Abstract
KRAS G12C mutation is prevalent in ~4% of colorectal cancer (CRC) and is associated with poor prognosis. Divarasib, a KRAS G12C inhibitor, has shown modest activity as a single agent in KRAS G12C-positive CRC at 400 mg. Epidermal growth factor receptor has been recognized as a major upstream activator of RAS–MAPK signaling, a proposed key mechanism of resistance to KRAS G12C inhibition in CRC. Here, we report on divarasib plus cetuximab (epidermal growth factor receptor inhibitor) in patients with KRAS G12C-positive CRC (n = 29) from arm C of an ongoing phase 1b trial. The primary objective was to evaluate safety. Secondary objectives included preliminary antitumor activity. The safety profile of this combination was consistent with those of single-agent divarasib and cetuximab. Treatment-related adverse events led to divarasib dose reductions in four patients (13.8%); there were no treatment withdrawals. The objective response rate was 62.5% (95% confidence interval: 40.6%, 81.2%) in KRAS G12C inhibitor-naive patients (n = 24). The median duration of response was 6.9 months. The median progression-free survival was 8.1 months (95% confidence interval: 5.5, 12.3). As an exploratory objective, we observed a decline in KRAS G12C variant allele frequency associated with response and identified acquired genomic alterations at disease progression that may be associated with resistance. The manageable safety profile and encouraging antitumor activity of divarasib plus cetuximab support the further investigation of this combination in KRAS G12C-positive CRC.
ClinicalTrials.gov identifier: NCT04449874
Similar content being viewed by others
Main
The Kirsten rat sarcoma virus oncogene homologue (KRAS) protein is a guanosine triphosphatase (GTPase) that cycles between the active GTP-bound and inactive GDP-bound states to regulate cell proliferation, migration and survival1,2. The glycine-to-cysteine mutation at position 12 (p.Gly12Cys) of the KRAS protein favors the active GTP-bound state, increasing downstream oncogenic signaling and uncontrolled cell growth. Oncogenic KRAS G12C mutations occur in ~4% of patients with CRC, and are associated with poor prognosis3,4,5,6,7. Patients with CRC who have tumors harboring a KRAS mutation (including KRAS G12C) do not benefit and thus are not eligible for anti-epidermal growth factor receptor-based therapies8,9.
Divarasib (GDC-6036) is an orally bioavailable, covalent KRAS G12C inhibitor that turns off its oncogenic signaling by irreversibly locking the protein in an inactive state. In vitro studies have also shown that divarasib is 5 to 20 times as potent and up to 50 times as selective as compared to the KRAS G12C inhibitors sotorasib and adagrasib10. Single-agent divarasib treatment at 400 mg achieved a confirmed objective response rate (ORR) of 56.4% in patients with non-small cell lung cancer and 35.9% in patients with CRC, with a median progression-free survival (PFS) of 13.1 and 6.9 months, respectively11.
Despite the encouraging antitumor activity with single-agent divarasib in patients with CRC, adaptive feedback reactivation of RAS–MAPK signaling, a proposed key mechanism of resistance to KRAS G12C inhibition in CRC, occurs frequently and ultimately limits efficacy12,13,14. Preclinical studies have identified EGFR as a major mediator of adaptive feedback, and concomitant blockade of EGFR has been shown to enhance the antitumor activity of KRAS G12C inhibition12,15. Therefore, the combination of an EGFR inhibitor with a KRAS G12C inhibitor may more effectively inhibit the KRAS–MAPK pathway, prevent adaptive feedback and lead to more robust clinical responses. Clinical studies of other KRAS G12C inhibitors, such as sotorasib and adagrasib, support this hypothesis, with higher response rates observed when used in combination with an EGFR inhibitor compared to when used as single-agent treatment in patients with CRC16,17,18,19,20.
Divarasib in combination with other anticancer therapies is currently being evaluated in an ongoing phase 1b study in patients with advanced or metastatic solid tumors harboring a KRAS G12C mutation. Here, we report results from arm C patients with CRC who received divarasib in combination with cetuximab, an EGFR inhibitor. The primary objective of this study was to evaluate safety; secondary objectives included characterization of preliminary antitumor activity and the pharmacokinetic profile, and exploratory objectives included the characterization of biomarkers of response and resistance.
Results
Patient disposition and baseline demographics
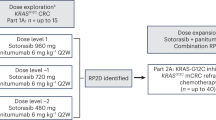
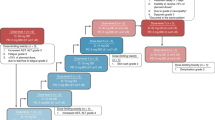
A total of 29 patients were enrolled into arm C from 17 sites in 10 countries between 28 July 2021 and 07 October 2022 (Fig. 1). Key inclusion criteria were CRC with documentation of the KRAS G12C mutation from either central testing of blood samples (FoundationOne Liquid CDx/F1LCDx) or local testing of tumor tissue or blood samples (using a validated molecular testing method) and evaluable or measurable disease per Response Evaluation Criteria in Solid Tumors (RECIST) v1.1. Patients with prior KRAS G12C inhibitor treatment must not have discontinued due to toxicity related to the prior KRAS G12C inhibitor. Key exclusion criteria were active, untreated central nervous system (CNS) metastases (previously treated CNS metastases were allowed); treatment with another anticancer therapy within 3 weeks or five half-lives before initiation of study treatment, whichever is shorter; and radiation therapy as cancer therapy within 4 weeks before initiation of divarasib.
Patients received oral divarasib at 200 mg (n = 3) or 400 mg (n = 26) once daily, plus intravenous cetuximab (400 mg/m2 on cycle 1 day 1 (C1D1) only, then 250 mg/m2 once weekly) in 21-d cycles. The selected expansion dose for the divarasib combination with cetuximab was 400 mg and ongoing dose investigations are still occurring in other combinations. The data cutoff date was 01 April 2023 with an enrollment cutoff of 07 October 2022. This analysis included patients who received at least one dose of divarasib and cetuximab. The median time on divarasib treatment was 7.1 months (range: 2.6–12.6), and on cetuximab treatment was 6.7 months (range: 0.5–12.2). Study treatment was discontinued in 20 patients (69.0%), with the reasons being progressive disease according to RECIST (n = 18, 62.1%), clinical progression (n = 1, 3.4%) or physician decision (n = 1, 3.4%).
Baseline demographics are summarized in Table 1. The median age was 59 years (range: 33–87) and patients received a median of 2 (range: 1–8) prior systemic therapies. All 29 (100.0%) patients received prior 5-fluorouracil (5-FU) or capecitabine, 27 (93.1%) received oxaliplatin, 24 (82.8%) received irinotecan, 24 (82.8%) received bevacizumab and 5 (17.2%) received a prior KRAS G12C inhibitor as a single agent or as a combination therapy (2 patients had single-agent divarasib, 1 had single-agent sotorasib, 1 had sotorasib with bevacizumab, and 1 had adagrasib with an inhibitor of Src homology region 2–containing protein tyrosine phosphatase-2 (SHP2)).
Safety
The primary objective of this study was to evaluate the safety of divarasib plus cetuximab. No dose-limiting toxicities were reported. All (100.0%) patients experienced at least one treatment-related adverse event (TRAE), with the majority of events being grade 1–2 (91.6%). The most common TRAEs included rash (96.6%; grouped term including dermatitis acneiform, rash, rash pustular, rash follicular and rash maculo-papular), diarrhea (82.8%), nausea (72.4%) and vomiting (48.3%). TRAEs occurring in ≥10% of patients are summarized in Table 2 and fully described in Extended Data Table 1. Grade 3 TRAEs occurred in 11 patients (37.9%) and included diarrhea (n = 3), increased lipase (n = 2), rash (n = 2) and abdominal pain, upper abdominal pain, dry skin, headache, hypomagnesemia, infusion-related reaction, insomnia, paronychia, pruritus and white blood cell count decrease (n = 1 for each). Grade 4 TRAEs occurred in 2 patients (6.9%) and included hypomagnesemia (n = 1) and neutropenia (n = 1). No grade 5 TRAEs occurred. Serious adverse events (AEs) occurred in 7 patients (24.1%), with none related to study treatment. Three deaths due to CRC progression occurred during safety follow-up.
TRAEs led to divarasib dose modifications (interruption, reduction or withdrawal) in 10 patients (34.5%), with divarasib dose interruptions in 7 patients (24.1%), divarasib dose reductions in 4 patients (13.8%) and no divarasib treatment withdrawals. TRAEs that led to divarasib dose reduction included diarrhea in 3 patients (10.3%, all grade 3) and vomiting in 1 patient (3.4%, grade 1). TRAEs led to cetuximab dose modifications (interruption, reduction or withdrawal) in 11 patients (37.9%), with cetuximab dose interruptions in 9 patients (31.0%), cetuximab dose reductions in 4 patients (13.8%) and cetuximab withdrawal in 1 patient (3.4%). TRAEs that led to cetuximab dose reduction include infusion-related reactions in 2 patients (6.9%, one grade 2 and one grade 1), and dermatitis acneiform, paronychia and pustular rash in 1 patient each (3.4%, grade 2 pustular rash, grade 2 paronychia and grade 3 dermatitis acneiform). Treatment-related grade 3 rash led to cetuximab withdrawal in 1 patient who continued to receive divarasib.
Antitumor activity
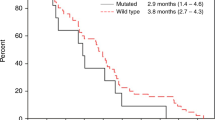
A secondary objective of this study was to assess the preliminary antitumor activity of divarasib plus cetuximab. Antitumor activity was determined by the investigator according to RECIST v1.1 (see the study end points in the Methods for further definitions), and all 29 patients had measurable disease at baseline (Fig. 2). Of the 24 patients who had not previously received a KRAS G12C inhibitor before enrollment, 1 patient received divarasib at 200 mg in combination with cetuximab and 23 patients received divarasib at 400 mg in combination with cetuximab. Among the 24 patients, 1 patient (4.2%; 1/1 confirmed) had a complete response (CR), 15 (62.5%; 14/15 confirmed) had a partial response (PR), and 8 (33.3%) had stable disease (SD) as their best response, for a confirmed ORR of 62.5% (95% confidence interval (CI): 40.6%, 81.2%). As an ad hoc end point, the median time to response was 1.4 months (range: 1.2–9.8). The median duration of response was 6.9 months (95% CI: 5.6, not estimable; Fig. 3). The median PFS was 8.1 months (95% CI: 5.5, 12.3). One patient experienced a 33.3% increase from baseline in the target lesion sum of diameters, but was assessed with a best response of SD based on the investigator’s interpretation of RECIST v1.1 in light of the response observed in the non-target lesions. Among the 5 patients who had received KRAS G12C inhibitors before enrollment, 3 (60.0%; 3/3 confirmed) had a PR and 2 (40.0%) had SD as their best response.
a, Waterfall plot showing the best percentage decrease from baseline in the tumor burden (defined as the sum of the longest diameters of all target lesions) in all 29 patients. b, Swimmer plot showing the time on study treatment, best response, and reason for treatment discontinuation for all 29 patients. c, Spider plot of the percentage changes from baseline in sum of tumor diameters over time in all 29 patients.
Pharmacokinetics
Another secondary objective in this study was to characterize the pharmacokinetic profile of divarasib plus cetuximab. We previously reported that the mean half-life of a single dose of divarasib 400 mg administered as a single agent was 17.6 ± 2.7 h and the corresponding accumulation index after daily dosing to steady state (area under the concentration–time curve from 0 to 24 h (RAUC0–24)) was 1.4 ± 0.4 (ref. 11). The steady-state pharmacokinetic profile and RAUC0–24 of divarasib (400 mg once daily) in combination with cetuximab were similar to single-agent divarasib (Extended Data Table 2 and Extended Data Fig. 1).
Biomarker analysis
Circulating tumor DNA (ctDNA) is an emerging biomarker for monitoring treatment effect, tracking tumor evolution and identifying potential mechanisms of resistance to treatment. As an exploratory objective in this study, retrospective ctDNA profiling was conducted with longitudinal plasma samples collected at baseline (C1D1), on-treatment (multiple time points) and end-of-treatment (EoT) visits.
KRAS G12C variant allele frequency (VAF) from circulating cell-free DNA (cfDNA) was monitored at baseline (C1D1) and at early on-treatment time points (cycle 1 day 15 (C1D15) and cycle 3 day 1 (C3D1)). At C1D1, the KRAS G12C mutation was detected from cfDNA in 22 of 25 patients evaluated. Decline in KRAS G12C VAF as early as C1D15 was observed in patients who had a response or SD. At C3D1, the VAF of KRAS G12C was <0.5% in 17 of these 22 patients (77.3%) including patients with a response or SD (Fig. 4a).
a, Shown is the KRAS G12C VAF at baseline (C1D1) and early treatment time points (C1D15, C3D1) among patients with a detectable KRAS G12C mutation from cfDNA at C1D1 (n = 20 shown, each line represents one patient). Two patients with missing C1D15 plasma samples are not shown in the plot. Box-and-whisker plots at each time point in each indication show the median (center line) with the minima and maxima box boundaries representing the 25th and 75th percentiles; whiskers represent the minimum and maximum values of the data that are within 1.5 times the interquartile range under the 25th and over the 75th percentiles. b, Shown are putative genetic mechanisms of acquired resistance to divarasib plus cetuximab combination treatment among 14 patients who had an EoT visit before 01 May 2023. Each row represents one patient with the first four columns describing assigned divarasib dose, best response, PFS, prior KRAS G12C inhibitor use and subsequent columns indicating acquired genomic alterations at EoT.
To explore the mechanisms of potential acquired resistance, we conducted ctDNA profiling from paired baseline and disease progression/EoT plasma samples. Of the 14 patients profiled, 13 patients (92.9%) had at least one acquired genomic alteration that may be associated with treatment resistance at EoT. Ten patients had at least one alteration in the KRAS gene (excluding the G12C mutation), including copy number gain/amplification, non-G12C pathogenic mutations that can result in oncogenic activation of KRAS, and secondary mutations that may diminish the binding of divarasib to the KRAS G12C protein. Other acquired genomic alterations observed at EoT include alterations in NRAS/HRAS (observed in 4/14 patients), genes in the MAPK pathway (8/14 patients), PI3K pathway (4/14 patients), RTK pathway (8/14 patients) and others (for example, MYC copy number gain; 2/14 patients; Fig. 4b).
Discussion
The KRAS G12C mutation is associated with worse overall survival in patients with CRC and is readily identified through standard-of-care testing, thus highlighting an area of need for more effective and targeted therapeutic approaches4. Current first-line standard of care for KRAS G12C-positive CRC includes 5-FU-based chemotherapy with irinotecan, oxaliplatin and/or capecitabine, but is limited by low tumor-specific selectivity and systemic toxicity21. Several KRAS G12C inhibitors, including divarasib, sotorasib and adagrasib, are currently being investigated as a single agent and in combination with EGFR inhibitors to target this unmet need, although they vary in their safety, antitumor activity and clinical benefit profiles.
The combination of divarasib and cetuximab appeared to be well tolerated, and AEs were manageable with supportive medications and dose modifications. The most common AEs observed in this study were low-grade gastrointestinal AEs and rash, which were consistent with single-agent divarasib and cetuximab safety profiles, respectively11,22. No patients withdrew divarasib treatment due to TRAEs and only 1 patient (3.4%) withdrew from cetuximab treatment due to a TRAE. Furthermore, there was a low rate of divarasib dose reductions (13.8%), with interruptions in 24.1% of patients. Among patients treated with adagrasib plus cetuximab in KRYSTAL-1 (n = 32), no patients discontinued adagrasib and 5 patients (16%) discontinued cetuximab due to TRAEs, with adagrasib dose reductions reported in 10 patients (31%)20. In the CodeBreaK300 study, TRAEs led to treatment discontinuation in 3.8% of patients in the 960 mg sotorasib plus panitumumab arm and 1.9% in the 240-mg sotorasib plus panitumumab arm17.
The improved antitumor activity observed with divarasib plus cetuximab in this study compared with single-agent divarasib is consistent with the hypothesis of EGFR-mediated adaptive feedback reactivation of the RAS–MAPK pathway following KRAS G12C inhibition. In patients with CRC who had not received prior KRAS G12C inhibitor treatment, single-agent divarasib achieved a response rate of 35.9%11, while the combination of divarasib and cetuximab achieved a response rate of 62.5%. Although cross-trial comparisons are difficult due to differences in patient populations, the ORR reported in this trial is numerically higher compared to that reported with 960 mg sotorasib plus panitumumab (26.4%) and adagrasib plus cetuximab (46%), as well as single-agent treatment of patients with CRC (35.9% for divarasib, 9.7% for sotorasib and 19% for adagrasib)16,17,20. Similarly, the median PFS of 8.1 months with divarasib observed in this trial is the longest reported for a KRAS G12C inhibitor combined with an EGFR inhibitor in patients with CRC (5.6 months (95% CI: 4.2, 6.3) with sotorasib plus panitumumab and 6.9 months (95% CI: 5.4, 8.1) for adagrasib plus cetuximab)17,20. Although preliminary evidence with the divarasib plus cetuximab combination is encouraging, this study was limited by the small number of patients enrolled into this arm and the lack of comparison of this divarasib and cetuximab combination to a standard-of-care treatment in KRAS G12C-positive CRC.
Divarasib has been shown to be 5 to 20 times more potent in vitro as compared to sotorasib and adagrasib10. This difference in potency may explain the numerically higher ORRs and longer PFS with divarasib compared to sotorasib and adagrasib, as a single-agent and in combination with an EGFR inhibitor in patients with CRC. This is further supported by a numerically higher ORR seen with divarasib in patients with non-small cell lung cancer (56.4%) compared with sotorasib (37% in a phase 2 trial and 28% in a phase 3 trial) and adagrasib (43% in a phase 2 trial)11,23,24,25. This current trial also included 5 patients who previously received a KRAS G12C inhibitor, with a PR observed in 3 of the 5 patients (60.0%) when treated with divarasib plus cetuximab. In contrast, patients who previously received single-agent adagrasib therapy showed no additional responses when subsequently treated with adagrasib plus cetuximab combination therapy20.
Divarasib plus cetuximab treatment led to a decrease in KRAS G12C ctDNA levels. Of note, divarasib plus cetuximab combination therapy showed a larger decline in KRAS G12C ctDNA levels compared to single-agent divarasib (77.3% versus 45.7% of patients with detectable KRAS G12C ctDNA at C1D1 had KRAS G12C VAF decline to below 0.5% at C3D1)11. Upon disease progression with divarasib plus cetuximab, the majority (92.9%) of patients profiled had at least one genomic alteration that may be associated with treatment resistance similarly to two previous reports26,27, which is higher than that of patients treated with divarasib (30.2%) or other KRAS G12C inhibitors as single agent11,28,29. This observation may be due to a more potent treatment effect with the combination of divarasib and cetuximab imposing a stronger selective pressure and/or potentially increasing the adaptive mutability of the residual cancer cells30.
In conclusion, divarasib in combination with cetuximab demonstrated a manageable safety profile and promising clinical activity. The further improvements in antitumor activity demonstrated with the addition of cetuximab to divarasib may represent an effective strategy for overcoming resistance to KRAS G12C inhibitors. Divarasib is continuing to be investigated with other anticancer therapies for patients with CRC in this study, including bevacizumab, GDC-1971 (a SHP2 inhibitor) and inavolisib (a PI3Kα inhibitor). Additionally, a study is ongoing to explore the effects of the combination of divarasib plus cetuximab with or without chemotherapy (FOLFOX: 5-FU, leucovorin and oxaliplatin; or FOLFIRI: leucovorin, 5-FU and irinotecan; ClinicalTrials.gov: NCT04929223) in patients with KRAS G12C-positive CRC.
Methods
Study design
Divarasib plus cetuximab combination data are reported from arm C of an ongoing phase 1b, open-label, multicenter, dose-escalation and dose-expansion study of divarasib in combination with other anticancer therapies in patients with advanced or metastatic solid tumors that harbor a KRAS G12C mutation (NCT04449874).
Patients received oral divarasib 200 or 400 mg once daily and intravenous cetuximab (400 mg/m2 on C1D1, then 250 mg/m2 once weekly) in 21-d cycles until intolerable toxicity, disease progression or patient withdrawal. Patients were enrolled in a 3 + 3 dose-escalation design at 200 mg and 400 mg divarasib, then enrolled into dose-expansion cohorts of 400 mg divarasib. As described in the protocol, an Internal Monitoring Committee was involved to ensure that appropriate patient safety oversight is maintained throughout the conduct of this study. The redacted protocol is available in the Supplementary Information.
This study was conducted in full conformance with the ICH E6 guideline for Good Clinical Practice and the Declaration of Helsinki, or the applicable laws and regulations of the country in which the research is conducted, whichever affords the greater protection to the individual. The protocol was approved by the institutional review boards at City of Hope Comprehensive Cancer Center, Princess Margaret Cancer Center, Peter MacCallum Cancer Center, Jewish General Hospital, Universitair Medisch Centrum Utrecht, Biokinetica Przychodnia Jozefow, Istituto Scientifico Romagnolo per lo Studio y la Cura dei Tumori, Asst Grande Ospedale Metropolitano Niguarda, UZ Antwerpen, Hospital Universitario Virgen Del Rocio, Hospital Universitari Vall D’hebron, Seoul National University Hospital, Seoul National University Bundang Hospital, Hospital Clinico Universitario De Valencia, Hospital Universitario 12 De Octubre, Sheba Medical Center and Abramson Cancer Center. Patients provided signed informed consent before enrollment.
Patients
Patients 18 years or older with locally advanced, recurrent or metastatic incurable adenocarcinoma of the colon or rectum harboring a KRAS G12C mutation documented by either central testing of blood samples (FoundationOne Liquid CDx/F1LCDx) or local testing of tumor tissue or blood samples (using a validated molecular testing method) were enrolled from 17 sites in 10 countries.
Patients were included based on the following inclusion criteria: disease that had progressed after at least one available standard therapy, or for which standard therapy has shown to be ineffective or intolerable, or for which a clinical trial of an investigational agent is a recognized standard of care; evaluable or measurable disease per RECIST v1.1; Eastern Cooperative Oncology Group performance status of 1 or lower (a five-point scale where higher numbers reflect greater disability); life expectancy ≥12 weeks; adequate hematologic and organ function within 14 d before initiation of study treatment defined as absolute neutrophil count ≥1,200/µl, hemoglobin ≥9 g dl−1, platelet count ≥100,000/µl, total bilirubin ≤1.5 times the upper limit of normal (ULN), serum albumin ≥2.5 g dl−1, aspartate transaminase (AST) and alanine transaminase (ALT) ≤ 2.5 times ULN (patients with documented liver metastases may have AST and ALT ≤ 5.0 times the ULN), serum creatinine ≤1.5 times the ULN or creatinine clearance ≥50 ml min−1 based on the Cockcroft–Gault estimation, and an international normalized ratio and activated partial thromboplastin time < 1.5 times the ULN (for those not receiving therapeutic anticoagulation); for women of childbearing potential to be abstinent or use contraception, and refrain from donating eggs/sperm for at least 6 months after divarasib and 2 months after cetuximab treatment; for nonsurgically sterile men to be abstinent or use contraception, and refrain from donating sperm for at least 4 months after divarasib and 2 months after cetuximab treatment; not have a known concomitant second oncogenic driver (for example, BRAFV600E mutation, ERBB2 amplification) as determined by Foundation Medicine; NGS assay or local testing of tumor tissue or blood samples (using a validated molecular testing method); and for patients required to provide pre-treatment and on-treatment biopsy samples, accessible lesion(s) that permit a total of at least two biopsy samples (pre-treatment and on-treatment) without unacceptable risk of a procedural complication. Patients with prior KRAS G12C inhibitor treatment must not have discontinued due to toxicity related to the prior KRAS G12C inhibitor and were required to provide tumor tissue specimens collected before and after treatment with the prior KRAS G12C inhibitor.
Patients were excluded based on the following criteria: inability/unwillingness to swallow pills; inability/unwillingness to comply with study or follow-up procedures; malabsorption syndrome or other condition that would interfere with enteral absorption; active, untreated CNS metastases (previously treated CNS metastases were allowed if they had measurable or evaluable disease outside the CNS, no history of intracranial or spinal cord hemorrhage, no ongoing requirement for corticosteroids for CNS metastases (with corticosteroids discontinued for ≥2 weeks before enrollment and no ongoing symptoms attributed to CNS metastases), no stereotactic radiation within 7 d or whole-brain radiation within 14 d before C1D1, and no evidence of interim progression between completion of CNS-directed therapy and screening radiographic study); leptomeningeal disease or carcinomatous meningitis; uncontrolled pleural effusion, pericardial effusion or ascites requiring recurrent drainage procedures biweekly or more frequently; any active infection that could impact patient safety or serious infection requiring intravenous antibiotics within 7 d before C1D1; clinical history of liver disease, including viral or other hepatitis, current alcohol abuse or cirrhosis; known HIV infection; any other diseases, active or uncontrolled pulmonary dysfunction, metabolic dysfunction, physical examination finding or clinical laboratory finding giving reasonable suspicion of a disease or condition that contraindicates the use of an investigational drug, that may affect the interpretation of the results, or renders the patients at high risk from treatment complications; uncontrolled hypercalcemia (>1.5 mmol−1 ionized calcium or 12 mg dl−1 calcium or ≥ corrected serum calcium ULN) or symptomatic hypercalcemia requiring continued use of bisphosphonate therapy or denosumab; traumatic injury or major surgical procedure within 4 weeks before C1D1; patients with chronic diarrhea, short bowel syndrome or upper gastrointestinal surgery including gastric resection, a history of inflammatory bowel disease or any active bowel inflammation (including diverticulitis); treatment with another anticancer therapy within 3 weeks or five half-lives before initiation of study treatment, whichever is shorter, or endocrine therapy within 2 weeks before initiation of study treatment, except for hormonal therapy with gonadotropin-releasing hormone agonists or antagonists for endocrine-sensitive cancers (approved kinase inhibitors may be used up to 2 weeks before initiation of study treatment, provided any drug-related toxicity has completely resolved); radiation therapy as cancer therapy within 4 weeks before initiation of study treatment; palliative radiation to bony metastases within 2 weeks before initiation of divarasib; AEs from prior anticancer therapy that have not resolved to grade 1 except for alopecia, vitiligo, endocrinopathy managed with replacement therapy or grade 2 peripheral neuropathy; history of other malignancy within 5 years before screening, with the exception of patients with a negligible risk of metastasis or death and/or treated with expected curative outcome; history of or active cardiovascular dysfunction, including history of stroke or transient ischemic attack within 6 months before first dose of study treatment, history of myocardial infarction within 6 months before first dose of study treatment, New York Heart Association Class III or IV cardiac disease or congestive heart failure requiring medication, uncontrolled arrhythmias, history of or active ventricular arrhythmia requiring medication, coronary heart disease that is symptomatic or unstable angina, congenital long QT syndrome or QT interval corrected through use of Fridericia’s formula >470 ms demonstrated by at least two electrocardiograms 30 min apart, or family history of sudden unexplained death or long QT syndrome, current treatment with medications that are well known to prolong the QT interval; pregnant or breastfeeding, or intending to become pregnant during the study or within 6 months after the final dose of divarasib (women of childbearing potential (including those who have had a tubal ligation) must have a negative serum pregnancy test result within 14 d before initiation of study drug); presence of appendiceal tumor; known hypersensitivity to study treatments; and history of idiopathic pulmonary fibrosis, organizing pneumonia (for example, bronchiolitis obliterans), drug-induced pneumonitis, or idiopathic pneumonitis, or evidence of active pneumonitis on screening chest computed tomography scan (history of radiation pneumonitis in the radiation field (fibrosis) was permitted).
Study assessments
Safety was assessed through the evaluation of AEs (NCI CTCAE v5.0), changes in laboratory test results and changes in vital signs and electrocardiograms, and included all patients who received at least one dose of both study drugs. Attribution of AEs to study drug was determined by the investigator. For each AE requiring a dose modification, only one action taken with study drug was selected according to the following hierarchy: withdrawal, reduction and interruption. Preliminary antitumor activity was determined by the investigator according to RECIST v1.1. Confirmed ORR was defined as the proportion of patients with measurable disease at baseline with CR or PR on two consecutive tumor assessments at least 4 weeks apart, while the best response did not require a confirmatory assessment. PFS was defined as the time from first treatment to the first occurrence of disease progression or death from any cause during the study (whichever occurred first).
Pharmacokinetics
Pharmacokinetic parameters were estimated using non-compartmental analysis of concentration–time data. Plasma samples were evaluated for divarasib using a validated liquid chromatography tandem mass spectrometry assay. The lower and upper limit of quantification for divarasib was 5 ng ml−1 and 5,000 ng ml−1, respectively. A stable labeled internal standard was used. Pharmacokinetic non-compartmental analysis was performed with nominal time using Phoenix WinNonlin (Certara USA, version 8.3). Graphical visualization was done using R (version 4.2.0; R Foundation for Statistical Computing). Pharmacokinetic samples at steady state were collected before dose, and at 0.5, 1, 2, 3, 4 and 8 h after dose on cycle 1 day 8 (C1D8) or on cycle 2 day 1 (C2D1). C1D8 or C2D1 AUC0–24 calculations included imputation of the 24-h time point based on pre-dose C1D8 or C2D1 plasma concentration of divarasib.
Statistical analysis
The study planned to enroll approximately 29 patients in the cetuximab combination cohorts. This study was intended to obtain preliminary safety, pharmacokinetic and antitumor activity information and the sample sizes do not reflect any power and type I considerations. The data cutoff date was 01 April 2023 with an enrollment cutoff of 07 October 2022. This analysis included all patients who received at least one dose of divarasib and cetuximab. The response rate was reported for patients with measurable disease at baseline and summarized with 95% CIs calculated using the Clopper–Pearson method. The time-to-event end points, including time to response, duration of response and PFS, were reported descriptively and were summarized using the Kaplan–Meier method; median estimates were reported with 95% CIs.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
KRAS G12C VAF data are available in the Supplementary Information. For eligible studies, qualified researchers may request access to individual patient-level clinical data through a data request platform. At the time of writing, this request platform is Vivli (https://vivli.org/ourmember/roche/). As this study is ongoing, access to patient-level data from this trial will not be available until at least 18 months after the last patient visit and a clinical study report has been completed. After that time, requests for data will be assessed by an independent review panel, which decides whether the data will be provided. On average, it takes a few months to access data in the Vivli platform, but the timeline will vary depending on the number of data contributors, the number of studies and your availability to respond to comments. Once approved, the data are available for up to 24 months.
For up-to-date details on Roche’s Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents, see https://www.roche.com/innovation/process/clinical-trials/data-sharing/. Anonymized records for individual patients across more than one data source external to Roche cannot, and should not, be linked due to a potential increase in risk of patient reidentification.
References
Khan, A. Q. et al. RAS-mediated oncogenic signaling pathways in human malignancies. Semin. Cancer Biol. 54, 1–13 (2019).
Moore, A. R., Rosenberg, S. C., McCormick, F. & Malek, S. RAS-targeted therapies: is the undruggable drugged? Nat. Rev. Drug Discov. 19, 533–552 (2020).
Henry, J. T. et al. Comprehensive clinical and molecular characterization of KRASG12C-mutant colorectal cancer. JCO Precis. Oncol. 5, PO.20.00256 (2021).
Jones, R. P. et al. Specific mutations in KRAS codon 12 are associated with worse overall survival in patients with advanced and recurrent colorectal cancer. Br. J. Cancer 116, 923–929 (2017).
Lee, J. K. et al. Comprehensive pan-cancer genomic landscape of KRAS altered cancers and real-world outcomes in solid tumors. NPJ Precis. Oncol. 6, 91 (2022).
Nassar, A. H., Adib, E. & Kwiatkowski, D. J. Distribution of KRASG12C somatic mutations across race, sex, and cancer type. N. Engl. J. Med. 384, 185–187 (2021).
Schirripa, M. et al. KRAS G12C metastatic colorectal cancer: specific features of a new emerging target population. Clin. Colorectal Cancer 19, 219–225 (2020).
Allegra, C. J. et al. American Society of Clinical Oncology provisional clinical opinion: testing for KRAS gene mutations in patients with metastatic colorectal carcinoma to predict response to anti-epidermal growth factor receptor monoclonal antibody therapy. J. Clin. Oncol. 27, 2091–2096 (2009).
Van Cutsem, E. et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann. Oncol. 27, 1386–1422 (2016).
Purkey, H. Discovery of GDC-6036, a clinical stage treatment for KRAS G12C-positive cancers. in AACR Annual Meeting (New Orleans, 2022).
Sacher, A. et al. Single-agent divarasib (GDC-6036) in solid tumors with a KRAS G12C mutation. N. Engl. J. Med. 389, 710–721 (2023).
Amodio, V. et al. EGFR blockade reverts resistance to KRASG12C inhibition in colorectal cancer. Cancer Discov. 10, 1129–1139 (2020).
Ryan, M. B. et al. KRASG12C-independent feedback activation of wild-type RAS constrains KRASG12C inhibitor efficacy. Cell Rep. 39, 110993 (2022).
Ryan, M. B. et al. Vertical pathway inhibition overcomes adaptive feedback resistance to KRASG12C Inhibition. Clin. Cancer Res. 26, 1633–1643 (2020).
Lito, P., Solomon, M., Li, L. S., Hansen, R. & Rosen, N. Allele-specific inhibitors inactivate mutant KRAS G12C by a trapping mechanism. Science 351, 604–608 (2016).
Fakih, M. G. et al. Sotorasib for previously treated colorectal cancers with KRASG12C mutation (CodeBreaK100): a prespecified analysis of a single-arm, phase 2 trial. Lancet Oncol. 23, 115–124 (2022).
Fakih, M. G. et al. Sotorasib plus panitumumab in refractory colorectal cancer with mutated KRAS G12C. N. Engl. J. Med. https://doi.org/10.1056/NEJMoa2308795 (2023).
Hong, D. S. et al. KRASG12C inhibition with sotorasib in advanced solid tumors. N. Engl. J. Med. 383, 1207–1217 (2020).
Ou, S. I. et al. First-in-human phase I/Ib dose-finding study of adagrasib (MRTX849) in patients with advanced KRASG12C solid tumors (KRYSTAL-1). J. Clin. Oncol. 40, 2530–2538 (2022).
Yaeger, R. et al. Adagrasib with or without cetuximab in colorectal cancer with mutated KRAS G12C. N. Engl. J. Med. 388, 44–54 (2023).
Xie, Y. H., Chen, Y. X. & Fang, J. Y. Comprehensive review of targeted therapy for colorectal cancer. Signal Transduct. Target Ther. 5, 22 (2020).
ERBITUX (cetuximab) [package insert]. Branchburg, NJ: ImClone (2004). Accessed 08 Nov 2023.
de Langen, A. J. et al. Sotorasib versus docetaxel for previously treated non-small-cell lung cancer with KRASG12C mutation: a randomised, open-label, phase 3 trial. Lancet 401, 733–746 (2023).
Janne, P. A. et al. Adagrasib in non-small-cell lung cancer harboring a KRASG12C mutation. N. Engl. J. Med. 387, 120–131 (2022).
Skoulidis, F. et al. Sotorasib for lung cancers with KRAS p.G12C mutation. N. Engl. J. Med. 384, 2371–2381 (2021).
Yaeger, R. et al. Molecular characterization of acquired resistance to KRASG12C–EGFR Inhibition in colorectal cancer. Cancer Discov. 13, 41–55 (2023).
Hong, D. S. et al. Abstract 2308: Biomarkers of acquired resistance to sotorasib (soto) plus panitumumab (pani) in chemorefractory KRAS G12C-mutated metastatic colorectal cancer (mCRC). Cancer Res. 83, 2308 (2023).
Awad, M. M. et al. Acquired resistance to KRASG12C inhibition in cancer. N. Engl. J. Med. 384, 2382–2393 (2021).
Li, B. T. et al. Largest evaluation of acquired resistance to sotorasib in KRAS p.G12C-mutated non–small cell lung cancer (NSCLC) and colorectal cancer (CRC): plasma biomarker analysis of CodeBreaK100. J. Clin. Oncol. 40, 102 (2022).
Russo, M. et al. Adaptive mutability of colorectal cancers in response to targeted therapies. Science 366, 1473–1480 (2019).
Acknowledgements
This study was sponsored by Genentech. We thank the patients and family members that were involved in this trial, as well as the clinical study teams. Writing assistance was provided by A. Occiano and S. Diaz of Genentech.
Author information
Authors and Affiliations
Consortia
Contributions
All authors participated in study design and conduct, in data acquisition, analysis and interpretation and in drafting and revising the manuscript. Y.C. performed statistical analyses. S.M. verified the pharmacokinetic data. Z.S. verified the biomarker data. All authors approved the final version before submission.
Corresponding authors
Ethics declarations
Competing interests
J.D. served as a consultant for BeiGene, Pierre Fabre, Bayer, GSK, Merck, Boehringer Ingelheim, Roche/Genentech, Daiichi Sankyo Europe, Novartis, Pfizer, Ellipses Pharma, Axelia Oncology and Amgen; and reports funding to their institution from Roche, GSK, Novartis, BeiGene, Lilly, Bristol Myers Squibb, AstraZeneca/MedImmune and Amgen. S.H.K. was a consultant on the advisory boards for Pfizer, Daiichi, Takeda and Astellas; and reports funding to their institution from Ono Pharma and Eisai Korea. A.C. received institutional research funding from Genentech, Merck Serono, Bristol Myers Squibb, Merck Sharp & Dohme, Roche, Beigene, Bayer, Servier, Lilly, Novartis, Takeda, Astellas, Natera and Fibrogen and advisory board or speaker fees from AbbVie, Amgen, Merck Serono, GlaxoSmithKline and Roche. T.K. has received honoraria from Incyte, Pfizer, Ipsen, AstraZeneca/MedImmune, Taiho Oncology, Elevar Therapeutics and Nucorion; and reports funding to their institution from Taiho Pharmaceutical, H3 Biomedicine, Bristol Myers Squibb, Lilly, Tempest Therapeutics, Xencor, Genentech, Incyte and Totus Medicines. L.M. has received travel and accommodation fees from Sevier, Novartis, Janssen, Bristol Myers Squibb; is on the speaker’s bureau for Novartis; has received research funding from AstraZeneca; and reports funding to their institution from Bristol Myers Squibb, AstraZeneca, Abbvie, Genmab, Nelum, Numab and Precision Medicine. E.S. reports funding to their institution from Amgen, Bristol Myers Squibb, Daiichi Sankyo, Genentech, Lutris Pharma, MSD, Novartis and Pangea Pharma. R.C. reports funding to their institution from Abbvie, AkesoBio, AcroImmune, ALX Oncology, Arcus, Ascendis Pharma, Amgen, ARIAD Pharmaceuticals, Array BioPharma, AstraZeneca, Bayer, Beigene, Biosplice Therapeutics, Bristol Myers Squibb, Blueprint Medicines Corporation, Boehringer, Boston Biomedical, BridgeBio, Carina Biotech, CBT Pharmaceuticals, Celgene, Corvus Pharmaceuticals, GSK, CSTONE Pharmaceuticals, Daichii Sankyo, Duality, DynamiCure, ENB Therapeutics, EtiraRX, Exelisis, Five Prime Therapeutics, Foghorn Therapeutics, Fortrea, Fusion Pharmaceuticals, GC Cell, Genentech, Gilead, Grey Wolf Therapeutics, Henlius, Biotech, ICON, IDEAYA Biosciences, Immunocore, Incyte, Innovent Biologics, InxMed, Janssen, Linnaeus Therapeutics, Lilly, Loxo, MacroGenics, Merck, Merrimack Pharmaceuticals, MSD, Moderna, Multitude Therapeutics, Myeloid Therapeutics, Nektar Therapeutics, Neoleukin Therapeutics, Novartis, NuvOx Pharma, Pfizer, QBiotics, Regeneron, Relay Therapeutics, Roche, Sanofi, Seagen, Starpharma, SERVIER AFFAIRES MÉDICALES, Takeda, TigerMed, ViroCure, Virogin Biotech, Xencore and Xennials Therapeutics. A.F. has received consultation fees from Seagen, Roche, Pfizer, Novartis, AstraZeneca, GSK and Esteve; and is on the speaker’s bureau for Seagen, Roche, Pfizer, Novartis, Lilly, AstraZeneca, Pierre Fabre, Daiichi, Gilead and Eisai. T.G. reports honoraria to their institution from Pierre Fabre. W.H.M is on the advisory boards of and has received consulting fees from Merck, Bristol Myers Squibb, Roche, GSK, Novartis, Amgen, Mylan, EMD Serono and Sanofi; is on the speaker’s bureau of and has received honoraria from Bristol Myers Squibb, Merck, Roche, GSK, Novartis, Amgen, Mylan, EMD Serono and Sanofi; reports grants paid to their institution from Merck, Canadian Institutes of Health Research, CRS, Terry Fox Research Institute, Samuel Waxman Cancer Research Foundation and Canadian Cancer Society Research Institute; and reports clinical trial support to their institution from Merck, MiMic, Astellas, Bristol Myers Squibb, Novartis, GSK, Incyte, Pfizer, Sanofi, Ocellaris Pharma, Alkermes, Genentech, Array, Exelixis, VelosBio, Esperas Pharma, Seagen, AstraZeneca and Roche. E.M. was an advisory board consultant for Lilly, Janssen Scientific Affairs, Bristol Myers Squibb, Daiichi Sankyo, AbbVie Consulting, Mirati Therapeutics, Fusion Pharmaceuticals, Iovance Biotherapeutics, Sanofi and Gilead; and was on the speaker’s bureau for AstraZeneca, Lilly, Takeda Pharmaceuticals and Mirati Therapeutics. L.P. has received consulting fees from Lilly, MSD, Roche, PharmaMar, Merck KgaA (Darmstadt, Germany), AstraZeneca, Novartis, Servier, Amgen, Pfizer, Sanofi, Bayer, Bristol Myers Squibb, Mirati, GSK, Janssen, Takeda, Regeneron and Sanofi; grants or contracts from MSD, AstraZeneca, Bristol Myers Squibb and Pfizer; and is on the speaker’s bureau and has received honoraria from AstraZeneca, Janssen, Merck, Mirati and Sanofi. H.P. has received honoraria from Amgen, Roche, Sanofi, AstraZeneca and Bayer. C.C. has received honoraria for speaker or advisory roles from Bayer, Roche, Merck Serono, Amgen, Servier, Mirati, Pierre Fabre, MSD, Nordic Pharma and Takeda; and research grants from Bayer, Servier, Merck and Amgen. T.W.K. reports funding to their institution from Genentech. V.M. has received consulting fees from Roche, Bayer, Bristol Myers Squibb, Janssen, Syneos, Affimed and AstraZeneca; and reports funding to their institution from Achilles, AbbVie, AceaBio, Adaptimmune, ADC Therapeutics, Arcus, Ascendis Pharma, Aduro, Agenus, Amcure, Amgen, Astellas, AstraZeneca Bayer, Beigene, Biomea, BioInvent International AB, BMS, Boheringer, Boston Therapeutics, Celgene, Daichii Sankyo, Debiopharm, Eisai, e-Therapeutics, Exelisis, Forma Therapeutics, Genmab, GSK, Harpoon, Hutchison, Immutep, Incyte, Inovio, Iovance, Janssen, Kyowa Kirin, Lilly, Loxo, MedSir, Menarini, Merck, Merus, Millennium, MSD, Nanobiotix, Nektar, Novartis, Odonate Therapeutics, Pfizer, PharmaMar, PharmaMar, Principia, PsiOxus, Puma, Regeneron, Relay Therapeutics, Rigontec, Roche, Sanofi, Sierra Oncology, Synthon, Taiho, Takeda, Tesaro, Transgene, Turning Point Therapeutics and Upsher-Smith. S.I.O. has received consulting fees from Pfizer, Janssen, Daiichi Sankyo, Lilly and AnHeart Therapeutics; reports funding to their institution from Pfizer, Mirati, Revolution Medicine, Nuvalent, Janssen and Genentech/Roche; and reports ownership of MBrace Therapeutics and Blossomhill Therapeutics. A.P. has received consulting fees from Amgen, Bayer, Merck, Servier and Pierre Fabre; and travel, accommodation and expenses from Pierre Fabre and Bayer. A. Sacher is a consultant and on the advisory board for Genentech-Roche and AstraZeneca; and is an institutional research and clinical trial principal investigator for AstraZeneca, Amgen, Genentech-Roche, Merck, Lilly, Pfizer, Bristol Myers Squibb, GSK, Spectrum, Iovance and CRISPR Therapeutics. A. Santoro is on the advisory board for Bristol Myers Squibb, Servier, Gilead, Pfizer, Eisai, Bayer and MSD; is a consultant for Sanofi and Incyte; and is on the speaker’s bureau for Takeda, Bristol Myers Squibb, Roche, Abbvie, Amgen, Celgene, Servier, Gilead, AstraZeneca, Pfizer, ArQule, Lilly, Sandoz, Eisai, Novartis, Bayers and MSD. R.S. has been a lecturer for Amgen, AstraZeneca, Bayer, Bristol Myers Squibb, Genentech, Gilead, Merck, MSD, Moderna, Novartis, Pfizer, Roche, Sanofi and Takeda. S.U. is on the advisory board for Eisai, AstraZeneca and IgM Biosciences; and reports funding to their institution from AbbVie, Adlai Nortye, ArQule, AstraZeneca, Atreca, Boehringer Ingelheim, Bristol Myers Squibb, Celgene Corporation, Ciclomed, Erasca, Evelo Biosciences, Exelexis, G1 Therapeutics, GSK, IGM Biosciences, Incyte, Isofol, Klus Pharma, Macrogenics, Merck, Mersana Therapeutics, OncoMed Pharmaceuticals, Pfizer, Regeneron, Revolution Medicines, Synermore Biologics, Takeda, Tarveda Therapeutics, Tesaro, Tempest, and Vigeo Therapeutics. K.A. has received personal financial compensation for advisory board participation from Amgen, Novartis, AstraZeneca, G1 Therapeutics and Sanofi-Genzyme; and reports financial support to their institution for conduct related to clinical trials from Genentech, Mirati and Revolution Medicines. P.L. has served on the advisory boards of Abbvie, GenMab, Genentech, CytomX, Takeda, Cybrexa, Agenus, IQVIA, TRIGR, Pfizer, ImmunoMet, Black Diamond, GSK, QED Therapeutics, AstraZeneca, EMD Serono, Shattuck, Astellas, Salarius, Silverback, MacroGenics, Kyowa Kirin Pharmaceutical Development, Kineta, Zentalis Pharmaceuticals, Molecular Templates, STCube Pharmaceuticals, Bayer, I-Mab, Seagen, imCheck, Relay Therapeutics, Stemline, Compass BADX, Mekanist, Mersana Therapeutics, BAKX Therapeutics, Scenic Biotech, Qualigen, NeuroTrials and Actuate Therapeutics; has served on the data safety monitoring board for Agios, Five Prime, Halozyme and Tyme; and has served as a consultant for Roche-Genentech, SOTIO, SK Life Science and Roivant Sciences. J.L. reports honoraria from Targeted Oncology, Physicians’ Education Resource, VJ Oncology, CancerGRACE and Community Cancer Education; advisory board participation from AstraZeneca and Astellas; research support to their institution from Erasca, Genentech, Kronos Bio, Novartis and Revolution Medicines; personal fees from Erasca, Blueprint Medicines and Daiichi Sankyo; and reports that a patent filed by Memorial Sloan Kettering Cancer Center related to multimodal features to predict response to immunotherapy (PCT/US2023/115872) is pending. M.R.P. is on the leadership board of ION Pharma; has received honoraria from Janssen Oncology; has a consulting or advisory role at Olema Pharmaceuticals, Daiichi Sankyo/UCB Japan and Accutar Biotech; and reports funding to their institution from Acerta Pharma, ADC Therapeutics, Agenus, Aileron Therapeutics, AstraZeneca, BioNTech AG, Boehringer Ingelheim, Celgene, CicloMed, Clovis Oncology, Cyteir Therapeutics, Daiichi Sankyo, Lilly, Evelo Therapeutics, Genentech/Roche, Gilead Sciences, GSK, H3 Biomedicine, Hengrui Therapeutics, Hutchison MediPharma, Jacobio, Janssen, Klus Pharma, Kymab, Loxo, LSK Biopartners, Lycera, Macrogenics, Merck, Millenium, Mirati Therapeutics, Moderna Therapeutics, Pfizer, Prelude Therapeutics, Ribon Therapeutics, Seven and Eight Biopharmaceuticals, Syndax, Taiho Pharmaceutical, Tesaro, TopAlliance BioSciences, Vigeo, ORIC Pharmaceuticals, Artios, Treadwell Therapeutics, Mabspace, IgM Biosciences, Puretech, BioTheryX, Black Diamond Therapeutics, NGM Biopharmaceuticals, Novartis, Nurix, Relay Therapeutics, Samumed, Silicon Therapeutics, TeneoBio, Zymeworks, Olema, Adagene, Astellas, NGM, Accutar Biotech, Compugen, Immunogen, Blueprint Pharmaceuticals, Bicycle Therapeutics, Cullinan Oncology, Erasca, Immune-Onc Therapeutics, Immunitas, Jazz Pharmaceuticals, Pionyr, Revolution Medicines, Step Pharma, Syndax, Synthorx, Xencor, Bristol Myers Squibb/Celgene, Incytes, Kineta, Hotspot Therapeutics, Conjupro Biotherapeutics and Allorion Therapeutics. Y.C., Z.S., S.M., M.T.L., S.R.J., J.C., T.J., N.V.D. and J.L.S. are employees of Genentech and are Roche stockholders. S.W.H. has received honoraria from IMBdx and Ono Pharmaceutical; and reports funding to their institution from IMBdx, Hanmi, Loxo, Roche, Genentech, Mirati Therapeutics, Turnstone Bio, Arcus Biosciences, MSD, BeyondBio, Jeil Pharmaceutical, Janssen, Seagen, Lilly, MedImmune and Leap Therapeutics. The other authors declare no completing interests.
Peer review
Peer review information
Nature Medicine thanks David Hong, Pashtoon Kasi, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Saheli Sadanand, in collaboration with the Nature Medicine team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Mean (SD) Plasma Concentration-Time Profiles of Divarasib as a Single Agent and in Combination with Cetuximab Following Multiple Doses.
Multiple-dose pharmacokinetic profile of divarasib was obtained on either C1D8 or C2D1 from blood samples collected over an 8 hour period following administration of divarasib 400 mg QD (n = 76 patients)1 or divarasib 400 mg QD plus 250 mg/m2 of cetuximab QW (n = 21 patients). Time (hr) is shown as nominal time and the error bars represent standard deviation around the mean divarasib plasma concentration (ng/mL) measured at each time point during the 8 hour post-dose interval.
Supplementary information
Supplementary Information
GO42144 investigators and study group, Supplementary Table 1 and clinical trial protocol
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Desai, J., Alonso, G., Kim, S.H. et al. Divarasib plus cetuximab in KRAS G12C-positive colorectal cancer: a phase 1b trial. Nat Med 30, 271–278 (2024). https://doi.org/10.1038/s41591-023-02696-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41591-023-02696-8
This article is cited by
-
Targeting KRAS in cancer
Nature Medicine (2024)