Abstract
Neoadjuvant immune checkpoint blockade (ICB) outperforms adjuvant ICB for treatment of stage IIIB–D melanoma, but potential biomarkers of response, such as interferon-gamma (IFNγ) signature and tumor mutational burden (TMB), are insufficient. Preclinical studies suggest that emotional distress (ED) can negatively affect antitumor immune responses via β-adrenergic or glucocorticoid signaling. We performed a post hoc analysis evaluating the association between pretreatment ED and clinical responses after neoadjuvant ICB treatment in patients with stage IIIB–D melanoma in the phase 2 PRADO trial (NCT02977052). The European Organisation for Research and Treatment of Cancer scale for emotional functioning was used to identify patients with ED (n = 28) versus those without (n = 60). Pretreatment ED was significantly associated with reduced major pathologic responses (46% versus 65%, adjusted odds ratio 0.20, P = 0.038) after adjusting for IFNγ signature and TMB, reduced 2-year relapse-free survival (74% versus 91%, adjusted hazard ratio 3.81, P = 0.034) and reduced 2-year distant metastasis-free survival (78% versus 95%, adjusted hazard ratio 4.33, P = 0.040) after adjusting for IFNγ signature. RNA sequencing analyses of baseline patient samples could not identify clear β-adrenergic- or glucocorticoid-driven mechanisms associated with these reduced outcomes. Pretreatment ED may be a marker associated with clinical responses after neoadjuvant ICB in melanoma and warrants further investigation. ClinicalTrials.gov registration: NCT02977052.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
RNA sequencing data generated during the study will be deposited in the European Genome-phenome Archive (EGA) under the accession code EGAS00001007601. To minimize the risk of patient re-identification, de-identified individual patient-level clinical data are available under restricted access. Upon scientifically sound request, data access can be obtained via the Netherlands Cancer Institute’s (NKI) scientific repository at repository@nki.nl, which will contact the corresponding author (L.V.v.d.P.-F.). Data requests will then be reviewed by the institutional review board of the NKI and will require the requesting researcher to sign a data access agreement with the NKI.
References
Ascierto, P. A. et al. Adjuvant nivolumab versus ipilimumab in resected stage IIIB-C and stage IV melanoma (CheckMate 238): 4-year results from a multicentre, double-blind, randomised, controlled, phase 3 trial. Lancet Oncol. 21, 1465–1477 (2020).
Dummer, R. et al. Five-year analysis of adjuvant dabrafenib plus trametinib in stage III melanoma. N. Engl. J. Med. 383, 1139–1148 (2020).
Eggermont, A. M. M. et al. Longer follow-up confirms recurrence-free survival benefit of adjuvant pembrolizumab in high-risk stage III melanoma: updated results from the EORTC 1325-MG/KEYNOTE-054 trial. J. Clin. Oncol. 38, 3925–3936 (2020).
Blank, C. U. et al. Neoadjuvant versus adjuvant ipilimumab plus nivolumab in macroscopic stage III melanoma. Nat. Med. 24, 1655–1661 (2018).
Patel, S. P. et al. Neoadjuvant–adjuvant or adjuvant-only pembrolizumab in advanced melanoma. N. Engl. J. Med. 388, 813–823 (2023).
Versluis, J. M. et al. Survival update of neoadjuvant ipilimumab + nivolumab in macroscopic stage III melanoma: the OpACIN and OpACIN-neo trials. J. Clin. Oncol. 40, 9572 (2022).
Reijers, I. L. M. Personalized response-directed surgery and adjuvant therapy after neoadjuvant ipilimumab and nivolumab in high-risk stage III melanoma: the PRADO trial. Nat. Med. 28, 1178–1188 (2022).
Rozeman, E. A. et al. Survival and biomarker analyses from the OpACIN-neo and OpACIN neoadjuvant immunotherapy trials in stage III melanoma. Nat. Med. 27, 256–263 (2021).
Reijers, D. P. et al. 6P response and survival according to the interferon-gamma (IFN-γ) signature and tumor mutational burden (tmb) in the PRADO trial testing neoadjuvant ipilimumab and nivolumab in stage III melanoma. Immuno-Oncol. Technol. 16, 100111 (2022).
Boesch, M. et al. Call for a holistic framework for cancer immunotherapy. Cancer 128, 3772–3774 (2022).
Niedzwiedz, C. L., Knifton, L., Robb, K. A., Katikireddi, S. V. & Smith, D. J. Depression and anxiety among people living with and beyond cancer: a growing clinical and research priority. BMC Cancer 19, 943 (2019).
Beesley, V. L. et al. Anxiety and depression after diagnosis of high-risk primary cutaneous melanoma: a 4-year longitudinal study. J. Cancer Surviv. 14, 712–719 (2020).
Liu, Y.-Z., Wang, Y.-X. & Jiang, C.-L. Inflammation: the common pathway of stress-related diseases. Front. Hum. Neurosci. 11, 316 (2017).
Eckerling, A., Ricon-Becker, I., Sorski, L., Sandbank, E. & Ben-Eliyahu, S. Stress and cancer: mechanisms, significance and future directions. Nat. Rev. Cancer 21, 767–785 (2021).
Tian, W. et al. Chronic stress: impacts on tumor microenvironment and implications for anti-cancer treatments. Front. Cell Dev. Biol. 9, 777018 (2021).
Cole, S. W., Nagaraja, A. S., Lutgendorf, S. K., Green, P. A. & Sood, A. K. Sympathetic nervous system regulation of the tumour microenvironment. Nat. Rev. Cancer 15, 563–572 (2015).
Volden, P. A. & Conzen, S. D. The influence of glucocorticoid signaling on tumor progression. Brain Behav. Immun. 30, S26–S31 (2013).
Sommershof, A., Scheuermann, L., Koerner, J. & Groettrup, M. Chronic stress suppresses anti-tumor TCD8+ responses and tumor regression following cancer immunotherapy in a mouse model of melanoma. Brain Behav. Immun. 65, 140–149 (2017).
Yang, H. et al. Stress–glucocorticoid–TSC22D3 axis compromises therapy-induced antitumor immunity. Nat. Med. 25, 1428–1441 (2019).
Acharya, N. et al. Endogenous glucocorticoid signaling regulates CD8+ T cell differentiation and development of dysfunction in the tumor microenvironment. Immunity 53, 658–671.e6 (2020).
Bucsek, M. J. et al. β-Adrenergic signaling in mice housed at standard temperatures suppresses an effector phenotype in CD8+ T cells and undermines checkpoint inhibitor therapy. Cancer Res. 77, 5639–5651 (2017).
Qiao, G. et al. Chronic adrenergic stress contributes to metabolic dysfunction and an exhausted phenotype in T cells in the tumor microenvironment. Cancer Immunol. Res. 9, 651–664 (2021).
Schmidt, D., Peterlik, D., Reber, S. O., Lechner, A. & Männel, D. N. Induction of suppressor cells and increased tumor growth following chronic psychosocial stress in male mice. PLoS ONE 11, e0159059 (2016).
Guereschi, M. G. et al. Beta2-adrenergic receptor signaling in CD4+ Foxp3+ regulatory T cells enhances their suppressive function in a PKA-dependent manner. Eur. J. Immunol. 43, 1001–1012 (2013).
Liu, J. et al. Improved efficacy of neoadjuvant compared to adjuvant immunotherapy to eradicate metastatic disease. Cancer Discov. 6, 1382–1399 (2016).
Zhou, Q. et al. Chronic psychological stress attenuates the efficacy of anti-PD-L1 immunotherapy for bladder cancer in immunocompetent mice. Cancer Invest 39, 571–581 (2021).
Menzies, A. M. et al. Pathological response and survival with neoadjuvant therapy in melanoma: a pooled analysis from the International Neoadjuvant Melanoma Consortium (INMC). Nat. Med. 27, 301–309 (2021).
Wu, F. et al. Correlation of psychological distress with quality of life and efficacy of immune checkpoint inhibitors in patients with newly diagnosed stage IIIB-IV NSCLC. J. Clin. Oncol. 40, 12001 (2022).
Bi, Z. et al. Negative correlations of psychological distress with quality of life and immunotherapy efficacy in patients with advanced NSCLC. Am. J. Cancer Res. 12, 805–815 (2022).
Feng, Z. et al. Chronic restraint stress attenuates p53 function and promotes tumorigenesis. Proc. Natl Acad. Sci. USA 109, 7013–7018 (2012).
Hara, M. R. et al. A stress response pathway regulates DNA damage through β2-adrenoreceptors and β-arrestin-1. Nature 477, 349–353 (2011).
Zhang, X. et al. Chronic stress promotes gastric cancer progression and metastasis: an essential role for ADRB2. Cell Death Dis. 10, 788 (2019).
Obradović, M. M. S. et al. Glucocorticoids promote breast cancer metastasis. Nature 567, 540–544 (2019).
Thaker, P. H. et al. Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nat. Med. 12, 939–944 (2006).
Xie, H. et al. Chronic stress promotes oral cancer growth and angiogenesis with increased circulating catecholamine and glucocorticoid levels in a mouse model. Oral Oncol. 51, 991–997 (2015).
Nagaraja, A. S. et al. Sustained adrenergic signaling leads to increased metastasis in ovarian cancer via increased PGE2 synthesis. Oncogene 35, 2390–2397 (2016).
Ben-Eliyahu, S., Shakhar, G., Page, G. G., Stefanski, V. & Shakhar, K. Suppression of NK cell activity and of resistance to metastasis by stress: a role for adrenal catecholamines and beta-adrenoceptors. Neuroimmunomodulation 8, 154–164 (2000).
Rosenne, E. et al. In vivo suppression of NK cell cytotoxicity by stress and surgery: glucocorticoids have a minor role compared to catecholamines and prostaglandins. Brain Behav. Immun. 37, 207–219 (2014).
Matyszak, M. K., Citterio, S., Rescigno, M. & Ricciardi-Castagnoli, P. Differential effects of corticosteroids during different stages of dendritic cell maturation. Eur. J. Immunol. 30, 1233–1242 (2000).
Hou, N. et al. A novel chronic stress-induced shift in the Th1 to Th2 response promotes colon cancer growth. Biochem. Biophys. Res. Commun. 439, 471–476 (2013).
Franchimont, D. et al. Inhibition of Th1 immune response by glucocorticoids: dexamethasone selectively inhibits IL-12-induced Stat4 phosphorylation in T lymphocytes. J. Immunol. 164, 1768–1774 (2000).
Mohammadpour, H. et al. β2 adrenergic receptor-mediated signaling regulates the immunosuppressive potential of myeloid-derived suppressor cells. J. Clin. Invest. 129, 5537–5552 (2019).
Colon-Echevarria, C. B., Lamboy-Caraballo, R., Aquino-Acevedo, A. N. & Armaiz-Pena, G. N. Neuroendocrine regulation of tumor-associated immune cells. Front. Oncol. 9, 1077 (2019).
Qiao, G., Chen, M., Bucsek, M. J., Repasky, E. A. & Hylander, B. L. Adrenergic signaling: a targetable checkpoint limiting development of the antitumor immune response. Front. Immunol. 9, 164 (2018).
Ben-Eliyahu, S., Page, G. G., Yirmiya, R. & Shakhar, G. Evidence that stress and surgical interventions promote tumor development by suppressing natural killer cell activity. Int. J. Cancer 80, 880–888 (1999).
Lutgendorf, S. K. et al. Depressed and anxious mood and T-cell cytokine expressing populations in ovarian cancer patients. Brain Behav. Immun. 22, 890–900 (2008).
Sanders, V. M. et al. Differential expression of the beta2-adrenergic receptor by Th1 and Th2 clones: implications for cytokine production and B cell help. J. Immunol. 158, 4200–4210 (1997).
Ramer-Quinn, D. S., Swanson, M. A., Lee, W. T. & Sanders, V. M. Cytokine production by naive and primary effector CD4+ T cells exposed to norepinephrine. Brain Behav. Immun. 14, 239–255 (2000).
Taves, M. D. & Ashwell, J. D. Glucocorticoids in T cell development, differentiation and function. Nat. Rev. Immunol. 21, 233–243 (2021).
Daher, C. et al. Blockade of β-adrenergic receptors improves CD8+ T-cell priming and cancer vaccine efficacy. Cancer Immunol. Res. 7, 1849–1863 (2019).
Messina, G. et al. Efficacy of IL-2 immunotherapy in metastatic renal cell carcinoma in relation to the psychic profile as evaluated using the Rorschach test. Anticancer Res. 27, 2985–2988 (2007).
Simoni, Y. et al. Bystander CD8+ T cells are abundant and phenotypically distinct in human tumour infiltrates. Nature 557, 575–579 (2018).
Attrill, G. H. et al. Higher proportions of CD39+ tumor-resident cytotoxic T cells predict recurrence-free survival in patients with stage III melanoma treated with adjuvant immunotherapy. J. Immunother. Cancer 10, e004771 (2022).
Danielsen, J. T. et al. Psychological and behavioral symptoms in patients with melanoma: a systematic review and meta-analysis. Psychooncology 32, 1208–1222 (2023).
Kokolus, K. M. et al. Beta blocker use correlates with better overall survival in metastatic melanoma patients and improves the efficacy of immunotherapies in mice. Oncoimmunology 7, e1405205 (2018).
Kennedy, O. J. et al. Prognostic and predictive value of β-blockers in the EORTC 1325/KEYNOTE-054 phase III trial of pembrolizumab versus placebo in resected high-risk stage III melanoma. Eur. J. Cancer 165, 97–112 (2022).
Gandhi, S. et al. Phase I clinical trial of combination propranolol and pembrolizumab in locally advanced and metastatic melanoma: safety, tolerability, and preliminary evidence of antitumor activity. Clin. Cancer Res. 27, 87–95 (2021).
Zhao, C. et al. The effects of acceptance and commitment therapy on the psychological and physical outcomes among cancer patients: a meta-analysis with trial sequential analysis. J. Psychosom. Res. 140, 110304 (2021).
Faller, H. et al. Effects of psycho-oncologic interventions on emotional distress and quality of life in adult patients with cancer: systematic review and meta-analysis. J. Clin. Oncol. 31, 782–793 (2013).
Xunlin, N. G., Lau, Y. & Klainin-Yobas, P. The effectiveness of mindfulness-based interventions among cancer patients and survivors: a systematic review and meta-analysis. Support Care Cancer 28, 1563–1578 (2020).
Paley, C. A. et al. Non-pharmacological interventions to manage psychological distress in patients living with cancer: a systematic review. BMC Palliat. Care 22, 88 (2023).
Machingura, A. et al. Clustering of EORTC QLQ-C30 health-related quality of life scales across several cancer types: validation study. Eur. J. Cancer 170, 1–9 (2022).
Schulte, T., Hofmeister, D., Mehnert-Theuerkauf, A., Hartung, T. & Hinz, A. Assessment of sleep problems with the Insomnia Severity Index (ISI) and the sleep item of the Patient Health Questionnaire (PHQ-9) in cancer patients. Support Care Cancer 29, 7377–7384 (2021).
Hofmeister, D., Schulte, T. & Hinz, A. Sleep problems in cancer patients: a comparison between the Jenkins Sleep Scale and the single-item sleep scale of the EORTC QLQ-C30. Sleep. Med. 71, 59–65 (2020).
Tetzlaff, M. T. et al. Pathological assessment of resection specimens after neoadjuvant therapy for metastatic melanoma. Ann. Oncol. 29, 1861–1868 (2018).
Aaronson, N. K. et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J. Natl Cancer Inst. 85, 365–376 (1993).
Fayers, P. M. et al. The EORTC QLQ-C30 Scoring Manual 3rd edn (European Organisation for Research and Treatment of Cancer, 2001).
Giesinger, J. M. et al. Thresholds for clinical importance were established to improve interpretation of the EORTC QLQ-C30 in clinical practice and research. J. Clin. Epidemiol. 118, 1–8 (2020).
Giesinger, J. M. et al. Thresholds for clinical importance for four key domains of the EORTC QLQ-C30: physical functioning, emotional functioning, fatigue and pain. Health Qual. Life Outcomes 14, 87 (2016).
Tavoli, A., Tavoli, Z. & Montazeri, A. The relationship between emotional functioning of the EORTC QLQ-C30 and a measure of anxiety and depression (HADS) in cancer patients. Int. J. Cancer Manag. 12, e94568 (2019).
Oort, Q. et al. Is the EORTC QLQ-C30 emotional functioning scale appropriate as an initial screening measure to identify brain tumour patients who may possibly have a mood disorder? Psychooncology 31, 995–1002 (2022).
Calderon, C. et al. Emotional functioning to screen for psychological distress in breast and colorectal cancer patients prior to adjuvant treatment initiation. Eur. J. Cancer Care 28, e13005 (2019).
Rodriguez-Gonzalez, A. et al. Using the emotional functioning in clinical practice to detect psychological distress in patients with advanced thoracic and colorectal cancer. Health Qual. Life Outcomes 21, 15 (2023).
van der Willik, K. D. et al. Inflammation markers and cognitive performance in breast cancer survivors 20 years after completion of chemotherapy: a cohort study. Breast Cancer Res. 20, 135 (2018).
Jiang, H., Lei, R., Ding, S.-W. & Zhu, S. Skewer: a fast and accurate adapter trimmer for next-generation sequencing paired-end reads. BMC Bioinformatics 15, 182 (2014).
Andrews S. Fastqc: A Quality Control Tool For High Throughput Sequence Data (Babraham Institute, 2010).
Dobin, A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 (2013).
Putri, G. H., Anders, S., Pyl, P. T., Pimanda, J. E. & Zanini, F. Analysing high-throughput sequencing data in Python with HTSeq 2.0. Bioinformatics 38, 2943–2945 (2022).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014).
Ayers, M. et al. IFN-γ-related mRNA profile predicts clinical response to PD-1 blockade. J. Clin. Invest. 127, 2930–2940 (2017).
Becht, E. et al. Estimating the population abundance of tissue-infiltrating immune and stromal cell populations using gene expression. Genome Biol. 17, 218 (2016).
Zhu, A., Ibrahim, J. G. & Love, M. I. Heavy-tailed prior distributions for sequence count data: removing the noise and preserving large differences. Bioinformatics 35, 2084–2092 (2018).
Acknowledgements
We thank all patients and their families for participation in the trial. We gratefully acknowledge the contributions of all participating study teams, and the support of all involved colleagues from the Netherlands Cancer Institute, Melanoma Institute Australia, Royal Prince Alfred Hospital, Royal North Shore and Mater Hospital, University Medical Center Utrecht, Erasmus Medical Center, Leiden University Medical Center and University Medical Center Groningen. We thank N. M. J. van den Heuvel and A.H. Boekhout for their contribution on the collection and analysis of the HRQoL data, H. Shehwana for assessment of the TMB calculation, and L. G. Grijpink-Ongering, A. Torres Acosta, R. Zucker, M. J. Gregorio, K. de Joode, A.M. van Eggermont, E. H .J. Tonk and J. Kingma-Veenstra for administrative support and data management. A.M.M.M. is supported by a National Health and Medical Research Council (NHMRC) Investigator Grant (no. 2021/GNT2009476), Melanoma Institute Australia and Nicholas and Helen Moore. G.V.L. is supported by an NHMRC Investigator Grant (no. 2021/GNT2007839) and the University of Sydney Medical Foundation. Financial support for the trial (NCT02977052) was provided by Bristol Myers Squibb.
Author information
Authors and Affiliations
Contributions
L.V.v.d.P.-F. designed the clinical emotional distress analysis. C.U.B. designed the clinical trial and wrote the trial protocol. G.V.L. reviewed the protocol. I.F., I.L.M.R., M.G., A.M.M.M., E.K., A.A.M.v.d.V., K.P.M.S., G.A.P.H., G.V.L. and C.U.B. recruited and treated patients and/or collected data. A.B. coordinated patient tumor sample processing and biobanking. I.F. and I.L.M.R. performed statistical analysis of the clinical data. P.D. performed RNA sequencing analyses. I.F., I.L.M.R., C.U.B. and L.V.v.d.P.-F. wrote the first draft of the manuscript. All authors interpreted the data, reviewed the manuscript and approved the final version.
Corresponding author
Ethics declarations
Competing interests
No author has received financial support for the work on this paper, and no medical writer was involved at any stage of the preparation of this paper. I.L.M.R. and P.D. report financial interest in Signature Oncology and will receive some possible revenues if the IFNγ signature is being developed as a clinical companion diagnostic. A.M.M.M. has served on advisory boards for Bristol Myers Squibb (BMS), Merck Sharp & Dohme (MSD), Novartis, Roche, Pierre Fabre and QBiotics. E.K. received honoraria for consultancy/advisory relationships (all paid to the institute) from BMS, Novartis, Merck, Lilly and Pierre Fabre, and received research grants not related to this paper from BMS, Pierre Fabre and Delcath. A.A.M.v.d.V. received compensation for advisory roles and honoraria (all paid to the institute) from BMS, MSD, Merck, Roche, Eisai, Pfizer, Sanofi, Novartis, Pierre Fabre and Ipsen. K.P.M.S. received compensation for advisory roles and honoraria (all paid to the institute) from BMS, MSD, Novartis, Pierre Fabre and Abbvie, and received research funding from Novartis, TigaTx and BMS. G.A.P.H. received compensation for consulting and advisory roles (all paid to the institute) from Amgen, Roche, MSD, BMS, Pfizer, Novartis and Pierre Fabre, and received research grants (paid to the institute) from BMS and Seerave. G.V.L. is consultant advisor for Agenus, Amgen, Array Biopharma, AstraZeneca, Boehringer Ingelheim, BMS, Evaxion, Hexal AG (Sandoz Company), Highlight Therapeutics, Innovent Biologics, MSD, Novartis, Oncosec, PHMR Ltd, Pierre Fabre, Provectus, QBiotics and Regeneron. C.U.B. reports receiving compensation for advisory roles from BMS, MSD, Roche, Novartis, GlaxoSmithKline, AstraZeneca, Pfizer, Eli Lilly, Genmab, Pierre Fabre and Third Rock Ventures, and receiving research funding from BMS, MSD, Novartis, 4SC and NanoString. Furthermore, C.U.B. reports to be co-founder of Immagene BV. All compensations and funding for C.U.B. were paid to the institute, except for Third Rock Ventures and Immagene. The remaining authors declare no competing interests.
Peer review
Peer review information
Nature Medicine thanks Cristiane Bergerot and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Saheli Sadanand, in collaboration with the Nature Medicine team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
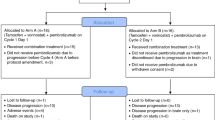
Extended Data Fig. 1 Flowchart for the Emotional Distress analyses of the PRADO trial.
Patients were defined as having emotional distress (emotional functioning score≤71) or no emotional distress (emotional functioning score >71) according to established clinically relevant thresholds using the EORTC QLQ-C30 questionnaire. HRQoL = health-related quality of life.
Extended Data Fig. 2 Baseline cortisol levels in patients with and without ED.
a, Cortisol levels in nmol/L as measured in the peripheral blood at baseline of patients with emotional distress (n = 26, purple) and without emotional distress (n = 53, orange). Patients with unknown baseline cortisol levels or unknown time of blood withdrawal were excluded. b, Two-tailed linear regression analysis (n = 79 patients) showing the association between cortisol levels and emotional distress status or time of blood withdrawal. c, Gene set enrichment analysis (n = 70 patients) using gene sets based on the Gene Ontology database showing the normalized enrichment score (NES) and corresponding unadjusted two-sided p-value of adrenergic and glucocorticoid-associated pathways. Orange bars indicate enrichment of pathways in patients without ED (n = 47), and purple bars indicate enrichment of pathways in patients with ED (n = 23). No corrections for multiple testing were performed.
Extended Data Fig. 3 Inflammation and T cell activation markers in patients with and without ED.
a-c, Comparison of genes associated with inflammation: COX2 (PTGS2), prostaglandin E2 (PGE2) and IL6 in the tumor. d-j, Comparison of T cell activation markers as measured by RNA sequencing. a-j, Patients with available RNA sequencing data (n = 23 patients with ED, n = 47 patients without ED) were included. P-values were calculated using two-tailed unpaired Student’s t-test. Bars represent mean +/− S.D.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fraterman, I., Reijers, I.L.M., Dimitriadis, P. et al. Association between pretreatment emotional distress and neoadjuvant immune checkpoint blockade response in melanoma. Nat Med 29, 3090–3099 (2023). https://doi.org/10.1038/s41591-023-02631-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41591-023-02631-x
This article is cited by
-
Beyond genetics: driving cancer with the tumour microenvironment behind the wheel
Nature Reviews Cancer (2024)
-
Integrative Oncology Approaches to Supporting Immune Checkpoint Inhibitor Treatment of Solid Tumours
Current Oncology Reports (2024)
-
Association between pretreatment emotional distress and immune checkpoint inhibitor response in non-small-cell lung cancer
Nature Medicine (2024)