and active smoking and breast cancer risk in Canada, 1994–97. Cancer Causes Control 11, 211–221 (2000).
Anderson, L. N., Cotterchio, M., Mirea, L., Ozcelik, H. & Kreiger, N. Passive cigarette smoke exposure during various periods of life, genetic variants, and breast cancer risk among never smokers. Am. J. Epidemiol. 175, 289–301 (2012).
Pirie, K. et al. Passive smoking and breast cancer in never smokers: prospective study and meta-analysis. Int. J. Epidemiol. 37, 1069–1079 (2008).
Shrubsole, M. J. et al. Passive smoking and breast cancer risk among non-smoking Chinese women. Int. J. Cancer 110, 605–609 (2004).
Li, B. et al. Passive smoking and breast cancer risk among non-smoking women: a case–control study in China. PLoS ONE 10, e0125894 (2015).
Liu, L. et al. Passive smoking and other factors at different periods of life and breast cancer risk in chinese women who have never smoked—a case–control study in Chongqing, People’s Republic of China. Asian Pac. J. Cancer Prev. 1, 131–137 (2000).
Reynolds, P. et al. Passive smoking and risk of breast cancer in the California teachers study. Cancer Epidemiol. Biomark. Prev. 18, 3389–3398 (2009).
Wartenberg, D. et al. Passive smoking exposure and female breast cancer mortality. J. Natl Cancer Inst. 92, 1666–1673 (2000).
Tong, J. et al. Passive smoking exposure from partners as a risk factor for ER+/PR+ double positive breast cancer in never-smoking Chinese urban women: a hospital-based matched case control study. PLoS ONE 9, e97498 (2014).
Niehoff, N. et al. Polycyclic aromatic hydrocarbons and postmenopausal breast cancer: an evaluation of effect measure modification by body mass index and weight change. Environ. Res. 152, 17–25 (2017).
De Silva, M., Senarath, U., Gunatilake, M. & Lokuhetty, D. Prolonged breastfeeding reduces risk of breast cancer in Sri Lankan women: a case–control study. Cancer Epidemiol. 34, 267–273 (2010).
Morabia, A., Bernstein, M., Héritier, S. & Khatchatrian, N. Relation of breast cancer with passive and active exposure to tobacco smoke. Am. J. Epidemiol. 143, 918–928 (1996).
Kariri, M. et al. Risk factors for breast cancer in Gaza Strip, Palestine: a case–control study. Clin. Nutr. Res 6, 161–171 (2017).
Hosseinzadeh, M. et al. Risk factors for breast cancer in Iranian women: a hospital-based case–control study in Tabriz, Iran. J. Breast Cancer 17, 236–243 (2014).
Bonner, M. R. et al. Secondhand smoke exposure in early life and the risk of breast cancer among never smokers (United States). Cancer Causes Control 16, 683–689 (2005).
Zahali, Z., Mitra, A., Abd Rashid, A. A. & Jan Mohamed, H. J. Serum cotinine and passive smoking status associated with non-smoking newly diagnosed women with breast cancer in Malaysia. Middle East J. Cancer 12, 87–96 (2021).
Pimhanam, C., Sangrajrang, S. & Ekpanyaskul, C. Tobacco smoke exposure and breast cancer risk in Thai urban females. Asian Pac. J. Cancer Prev. 15, 7407–7411 (2014).
Metsola, K. et al. XRCC1 and XPD genetic polymorphisms, smoking and breast cancer risk in a Finnish case–control study. Breast Cancer Res. 7, R987–R997 (2005).
Dwedar, I. A. & El Sayed, M. A. Association between passive smoking and community-acquired pneumonia among the adult population. Egypt. J. Chest Dis. Tuberc. 67, 457 (2018).
Ramesh Bhat, Y., Manjunath, N., Sanjay, D. & Dhanya, Y. Association of indoor air pollution with acute lower respiratory tract infections in children under 5 years of age. Paediatr. Int. Child Health 32, 132–135 (2012).
Schulte-Hobein, B., Schwartz-Bickenbach, D., Abt, S., Plum, C. & Nau, H. Cigarette smoke exposure and development of infants throughout the first year of life: influence of passive smoking and nursing on cotinine levels in breast milk and infant’s urine. Acta Paediatr. 81, 550–557 (1992).
Baker, R. J. et al. Coal home heating and environmental tobacco smoke in relation to lower respiratory illness in Czech children, from birth to 3 years of age. Environ. Health Perspect. 114, 1126–1132 (2006).
Nuesslein, T. G., Beckers, D. & Rieger, C. H. Cotinine in meconium indicates risk for early respiratory tract infections. Hum. Exp. Toxicol. 18, 283–290 (1999).
Arlington, L. et al. Duration of solid fuel cookstove use is associated with increased risk of acute lower respiratory infection among children under six months in rural central India. PLoS ONE 14, e0224374 (2019).
Islam, M., Sultana, Z. Z., Iqbal, A., Ali, M. & Hossain, A. Effect of in-house crowding on childhood hospital admissions for acute respiratory infection: a matched case–control study in Bangladesh. Int. J. Infect. Dis. 105, 639–645 (2021).
Bermúdez Barrezueta, L. et al. Effect of prenatal and postnatal exposure to tobacco in the development of acute bronchiolitis in the first two years of life. An. Pediatr. (Engl. Ed.) 94, 385–395 (2021).
Loeb, M. et al. Environmental risk factors for community-acquired pneumonia hospitalization in older adults. J. Am. Geriatr. Soc. 57, 1036–1040 (2009).
Miyahara, R. et al. Exposure to paternal tobacco smoking increased child hospitalization for lower respiratory infections but not for other diseases in Vietnam. Sci. Rep. 7, 45481 (2017).
Barsam, F. J. B. G. et al. Factors associated with community-acquired pneumonia in hospitalised children and adolescents aged 6 months to 13 years old. Eur. J. Pediatr. 172, 493–499 (2013).
Goetghebuer, T., Kwiatkowski, D., Thomson, A. & Hull, J. Familial susceptibility to severe respiratory infection in early life. Pediatr. Pulmonol. 38, 321–328 (2004).
Roda, C. et al. Formaldehyde exposure and lower respiratory infections in infants: findings from the PARIS cohort study. Environ. Health Perspect. 119, 1653–1658 (2011).
le Roux, D. M., Myer, L., Nicol, M. P. & Zar, H. J. Incidence and severity of childhood pneumonia in the first year of life in a South African birth cohort: the Drakenstein Child Health Study. Lancet. Glob. Health 3, e95–e103 (2015).
Colley, J. R., Holland, W. W. & Corkhill, R. T. Influence of passive smoking and parental phlegm on pneumonia and bronchitis in early childhood. Lancet 2, 1031–1034 (1974).
Taylor, B. & Wadsworth, J. Maternal smoking during pregnancy and lower respiratory tract illness in early life. Arch. Dis. Child 62, 786–791 (1987).
Duijts, L. et al. Maternal smoking in prenatal and early postnatal life and the risk of respiratory tract infections in infancy. The Generation R study. Eur. J. Epidemiol. 23, 547–555 (2008).
Nenna, R. et al. Modifiable risk factors associated with bronchiolitis. Ther. Adv. Respir. Dis. 11, 393–401 (2017).
Fergusson, D. M. & Horwood, L. J. Parental smoking and respiratory illness during early childhood: a six-year longitudinal study. Pediatr. Pulmonol. 1, 99–106 (1985).
McConnochie, K. M. & Roghmann, K. J. Parental smoking, presence of older siblings, and family history of asthma increase risk of bronchiolitis. Am. J. Dis. Child 140, 806–812 (1986).
Rylander, E., Pershagen, G., Eriksson, M. & Bermann, G. Parental smoking, urinary cotinine, and wheezing bronchitis in children. Epidemiology 6, 289–293 (1995).
Almirall, J. et al. Passive smoking at home is a risk factor for community-acquired pneumonia in older adults: a population-based case–control study. BMJ Open 4, e005133 (2014).
Tupasi, T. E. et al. Patterns of acute respiratory tract infection in children: a longitudinal study in a depressed community in Metro Manila. Rev. Infect. Dis. 12, S940–S949 (1990).
Fuentes-Leonarte, V. et al. Pre- and postnatal exposure to tobacco smoke and respiratory outcomes during the first year. Indoor Air 25, 4–12 (2015).
Behrooz, L. et al. Prenatal and postnatal tobacco smoke exposure and risk of severe bronchiolitis during infancy. Respir. Med. 140, 21–26 (2018).
Lanari, M. et al. Prenatal tobacco smoke exposure increases hospitalizations for bronchiolitis in infants. Respir. Res. 16, 152 (2015).
Broughton, S. et al. Prospective study of healthcare utilisation and respiratory morbidity due to RSV infection in prematurely born infants. Thorax 60, 1039–1044 (2005).
Wright, A. L., Holberg, C., Martinez, F. D. & Taussig, L. M. Relationship of parental smoking to wheezing and nonwheezing lower respiratory tract illnesses in infancy. Group Health Medical Associates. J. Pediatr. 118, 207–214 (1991).
Koch, A. et al. Risk factors for acute respiratory tract infections in young Greenlandic children. Am. J. Epidemiol. 158, 374–384 (2003).
Törmänen, S. et al. Risk factors for asthma after infant bronchiolitis. Allergy 73, 916–922 (2018).
Farr, B. M. et al. Risk factors for community-acquired pneumonia diagnosed by general practitioners in the community. Respir. Med. 94, 422–427 (2000).
Farr, B. M., Bartlett, C. L., Wadsworth, J. & Miller, D. L. Risk factors for community-acquired pneumonia diagnosed upon hospital admission. British Thoracic Society Pneumonia Study Group. Respir. Med. 94, 954–963 (2000).
Grant, C. C. et al. Risk factors for community-acquired pneumonia in pre-school-aged children. J. Paediatr. Child Health 48, 402–412 (2012).
Victora, C. G., Fuchs, S. C., Flores, J. A., Fonseca, W. & Kirkwood, B. Risk factors for pneumonia among children in a Brazilian metropolitan area. Pediatrics 93, 977–985 (1994).
Verani, J. R. et al. Risk factors for presumed bacterial pneumonia among HIV-uninfected children hospitalized in Soweto, South Africa. Pediatr. Infect. Dis. J. 35, 1169–1174 (2016).
Broor, S. et al. Risk factors for severe acute lower respiratory tract infection in under-five children. Indian Pediatr. 38, 1361–1369 (2001).
Robledo-Aceves, M. et al. Risk factors for severe bronchiolitis caused by respiratory virus infections among Mexican children in an emergency department. Medicine 97, e0057 (2018).
Hassan, M. K. & Al-Sadoon, I. Risk factors for severe pneumonia in children in Basrah. Trop. Doct. 31, 139–141 (2001).
Farzana, R., Hoque, M., Kamal, M. S. & Choudhury, M. M. U. Role of parental smoking in severe bronchiolitis: a hospital based case–control study. Int. J. Pediatr. 2017, 9476367 (2017).
Liyanage, G., Kaneshapillai, A. & Kanthasamy, S. Serum vitamin D level and risk of community-acquired pneumonia: a case–control study. Interdiscip. Perspect. Infect. Dis. 2021, 2157337 (2021).
Johnson, A. W. & Aderele, W. I. The association of household pollutants and socio-economic risk factors with the short-term outcome of acute lower respiratory infections in hospitalized pre-school Nigerian children. Ann. Trop. Paediatr. 12, 421–432 (1992).
Liu, Y., Lu, C., Deng, M., Norbäck, D. & Sun, S. The effect of prenatal and early-postnatal exposure to classical air pollution on childhood pneumonia in China. Indoor Built Environ. 31, 170–185 (2022).
Keskinoglu, P., Cimrin, D. & Aksakoglu, G. The impact of passive smoking on the development of lower respiratory tract infections in children. J. Trop. Pediatr. 53, 319–324 (2007).
Dina, A. D. & Djuwita, R. The role of exclusive breastfeeding in reducing pneumonia prevalence in children under five. J. Gizi dan Pangan 16, 89–98 (2021).
Wenten, M. et al. TNF-308 modifies the effect of second-hand smoke on respiratory illness-related school absences. Am. J. Respir. Crit. Care Med. 172, 1563–1568 (2005).
Pullan, C. R. & Hey, E. N. Wheezing, asthma, and pulmonary dysfunction 10 years after infection with respiratory syncytial virus in infancy. Br. Med. J. 284, 1665–1669 (1982).
Prins-van Ginkel, A. C. et al. Acute otitis media during infancy: parent-reported incidence and modifiable risk factors. Pediatr. Infect. Dis. J. 36, 245–249 (2017).
Niclasen, J., Obel, C., Homøe, P., Kørvel-Hanquist, A. & Dammeyer, J. Associations between otitis media and child behavioural and learning difficulties: results from a Danish cohort. Int. J. Pediatr. Otorhinolaryngol. 84, 12–20 (2016).
Koch, A., Homøe, P., Pipper, C., Hjuler, T. & Melbye, M. Chronic suppurative otitis media in a birth cohort of children in Greenland: population-based study of incidence and risk factors. Pediatr. Infect. Dis. J. 30, 25–29 (2011).
Alho, O. P., Kilkku, O., Oja, H., Koivu, M. & Sorri, M. Control of the temporal aspect when considering risk factors for acute otitis media. Arch. Otolaryngol. Head. Neck Surg. 119, 444–449 (1993).
Bentdal, Y. E., Karevold, G., Nafstad, P. & Kvaerner, K. J. Early acute otitis media: predictor for AOM and respiratory infections in schoolchildren? Int. J. Pediatr. Otorhinolaryngol. 71, 1251–1259 (2007).
Daly, K. A., Pirie, P. L., Rhodes, K. L., Hunter, L. L. & Davey, C. S. Early otitis media among Minnesota American Indians: the Little Ears Study. Am. J. Public Health 97, 317–322 (2007).
Yang, C. Y. et al. Effects of indoor environmental factors on risk for acute otitis media in a subtropical area. J. Toxicol. Environ. Health A 56, 111–119 (1999).
Adair-Bischoff, C. E. & Sauve, R. S. Environmental tobacco smoke and middle ear disease in preschool-age children. Arch. Pediatr. Adolesc. Med. 152, 127–133 (1998).
Teele, D. W., Klein, J. O. & Rosner, B. Epidemiology of otitis media during the first seven years of life in children in greater Boston: a prospective, cohort study. J. Infect. Dis. 160, 83–94 (1989).
Daly, K. A. et al. Epidemiology of otitis media onset by six months of age. Pediatrics 103, 1158–1166 (1999).
Stenstrom, R., Bernard, P. A. & Ben-Simhon, H. Exposure to environmental tobacco smoke as a risk factor for recurrent acute otitis media in children under the age of five years. Int. J. Pediatr. Otorhinolaryngol. 27, 127–136 (1993).
Clamp, P. J. et al. Factors associated with the development of paediatric chronic otitis media by age nine: a prospective longitudinal cohort study of 6560 children. J. Laryngol. Otol. https://doi.org/10.1017/S0022215120002182 (2020).
Samson, D. et al. Follow up of a birth cohort to identify prevalence and risk factors for otitis media among Indian children in the eighth year of life. Int. J. Pediatr. Otorhinolaryngol. 137, 110201 (2020).
da Costa, J. L., Navarro, A., Neves, J. B. & Martin, M. Household wood and charcoal smoke increases risk of otitis media in childhood in Maputo. Int. J. Epidemiol. 33, 573–578 (2004).
Collet, J. P., Larson, C. P., Boivin, J. F., Suissa, S. & Pless, I. B. Parental smoking and risk of otitis media in pre-school children. Can. J. Public Health 86, 269–273 (1995).
Ey, J. L. et al. Passive smoke exposure and otitis media in the first year of life. Group Health Medical Associates. Pediatrics 95, 670–677 (1995).
Håberg, S. E. et al. Prenatal and postnatal parental smoking and acute otitis media in early childhood. Acta Paediatr. 99, 99–105 (2010).
Pukander, J., Luotonen, J., Timonen, M. & Karma, P. Risk factors affecting the occurrence of acute otitis media among 2–3-year-old urban children. Acta Otolaryngol. 100, 260–265 (1985).
Wijayanti, S. P. M. et al. Risk factors for acute otitis media in primary school children: a case–control study in Central Java, Indonesia. J. Public Health Res. 10, 1909 (2021).
Tainio, V. M. et al. Risk factors for infantile recurrent otitis media: atopy but not type of feeding. Pediatr. Res. 23, 509–512 (1988).
Ståhlberg, M. R., Ruuskanen, O. & Virolainen, E. Risk factors for recurrent otitis media. Pediatr. Infect. Dis. 5, 30–32 (1986).
Jensen, R. G., Koch, A., Homøe, P. & Bjerregaard, P. Tobacco smoke increases the risk of otitis media among Greenlandic Inuit children while exposure to organochlorines remain insignificant. Environ. Int. 54, 112–118 (2013).
Zheng, P. et al. The Burden of Proof studies: assessing the evidence of risk. Nat. Med. 28, 2038–2044 (2022).
Khoramdad, M. et al. Association between passive smoking and cardiovascular disease: a systematic review and meta-analysis. IUBMB Life 72, 677–686 (2020).
Zhang, D. et al. Dose-related effect of secondhand smoke on cardiovascular disease in nonsmokers: systematic review and meta-analysis. Int. J. Hyg. Environ. Health 228, 113546 (2020).
Lee, P. N. & Hamling, J. S. Environmental tobacco smoke exposure and risk of breast cancer in nonsmoking women. An updated review and meta-analysis. Inhal. Toxicol. 28, 431–454 (2016).
Lee, P. N., Thornton, A. J., Forey, B. A. & Hamling, J. S. Environmental tobacco smoke exposure and risk of stroke in never smokers: an updated review with meta-analysis. J. Stroke Cerebrovasc. Dis. 26, 204–216 (2017).
Lee, P. N., Forey, B. A., Coombs, K. J., Hamling, J. S. & Thornton, A. J. Epidemiological evidence relating environmental smoke to COPD in lifelong non-smokers: a systematic review. F1000Res 7, 146 (2018).
Huang, J. et al. Influencing factors of lung cancer in nonsmoking women: systematic review and meta-analysis. J. Public Health 44, 259–268 (2022).
DiFranza, J. R. & Lew, R. A. Morbidity and mortality in children associated with the use of tobacco products by other people. Pediatrics 97, 560–568 (1996).
Pan, A., Wang, Y., Talaei, M., Hu, F. B. & Wu, T. Relation of active, passive, and quitting smoking with incident type 2 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 3, 958–967 (2015).
Zhang, Y. et al. Risk factors for chronic and recurrent otitis media—a meta-analysis. PLoS ONE 9, e86397 (2014).
Liu, W., Wang, B., Xiao, Y., Wang, D. & Chen, W. Secondhand smoking and neurological disease: a meta-analysis of cohort studies. Rev. Environ. Health 36, 271–277 (2021).
Zhu, B., Wu, X., Wang, X., Zheng, Q. & Sun, G. The association between passive smoking and type 2 diabetes: a meta-analysis. Asia Pac. J. Public Health 26, 226–237 (2014).
He, J. et al. Passive smoking and the risk of coronary heart disease—a meta-analysis of epidemiologic studies. N. Engl. J. Med. 340, 920–926 (1999).
Lee, P. N., Forey, B. A., Hamling, J. S. & Thornton, A. J. Environmental tobacco smoke exposure and heart disease: a systematic review. World J. Meta-Anal. 5, 14–40 (2017).
Fischer, F. & Kraemer, A. Meta-analysis of the association between second-hand smoke exposure and ischaemic heart diseases, COPD and stroke. BMC Public Health 15, 1202 (2015).
Lv, X. et al. Risk of all-cause mortality and cardiovascular disease associated with secondhand smoke exposure: a systematic review and meta-analysis. Int. J. Cardiol. 199, 106–115 (2015).
Law, M. R., Morris, J. K. & Wald, N. J. Environmental tobacco smoke exposure and ischaemic heart disease: an evaluation of the evidence. BMJ 315, 973–980 (1997).
Whincup, P. H. et al. Passive smoking and risk of coronary heart disease and stroke: prospective study with cotinine measurement. BMJ 329, 200–205 (2004).
Oono, I. P., Mackay, D. F. & Pell, J. P. Meta-analysis of the association between secondhand smoke exposure and stroke. J. Public Health 33, 496–502 (2011).
Vos, T. et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet 396, 1204–1222 (2020).
Egan, K. M. et al. Active and passive smoking in breast cancer: prospective results from the Nurses’ Health Study. Epidemiology 13, 138–145 (2002).
Kocarnik, J. M. et al. Cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life years for 29 cancer groups from 2010 to 2019. JAMA Oncol. 8, 420–444 (2022).
Sio, Y. Y. & Chew, F. T. Risk factors of asthma in the Asian population: a systematic review and meta-analysis. J. Physiol. Anthropol. 40, 22 (2021).
He, Z. et al. The association between secondhand smoke and childhood asthma: a systematic review and meta-analysis. Pediatr. Pulmonol. 55, 2518–2531 (2020).
Protection from Exposure to Second-Hand Tobacco Smoke: Policy Recommendations (World Health Organization, 2007).
Stallings-Smith, S., Zeka, A., Goodman, P., Kabir, Z. & Clancy, L. Reductions in cardiovascular, cerebrovascular, and respiratory mortality following the National Irish Smoking Ban: interrupted time-series analysis. PLoS ONE 8, e62063 (2013).
Akter, S. et al. Evaluation of population-level tobacco control interventions and health outcomes: a systematic review and meta-analysis. JAMA Netw. Open 6, e2322341 (2023).
Chu, M. et al. Effects of a smoke-free policy in Xi’an, China: impact on hospital admissions for acute ischemic heart disease and stroke. Front. Public Health 10, 898461 (2022).
Rando-Matos, Y. et al. Smokefree legislation effects on respiratory and sensory disorders: a systematic review and meta-analysis. PLoS ONE 12, e0181035 (2017).
Gao, M. et al. The effect of smoke-free legislation on the mortality rate of acute myocardial infarction: a meta-analysis. BMC Public Health 19, 1269 (2019).
Huque, R. & Siddiqi, K. Smoke-free homes: the final frontier. Tob. Prev. Cessat. 7, 1–3 (2021).
Gallus, S. et al. Voluntary home smoking ban: prevalence, trend and determinants in Italy. Eur. J. Public Health 26, 841–844 (2016).
Du, Y. et al. Lung cancer occurrence attributable to passive smoking among never smokers in China: a systematic review and meta-analysis. Transl. Lung Cancer Res. 9, 204–217 (2020).
The Health Consequences of Involuntary Exposure to Tobacco Smoke: A Report of the Surgeon General (US Department of Health and Human Services, 2006).
Dai, X. et al. Health effects associated with smoking: a Burden of Proof study. Nat. Med. 28, 2045–2055 (2022).
Razo, C. et al. Effects of elevated systolic blood pressure on ischemic heart disease: a Burden of Proof study. Nat. Med. 28, 2056–2065 (2022).
Lescinsky, H. et al. Health effects associated with consumption of unprocessed red meat: a Burden of Proof study. Nat. Med. 28, 2075–2082 (2022).
Stanaway, J. D. et al. Health effects associated with vegetable consumption: a Burden of Proof study. Nat. Med. 28, 2066–2074 (2022).
Stevens, G. A. et al. Guidelines for accurate and transparent health estimates reporting: the GATHER statement. Lancet 388, e19–e23 (2016).
Guyatt, G. H. et al. GRADE guidelines: 4. Rating the quality of evidence—study limitations (risk of bias). J. Clin. Epidemiol. 64, 407–415 (2011).
Zheng, P., Barber, R., Sorensen, R. J. D., Murray, C. J. L. & Aravkin, A. Y. Trimmed constrained mixed effects models: formulations and algorithms. J. Comput. Graph. Stat. 30, 544–556 (2021).
Acknowledgements
Research reported in this publication was supported by the Bill & Melinda Gates Foundation (award OPP1152504, E.G.) and Bloomberg Philanthropies (award 47386, E.G.). The funders of the study had no role in study design, data collection, data analysis, data interpretation, writing of the final report or the decision to publish.
Author information
Authors and Affiliations
Contributions
J.W., J.A.A., N.A., C.O., G.F.G. and L.S.F. were primarily responsible for seeking, cataloging, extracting or cleaning data. S.C., C.O., G.F.G., J.A.A., J.W., L.S.F., N.A. and X.D. designed or coded figures and tables. M.J.M., R.J.D.S., L.S.F. and E.G. provided data or critical feedback on data sources. C.J.L.M., E.G., G.F.G., L.S.F., R.J.D.S., S.I.H., X.D. and S.C. provided critical feedback on methods or results. A.A., C.J.L.M., E.G., L.S.F., S.I.H. and S.A.M. drafted the work or revised it critically for important intellectual content. E.C.M., E.M.O., E.G., L.S.F. and S.I.H. managed the overall research enterprise. A.A., C.J.L.M., P.Z., R.J.D.S. and S.C. developed methods or computational machinery. L.S.F. was primarily responsible for applying analytical methods to produce estimates. L.S.F. wrote the first draft of the manuscript. S.I.H., E.M.O., E.G. and L.S.F. managed the estimation or publication process.
Corresponding author
Ethics declarations
Competing interests
The authors of this manuscript declare no competing interests.
Peer review
Peer review information
Nature Medicine thanks Bo Xi and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editors: Jennifer Sargent and Ming Yang, in collaboration with the Nature Medicine team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Forest plot of the association between secondhand smoke exposure and ischemic heart disease.
This forest plot presents the estimated mean relative risk, its 95% uncertainty intervals (UI), and the data points underlying the estimates for ischemic heart disease in association with secondhand smoke exposure (two-star rating of the risk-outcome relationship). The color of the point indicates whether the point was detected and trimmed as an outlier. The light blue interval corresponds to the 95% UI incorporating between-study heterogeneity; the dark blue interval corresponds to the 95% UI without between-study heterogeneity. The black vertical dotted line reflects the null relative risk value (one) and the red vertical line is the burden of proof function at the 5th quantile for this harmful risk-outcome association. The black data points and horizontal lines each correspond to a mean effect size and 95% UI from the included study identified on the y-axis. We included multiple observations from a single study when effects were reported by location or source of exposure and/or separately by sex or other subgroups. See Supplementary Table 4 for more details on included observations from each study (n = 37 studies).
Extended Data Fig. 2 Forest plot of the association between secondhand smoke exposure and stroke.
This forest plot presents the estimated mean relative risk, its 95% uncertainty intervals (UI), and the data points underlying the estimates for ischemic heart disease in association with secondhand smoke exposure (two-star rating of the risk-outcome relationship). The color of the point indicates whether the point was detected and trimmed as an outlier. The light blue interval corresponds to the 95% UI incorporating between-study heterogeneity; the dark blue interval corresponds to the 95% UI without between-study heterogeneity. The black vertical dotted line reflects the null relative risk value (one) and the red vertical line is the burden of proof function at the 5th quantile for this harmful risk-outcome association. The black data points and horizontal lines each correspond to a mean effect size and 95% UI from the included study identified on the y-axis. We included multiple observations from a single study when effects were reported by location or source of exposure and/or separately by sex or other subgroups. See Supplementary Table 5 for more details on included observations from each study (n = 20 studies).
Extended Data Fig. 3 Forest plot of the association between secondhand smoke exposure and lung cancer.
This forest plot presents the estimated mean relative risk, its 95% uncertainty intervals (UI), and the data points underlying the estimates for ischemic heart disease in association with secondhand smoke exposure (two-star rating of the risk-outcome relationship). The color of the point indicates whether the point was detected and trimmed as an outlier. The light blue interval corresponds to the 95% UI incorporating between-study heterogeneity; the dark blue interval corresponds to the 95% UI without between-study heterogeneity. The black vertical dotted line reflects the null relative risk value (one) and the red vertical line is the burden of proof function at the 5th quantile for this harmful risk-outcome association. The black data points and horizontal lines each correspond to a mean effect size and 95% UI from the included study identified on the y-axis. We included multiple observations from a single study when effects were reported by location or source of exposure and/or separately by sex or other subgroups. See Supplementary Table 6 for more details on included observations from each study (n = 104 studies).
Extended Data Fig. 4 Forest plot of the association between secondhand smoke exposure and breast cancer.
This forest plot presents the estimated mean relative risk, its 95% uncertainty intervals (UI), and the data points underlying the estimates for ischemic heart disease in association with secondhand smoke exposure (one-star rating of the risk-outcome relationship). The color of the point indicates whether the point was detected and trimmed as an outlier. The light blue interval corresponds to the 95% UI incorporating between-study heterogeneity; the dark blue interval corresponds to the 95% UI without between-study heterogeneity. The black vertical dotted line reflects the null relative risk value (one) and the red vertical line is the burden of proof function at the 5th quantile for this harmful risk-outcome association. The black data points and horizontal lines each correspond to a mean effect size and 95% UI from the included study identified on the y-axis. We included multiple observations from a single study when effects were reported by location or source of exposure and/or separately by sex or other subgroups. See Supplementary Table 7 for more details on included observations from each study (n = 51 studies).
Extended Data Fig. 5 Forest plot of the association between secondhand smoke exposure and asthma.
This forest plot presents the estimated mean relative risk, its 95% uncertainty intervals (UI), and the data points underlying the estimates for ischemic heart disease in association with secondhand smoke exposure (one-star rating of the risk-outcome relationship). The color of the point indicates whether the point was detected and trimmed as an outlier. The light blue interval corresponds to the 95% UI incorporating between-study heterogeneity; the dark blue interval corresponds to the 95% UI without between-study heterogeneity. The black vertical dotted line reflects the null relative risk value (one) and the red vertical line is the burden of proof function at the 5th quantile for this harmful risk-outcome association. The black data points and horizontal lines each correspond to a mean effect size and 95% UI from the included study identified on the y-axis. We included multiple observations from a single study when effects were reported by location or source of exposure and/or separately by sex or other subgroups. See Supplementary Table 8 for more details on included observations from each study (n = 125 studies).
Extended Data Fig. 6 Forest plot of the association between secondhand smoke exposure and lower respiratory infections.
This forest plot presents the estimated mean relative risk, its 95% uncertainty intervals (UI), and the data points underlying the estimates for ischemic heart disease in association with secondhand smoke exposure (one-star rating of the risk-outcome relationship). The color of the point indicates whether the point was detected and trimmed as an outlier. The light blue interval corresponds to the 95% UI incorporating between-study heterogeneity; the dark blue interval corresponds to the 95% UI without between-study heterogeneity. The black vertical dotted line reflects the null relative risk value (one) and the red vertical line is the burden of proof function at the 5th quantile for this harmful risk-outcome association. The black data points and horizontal lines each correspond to a mean effect size and 95% UI from the included study identified on the y-axis. We included multiple observations from a single study when effects were reported by location or source of exposure and/or separately by sex or other subgroups. See Supplementary Table 9 for more details on included observations from each study (n = 50 studies).
Extended Data Fig. 7 Forest plot of the association between secondhand smoke exposure and chronic obstructive pulmonary disease.
This forest plot presents the estimated mean relative risk, its 95% uncertainty intervals (UI), and the data points underlying the estimates for ischemic heart disease in association with secondhand smoke exposure (one-star rating of the risk-outcome relationship). The color of the point indicates whether the point was detected and trimmed as an outlier. The light blue interval corresponds to the 95% UI incorporating between-study heterogeneity; the dark blue interval corresponds to the 95% UI without between-study heterogeneity. The black vertical dotted line reflects the null relative risk value (one) and the red vertical line is the burden of proof function at the 5th quantile for this harmful risk-outcome association. The black data points and horizontal lines each correspond to a mean effect size and 95% UI from the included study identified on the y-axis. We included multiple observations from a single study when effects were reported by location or source of exposure and/or separately by sex or other subgroups. See Supplementary Table 10 for more details on included observations from each study (n = 21 studies).
Extended Data Fig. 8 plot of the association between secondhand smoke exposure and type 2 diabetes mellitus.
This forest plot presents the estimated mean relative risk, its 95% uncertainty intervals (UI), and the data points underlying the estimates for ischemic heart disease in association with secondhand smoke exposure (two-star rating of the risk-outcome relationship). The color of the point indicates whether the point was detected and trimmed as an outlier. The light blue interval corresponds to the 95% UI incorporating between-study heterogeneity; the dark blue interval corresponds to the 95% UI without between-study heterogeneity. The black vertical dotted line reflects the null relative risk value (one) and the red vertical line is the burden of proof function at the 5th quantile for this harmful risk-outcome association. The black data points and horizontal lines each correspond to a mean effect size and 95% UI from the included study identified on the y-axis. We included multiple observations from a single study when effects were reported by location or source of exposure and/or separately by sex or other subgroups. See Supplementary Table 11 for more details on included observations from each study (n = 9 studies).
Extended Data Fig. 9 plot of the association between secondhand smoke exposure and otitis media.
This forest plot presents the estimated mean relative risk, its 95% uncertainty intervals (UI), and the data points underlying the estimates for ischemic heart disease in association with secondhand smoke exposure (one-star rating of the risk-outcome relationship). The color of the point indicates whether the point was detected and trimmed as an outlier. The light blue interval corresponds to the 95% UI incorporating between-study heterogeneity; the dark blue interval corresponds to the 95% UI without between-study heterogeneity. The black vertical dotted line reflects the null relative risk value (one) and the red vertical line is the burden of proof function at the 5th quantile for this harmful risk-outcome association. The black data points and horizontal lines each correspond to a mean effect size and 95% UI from the included study identified on the y-axis. We included multiple observations from a single study when effects were reported by location or source of exposure and/or separately by sex or other subgroups. See Supplementary Table 12 for more details on included observations from each study (n = 24 studies).
Extended Data Fig. 10 Summarized results of the primary model and sensitivity analyses conducted across all nine health outcomes.
This heatmap reports the summarized results of the main model and the sensitivity analyses (columns) conducted for each of the nine health outcomes (rows) reported in this study. Detailed results for each of the sensitivity models are presented in the Supplementary Information (Supplementary Tables 13–16). Sensitivity analyses reflect the impact of restricting the input data to 1) prospective cohort studies, 2) observations associated with never-smokers, and 3) both prospective cohort studies and never-smoking samples. For asthma, we additionally restrict the data to children population aged 16 or less. General model parameters remained constant across models; we trimmed 10% of the data if more than 10 observations were available for the specific model. The color of the blue boxes and the number depicted in each box corresponds to the resulting risk-outcome score (ROS) calculated for models in which the estimates of association without incorporating between-study heterogeneity were statistically significant. Grey boxes depict models that did not pass this threshold and, thus, ROS did not apply (NA). For models that did pass this threshold, the ROS reflects a conservative interpretation of the data that aligns with the Burden of Proof approach incorporating between-study heterogeneity and other sources of uncertainty. The ROS is translated into a star rating from 1 to 5 stars based on thresholds outlined in Zheng et al. The star rating for each model result is reported as the yellow stars in each box. A one-star association suggests that there is weak evidence supporting estimates of an association between the risk and the outcome. A two-star association reflects that there is weak-to-moderate evidence suggesting an association between the risk and outcome, and additional stars illustrate increasing strength of evidence.
Supplementary information
Supplementary Information
Supplementary information.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Flor, L.S., Anderson, J.A., Ahmad, N. et al. Health effects associated with exposure to secondhand smoke: a Burden of Proof study. Nat Med 30, 149–167 (2024). https://doi.org/10.1038/s41591-023-02743-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41591-023-02743-4
This article is cited by
-
Secondhand smoke increases the risk of developing chronic obstructive pulmonary disease
Scientific Reports (2024)