Abstract
Axicabtagene ciloleucel (axi-cel) demonstrated superior efficacy compared to standard of care as second-line therapy in patients with high-risk relapsed/refractory (R/R) large B cell lymphoma (LBCL) considered eligible for autologous stem cell transplantation (ASCT); however, in clinical practice, roughly half of patients with R/R LBCL are deemed unsuitable candidates for ASCT. The efficacy of axi-cel remains to be ascertained in transplant-ineligible patients. ALYCANTE, an open-label, phase 2 study, evaluated axi-cel as a second-line therapy in 62 patients with R/R LBCL who were considered ineligible for ASCT. The primary end point was investigator-assessed complete metabolic response at 3 months from the axi-cel infusion. Key secondary end points included progression-free survival, overall survival and safety. The study met its primary end point with a complete metabolic response of 71.0% (95% confidence interval, 58.1–81.8%) at 3 months. With a median follow-up of 12.0 months (range, 2.1–17.9), median progression-free survival was 11.8 months (95% confidence interval, 8.4–not reached) and overall survival was not reached. There was no unexpected toxicity. Grade 3–4 cytokine release syndrome and neurologic events occurred in 8.1% and 14.5% of patients, respectively. These results support axi-cel as second-line therapy in patients with R/R LBCL ineligible for ASCT. ClinicalTrials.gov Identifier: NCT04531046.
Similar content being viewed by others
Main
Large B cell lymphoma (LBCL) is successfully treated in approximately two-thirds of patients with rituximab-based chemoimmunotherapy1,2. Until recently, the standard of care (SOC) for second-line therapy consisted of salvage chemoimmunotherapy followed, if possible, by consolidation with high-dose chemotherapy (HDCT) and autologous stem cell transplantation (ASCT) in fit and responding patients3. However, patients who are primary refractory or who relapse early after a rituximab-containing first-line therapy, notably within a year from initial therapy, have a poor prognosis with standard salvage chemoimmunotherapy4,5,6. The recent advent of chimeric antigen receptor (CAR)-T cell therapy has led to an important paradigm shift in the management of these patients with high-risk relapsed/refractory (R/R) LBCL7,8,9,10.
In patients with high-risk R/R LBCL considered eligible for ASCT, axicabtagene ciloleucel (axi-cel), an autologous anti-CD19 CAR-T cell therapy, demonstrated superior efficacy over the SOC as a second-line therapy in the ZUMA-7 trial8. In this international, phase 3 trial, patients intended for transplant were randomized to receive either a single infusion of axi-cel after fludarabine and cyclophosphamide lymphodepleting chemotherapy or SOC consisting of two or three cycles of chemoimmunotherapy followed by HDCT/ASCT in patients who achieved a complete or partial remission. At a median follow-up of 24.9 months, axi-cel demonstrated superior efficacy compared to SOC, with an estimated median event-free survival (EFS) of 8.3 versus 2.0 months, respectively (P < 0.001)8. Moreover, at a median follow-up of 47.2 months, overall survival (OS) was superior in the axi-cel arm compared to the SOC (median not reached versus 31.1 months, respectively; P = 0.03)11.
In clinical practice, about half of patients with R/R LBCL are considered ineligible for HDCT/ASCT3. This population has not been evaluated in the ZUMA-7 trial8. Several factors may preclude patients from receiving HDCT/ASCT including advanced age, frailty and coexisting medical conditions12. Furthermore, patients who have undergone a previous ASCT as first-line consolidation are usually considered ineligible for a second ASCT12.
The prognosis of patients with R/R LBCL who are ineligible for HDCT/ASCT is usually poor with standard salvage chemoimmunotherapy6,13. Typical therapeutic choices within this context encompass rituximab, gemcitabine and oxaliplatin (R-GemOx)14; polatuzumab vedotin plus bendamustine and rituximab (Pola-BR)15; and tafasitamab plus lenalidomide (Tafa-Len)16. The R-GemOx regimen is one of the most widely used because of its acceptable tolerability. The real-world use of R-GemOx was evaluated in a large retrospective analysis of 196 patients with R/R LBCL who were not eligible for HDCT/ASCT6. At a median follow-up of 22 months, R-GemOx demonstrated modest efficacy, with a complete response rate of 33% and median progression-free survival (PFS) and OS of 5 and 10 months, respectively6. Patients who were refractory or who relapsed within 1 year after first-line chemoimmunotherapy with rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone (R-CHOP) (n = 60) had a particularly poor prognosis when treated with second-line R-GemOx, with a complete response rate of 12% and a PFS of 22% at 6 months and 14% at 1 year17. Thus, in patients with R/R LBCL who are not eligible for HDCT/ASCT, the outcome is poor after second-line chemoimmunotherapy, notably in patients who are refractory or relapse early (within 12 months from first-line therapy).
Although axi-cel has not been evaluated as a second-line therapy in patients with R/R LBCL who are ineligible for HDCT/ASCT, clinical trial data and real-world evidence have shown that CAR-T cell therapy is feasible in a subset of transplant-ineligible patients, notably in elderly and less-fit patients12,18,19,20,21,22,23. In this context, we conducted a phase 2 study (ALYCANTE) to assess the efficacy and safety of a single axi-cel infusion as a second-line therapy in patients with high-risk R/R LBCL deemed ineligible for ASCT but eligible for CAR-T cell therapy.
Results
The primary end point was investigator-assessed complete metabolic response (CMR) at 3 months from the axi-cel infusion. Secondary end points were objective response rate (ORR) at 3 months from the axi-cel infusion, CMR at 6 months from the axi-cel infusion, best ORR, best CMR, duration of response (DOR), EFS from leukapheresis, PFS from infusion, OS from infusion and the incidence, nature and severity of adverse events. Additional planned secondary end points not reported in this manuscript include health-related quality of life and the cell product characteristics and cellular kinetics of axi-cel.
In the initial study protocol, we calculated that a sample size of 40 patients with aggressive B cell non-Hodgkin lymphoma that was refractory to or had relapsed no more than 12 months after first-line chemoimmunotherapy and who were ineligible for HDCT/ASCT based on a physician’s assessment was sufficient to test the efficacy of axi-cel infusion. Our initial part of the study (n = 40) met its primary end point with an investigator-assessed CMR of 67.5% at 3 months after infusion. The protocol was amended to enroll additional patients to enable a balanced comparison of the efficacy and toxicity of axi-cel in different age subgroups (<70 and ≥70 years). Here, we report the results of the study cohort of 62 patients who received axi-cel infusion. Of note, patient characteristics and outcomes were similar between the initial (n = 40) and expanded (n = 62) cohorts (Extended Data Table 1).
Patients and treatment
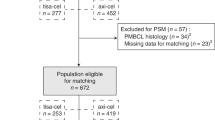
Between 19 March 2021 and 4 May 2022, a total of 69 patients were enrolled and underwent leukapheresis, representing the full analysis set (FAS). Of these 69 patients, 62 (89.8%) received a single axi-cel infusion between 26 April 2021 and 16 June 2022 and were consequently included in the modified full analysis set (mFAS). There were five important protocol deviations from inclusion criteria: three related to a relapsed disease occurring beyond 12 months from completion of first-line chemoimmunotherapy and two related to patients with a histological diagnosis of grade 1–3A follicular lymphoma after central review. The patient disposition flow diagram is shown in Fig. 1. The study design is summarized in Extended Data Fig. 1. Overall, seven patients underwent leukapheresis but did not receive axi-cel infusion because of disease progression (n = 1), investigational medicinal product out of specification (n = 1), CMR before axi-cel infusion (n = 1) based on the investigator’s assessment, absence of documented relapse on biopsy before axi-cel infusion (n = 1), consent withdrawal (n = 1), occurrence of cutaneous nocardiosis (n = 1) and lymphoma-related death (n = 1) (Fig. 1). At the data cutoff date of 19 January 2023, median time between study inclusion and axi-cel infusion was 41.5 days (interquartile range (IQR), 38.0–48.0) and median follow-up duration from axi-cel infusion was 12.0 months (IQR, 9.1–12.6).
The demographics and disease characteristics of patients in the mFAS (N = 62) are summarized in Table 1. Median age was 70 years (range, 49–81), 15 patients (24.2%) were female and 35 patients (56.5%) had an international prognostic index (IPI) of 3 or higher. Almost all patients had an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1 (n = 61; 98.4%). The majority of patients (n = 52; 83.9%) were histologically diagnosed with diffuse large B cell lymphoma (DLBCL). In total, 34 patients (54.8%) had primary refractory disease to first-line chemoimmunotherapy. Patients were deemed ineligible for HDCT/ASCT because of age ≥65 years (88.7%), high hematopoietic cell transplantation-specific comorbidity index (HCT-CI) score ≥3 (32.3%)24 and/or previous ASCT (3.2%) (Extended Data Fig. 2). Overall, 52 patients (83.9%) received bridging therapy after leukapheresis at the investigator’s discretion. In 51 of these 52 patients (98.1%), bridging therapy consisted of R-GemOx administered for one cycle (n = 25), two cycles (n = 25) or three cycles (n = 1). Additionally, 9 out of 52 patients (17.3%) received corticosteroids, of whom one patient received corticosteroids alone without R-GemOx. Among patients who received bridging therapy, 63.4% did not respond (stable disease or progressive disease).
Primary efficacy outcome
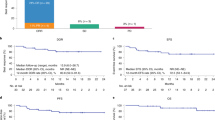
The CMR at 3 months from the axi-cel infusion, as assessed by the investigator according to the Lugano response criteria25, was 71.0% (95% confidence interval (CI), 58.1–81.8%) in the mFAS (N = 62) (Table 2). Compared to the mFAS, the sensitivity analysis on the FAS (N = 69) did not show a notable difference in the investigator-assessed CMR at 3 months, which was 66.7% (95% CI, 54.3–77.6%). Likewise, a post-hoc analysis, which excluded two patients with grade 1–3A follicular lymphoma and four patients who achieved complete response with bridging therapy, found an investigator-assessed CMR at 3 months of 67.9% (95% CI, 54.0–79.7%), which is comparable to the 3-month CMR reported in the mFAS. Of note, out of ten patients with a partial metabolic response (PMR) at 1 month after axi-cel infusion, five patients converted to a CMR at 3 months without any additional therapy (Extended Data Fig. 3).
Secondary efficacy outcomes
At 3 months from the axi-cel infusion, the investigator-assessed ORR was 75.8% (95% CI, 63.3–85.8%). In total, 37 patients (59.7%) remained in CMR, as assessed by the investigator, at 6 months from the axi-cel infusion (95% CI, 46.5–72.0%) (Extended Data Fig. 3). The investigator-assessed best ORR and best CMR were 90.3% and 79.0%, respectively. When assessed by a central review panel, CMR and ORR at 3 months were 66.1% (95% CI, 53.0–77.7%) and 69.4% (95% CI, 56.4–80.4%), respectively. The best ORR and best CMR as assessed by the central review panel were 91.9% and 82.3%, respectively (Table 2).
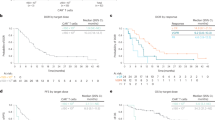
At a median follow-up of 12.0 months, the median EFS from leukapheresis was 12.3 months (95% CI, 7.2–not reached; Fig. 2a) in the FAS (n = 69). The estimated EFS rates at 6 and 12 months were 66.7% (95% CI, 54.2–76.4%) and 51.2% (95% CI, 38.2–62.8%), respectively. Median PFS from axi-cel infusion was 11.8 months (95% CI, 8.4–not reached; Fig. 2b) in the mFAS (N = 62). The estimated PFS rates at 6 and 12 months were 67.7% (95% CI, 54.5–77.8%) and 48.8% (95% CI, 34.0–62.0%), respectively. Median OS from axi-cel infusion was not reached (Fig. 2c) in the mFAS (N = 62). The estimated OS rates at 6 and 12 months were 91.9% (95% CI, 81.6–96.5%) and 78.3% (95% CI, 64.7–87.1%), respectively. Median DOR was not reached (Fig. 2d).
Safety
All study participants had at least one adverse effect of any grade. Adverse effects of grade 3 or higher occurred in 59 out of 62 patients (95.2%). The most commonly reported adverse effects of grade 3 or higher were neutropenia (occurring in 66.1% of patients), anemia (38.7%) and thrombocytopenia (38.7%) (Extended Data Table 2). Various infections of any grade (that is, COVID-19, urinary tract infection, sepsis, respiratory tract infection, skin infection) occurred in 33 patients (53.2%). The most frequent infection was COVID-19, which was reported in 15 patients (24.2%), including 7 with grade ≥3 and 2 with grade 5.
Adverse effects of special interest related to CAR-T cell toxicities are reported in Table 3. Cytokine release syndrome (CRS) occurred in 93.5% of patients, with CRS of grade 3 or higher reported in 8.1% of patients. The median time to onset of CRS was 1.5 days (IQR, 1.0–3.0) after axi-cel infusion and the median CRS duration was 5.0 days (IQR, 4.0–9.0). Immune effector cell-associated neurotoxicity syndrome (ICANS) occurred in 51.6% of patients, with ICANS of grade 3 or higher reported in 14.5% of patients. The median time to onset of ICANS was 6.0 days (IQR, 5.0–8.0) after axi-cel infusion and the median ICANS duration was 5.0 days (IQR, 3.0–8.0). No deaths related to CRS or neurologic events occurred. CAR-T cell toxicities were mainly managed with the interleukin-6 receptor antagonist tocilizumab (administered in 77.4% of patients) and/or corticosteroids (64.5% of patients). A total of 16 patients (25.8%) were admitted to the intensive care unit (ICU) as a result of CAR-T cell toxicities. Grade 3 or worse prolonged cytopenias (defined as a grade ≥3 laboratory result of anemia, neutropenia and/or thrombocytopenia not resolved 30 days after axi-cel infusion) occurred in 23 out of 62 patients (37.1%).
At the time of data cutoff, 12 patients died, 5 of whom from lymphoma and 1 of unknown reason. Nonrelapse mortality (NRM) was recorded in six patients (9.7%). All fatal adverse effects occurred late (beyond 2 months after axi-cel infusion) and were infections: two COVID-19, one aspergillosis, one mucormycosis, one sepsis and one perineal infection (Extended Data Table 3).
Subgroup analysis
We investigated whether the efficacy and safety of axi-cel were consistent among different patient populations, especially in patients aged ≥70 years and with comorbidities defined by a HCT-CI score ≥3. In patients aged ≥70 years (n = 33), the 3-month CMR was 72.7% versus 69.0% in patients <70 years (n = 29) (Extended Data Table 4). Survival outcomes and DOR were comparable between patients aged <70 years and ≥70 years (Fig. 3). Patients aged ≥70 years did not show increased toxicity compared to those aged <70 years, with similar rates of CRS, ICANS and ICU transfer (Extended Data Table 4). Likewise, patients with a HCT-CI score ≥3 (n = 20) reported a 3-month CMR of 80.0%, with similar survival and no increase in toxicity compared to those with a HCT-CI score < 3 (n = 42) (Extended Data Table 5). Finally, a similar investigator-assessed CMR at 3 months was observed across all evaluated subgroups but one. The only exception was total metabolic tumor volume (TMTV), as a high TMTV > 80 ml at inclusion was associated with a reduced CMR at 3 months (Extended Data Fig. 4)26.
Discussion
Patients with LBCL who are refractory or who relapse early after first-line chemoimmunotherapy are often chemorefractory and have a very limited cure rate after standard chemoimmunotherapy27. The ALYCANTE study is the first to assess the efficacy and safety of axi-cel as a second-line therapy in patients with high-risk R/R LBCL who are deemed ineligible for HDCT/ASCT. In this prospective, multicenter, open-label, phase 2 trial, a single axi-cel infusion was associated with a manageable safety profile and a high antitumor activity. The study met its primary end point with a CMR at 3 months of 71% versus 12% with SOC (second-line chemoimmunotherapy) based on historical controls6.
In this patient population with poor prognostic features, including 54.8% with primary refractory disease and 63.4% who were refractory to bridging therapy, treatment with axi-cel resulted in high response rates and durable remissions. The investigator-assessed best ORR and best CMR were 90.3% and 79.0%, respectively. Median PFS was 11.8 months and median OS was not reached. Furthermore, the efficacy of axi-cel was maintained across key subgroups, including patients with high-risk features, such as age ≥70 years, HCT-CI score ≥3, IPI ≥ 3 and primary refractory disease. The only exception was in patients with a high TMTV at inclusion, as we observed a reduced CMR at 3 months among these patients. This observation is consistent with other studies evaluating CAR-T cell therapy for R/R LBCL, in which a high TMTV was associated with an increased risk of early relapse or progression26,28,29,30,31.
The findings of ALYCANTE are overall consistent with the results of the phase 3 ZUMA-7 trial8,11 evaluating axi-cel as second-line therapy in patients with high-risk R/R LBCL deemed eligible for HDCT/ASCT. In ZUMA-7, axi-cel resulted in a CMR of 65% and a median PFS of 14.7 months. At a median follow-up of 24.9 months, median OS was not reached8. There are, however, many differences in design and patient populations between ALYCANTE and ZUMA-7 (ref. 8). For instance, no bridging therapy was allowed in ZUMA-7, except for corticosteroid use, in contrast to ALYCANTE where bridging with R-GemOx was permitted to reflect real-world clinical practice. Indeed, many patients with aggressive lymphomas cannot be spared from bridging chemoimmunotherapy after leukapheresis21. As expected, patients were notably older in the ALYCANTE study than in the ZUMA-7 study with a median age of 70 versus 59 years, respectively. Despite the advanced age and comorbidity burden in ALYCANTE, the observed toxicity of axi-cel was overall consistent with that in ZUMA-7 (ref. 8). For instance, the incidences of severe CRS and ICANS were comparable, at 8% and 15% for grade 3–4 CRS and ICANS in ALYCANTE versus 6% and 21% in ZUMA-7, respectively8. Likewise, the rate of patients admitted to the ICU was 26% in ALYCANTE and 25% in ZUMA-7. NRM was also similar in ALYCANTE and ZUMA-7 (refs. 8,11), at 10% in both studies. It is nevertheless important to note that ZUMA-7 was conducted before the COVID-19 pandemic (between January 2018 and October 2019), whereas ALYCANTE was conducted between March 2021 and May 2022. If we omit the two cases of fatal COVID-19 infection that contribute to one-third of the NRM, the NRM in ALYCANTE would be 6%. This number is in a similar range to a post-marketing cohort study by Nastoupil et al. conducted before the COVID-19 pandemic, in which the NRM was 4% among 275 patients who received axi-cel for R/R LBCL32. Overall, the safety and efficacy of axi-cel seem comparable in the ALYCANTE and ZUMA-7 trials, supporting the role of axi-cel as a second-line therapy in a broad population of patients with R/R LBCL, regardless of transplant-eligibility.
Another CD19-directed CAR-T cell product, lisocabtagene maraleucel (liso-cel), has also been evaluated as a second-line therapy in the open-label, phase 2 PILOT study performed in 61 patients with R/R LBCL who are ineligible for ASCT33. In PILOT, liso-cel yielded a best ORR and a best CMR, as assessed by an independent review committee, of 80% and 54%, respectively, with a median PFS of 9.0 months33. These results are overall consistent with those of ALYCANTE, in which centrally assessed best ORR and best CMR were 92% and 82%, respectively. The results of PILOT also complement the TRANSFORM trial, a phase 3 trial evaluating liso-cel as second-line therapy in patients with R/R LBCL intended to receive HDCT/ASCT7,10. In TRANSFORM, at a median follow-up of 17.5 months, the ORR and the CMR were 87% and 74%, respectively and the median PFS was not reached. As previously reported with liso-cel7,10, acute toxicities appeared particularly low in the PILOT study, notably grade ≥3 CRS and ICANS (2% and 5% respectively). In PILOT, 15% of patients were admitted to the ICU and 3% experienced NRM33. Regarding delayed toxicities, 30% of patients experienced grade ≥3 prolonged cytopenias in PILOT compared to 37% in ALYCANTE.
Despite the comparable study designs and sample sizes of ALYCANTE and PILOT33, cross-trial comparisons should be approached with caution, particularly as eligibility criteria differed between these two studies. First, late relapses (beyond 12 months) were allowed in PILOT33, whereas only early relapses were allowed in ALYCANTE and TRANSFORM7,10. Overall, 25% of patients included in PILOT had late relapses33. Second, although ASCT ineligibility in both studies was based on physician’s assessment (subjective assessment), the objective criteria for ASCT ineligibility differed between ALYCANTE and PILOT33. In ALYCANTE, patients had to meet at least one of the three protocol-defined criteria for ASCT ineligibility: age ≥65 years, HCT-CI score ≥3, or previous ASCT. On the other hand, patients in PILOT were required to meet at least one of the following criteria to define ASCT ineligibility: age ≥70 years; ECOG performance status of 2; diffusing capacity of the lung for carbon monoxide ≤60%; left ventricular ejection fraction <50%; creatinine clearance <60 ml min−1; aspartate aminotransferase (AST) or alanine aminotransferase (ALT) concentrations more than two times the upper limit of normal33.
Currently, there are no standard criteria nor consensus to determine whether a patient can undergo HDCT/ASCT. In most countries, a theoretical age limit for HDCT/ASCT is set to 65–70 years. The age cutoff to define ASCT ineligibility differed between ALYCANTE (65 years) and PILOT (70 years); however, in the present study, subgroup analysis demonstrated that efficacy and safety outcomes after axi-cel infusion were overall similar between patients aged <70 years and ≥70 years. Numerous investigations have additionally found that HCT-CI, a well-established prognostic model for comorbidities, can predict survival outcomes for both autologous and allogeneic stem cell transplantation24,34,35. A HCT-CI score ≥3 was shown to be independently associated with a higher risk of NRM (P < 0.001) and shorter survival (P = 0.03) among recipients of ASCT34,35. In ALYCANTE, the efficacy and safety of axi-cel were not altered in patients with high HCT-CI scores.
This study is limited by its single-arm design with no active control group. Therefore, selection bias cannot be ruled out. Although axi-cel compares favorably to second-line chemoimmunotherapy based on historical controls6, it remains to be compared to more recent regimens such as Tafa-Len, Pola-BR and bispecific antibodies15,16,36,37,38,39,40,41,42,43. Our study is also limited by a small sample size and a relatively short follow-up duration (median of 12.0 months); however, the ALYCANTE trial is still ongoing, with a planned follow-up of up to 3 years per patient to determine the long-term efficacy and safety of axi-cel in this patient population.
In conclusion, axi-cel as a second-line treatment resulted in high response rates and durable remissions in patients with high-risk R/R LBCL with poor prognostic features and who were not eligible for HDCT/ASCT. Moreover, despite advanced age and comorbidities, axi-cel had an acceptable safety profile in this population considered unfit for HDCT/ASCT. Together, these results support axi-cel as a second-line treatment in patients with R/R LBCL who are deemed ineligible for HDCT/ASCT.
Methods
Study design
ALYCANTE (ClinicalTrials.gov ID NCT04531046) is an ongoing prospective, single-arm, multicenter, open-label, phase 2 trial. Study participants were enrolled in 18 centers across France. The full study protocol is provided in the Supplementary Information. Supplementary Table 1 provides information on the ALYCANTE study team as well as the study’s investigators and co-investigators.
Inclusion and ethics
The study protocol was approved by French Ethics Committee Est I (Dijon) N°20.07.08.66206, in accordance with applicable French laws and regulations. The study was performed in accordance with the Declaration of Helsinki and the International Conference on Harmonization Good Clinical Practice guidelines. Written informed consent was obtained from each participant before any study procedure.
Participants
Eligible patients were aged 18 years or older with histologically confirmed aggressive B cell non-Hodgkin lymphoma, diagnosed according to the 2016 World Health Organization classification criteria44, as DLBCL, high-grade B cell lymphoma or follicular lymphoma grade 3B. Disease had to be refractory to or had relapsed no more than 12 months after the completion of first-line chemoimmunotherapy containing a monoclonal CD20 antibody and an anthracycline-containing regimen (CHOP or CHOP-like regimen). Refractory disease was defined as a lack of complete response to first-line therapy and relapsed disease as biopsy-proven disease relapse within 12 months from completion of first-line therapy. Patients must also have been ineligible for HDCT/ASCT based on a physician’s assessment and with at least one of the following criteria: age ≥65 years, HCT-CI score ≥3 (as reported by investigators)24 or previous ASCT (as first-line consolidation). Patients were enrolled regardless of sex, which was collected according to the identity information provided by the patients.
There were in total, three important protocol deviations from the inclusion criteria in the present study, which were all related to relapsed disease longer than 12 months after first-line chemoimmunotherapy. For two patients, the central histological diagnosis was not aggressive B cell non-Hodgkin lymphoma but grade 1–3A follicular lymphoma.
Patients were deemed eligible for CAR-T cell therapy based on a physician’s assessment and all of the following:
-
1.
ECOG performance status of 0, 1 or 2.
-
2.
Adequate vascular access for leukapheresis (peripheral or central venous line).
-
3.
Absolute neutrophil count ≥1.0 × 109 l−1.
-
4.
Platelet count ≥75 × 109 l−1.
-
5.
Absolute lymphocyte count ≥0.1 × 109 l−1.
-
6.
Creatinine clearance (according to the Cockcroft–Gault equation or the modification of diet in renal disease equation) ≥40 ml min−1.
-
7.
Serum ALT/AST ≤ 2.5 × upper limit of normal.
-
8.
Total bilirubin ≤26 μmol l−1, except in patients with Gilbert’s syndrome.
-
9.
Left ventricular ejection fraction ≥45%.
-
10.
Oxygen saturation ≥92% on room air.
Key exclusion criteria included:
-
1.
Receipt of more than one prior line of systemic therapy.
-
2.
Previous CD19-targeted therapy.
-
3.
Cardiac involvement.
-
4.
Requirement for urgent therapy due to tumor mass effects such as bowel obstruction or blood vessel compression.
-
5.
Clinically significant pleural effusion.
-
6.
Known central nervous system disease.
-
7.
History of cardiovascular disease within the past 6 months.
-
8.
History of autoimmune disease requiring systemic immunosuppression and/or disease-modifying agents within the last year.
-
9.
History of idiopathic pulmonary fibrosis, organizing pneumonia, drug-induced pneumonitis, idiopathic pneumonitis or evidence of active pneumonitis on a chest computed tomography scan at screening.
-
10.
Active hepatitis B or C infection or positive HIV serology at the time of screening.
Procedures
Extended Data Fig. 1 provides an overview of the study procedures. Within 15 days after screening, all eligible patients underwent leukapheresis to obtain enough peripheral blood mononuclear cells to produce axi-cel. Optional bridging therapy after leukapheresis consisting of 1–2 cycles of R-GemOx (rituximab at 375 mg m−2, gemcitabine at 1,000 mg m−2 and oxaliplatin at 100 mg m−2, intravenously administered every 2 weeks for one or two cycles), corticosteroids (type and dose at the investigator’s discretion) or both was allowed. Upon the availability of axi-cel, patients received lymphodepleting conditioning chemotherapy for 3 days with cyclophosphamide (at a dose of 500 mg m−2 d−1) and fludarabine (30 mg m−2 d−1), followed 2–7 days later by a single intravenous infusion of axi-cel (at a target dose of 2 × 106 CAR-T cells per kilogram of body weight). Before lymphodepleting chemotherapy was administered, reconfirmation of study eligibility, including positron emission tomography (PET)-positive disease, was required. Hence, disease assessment by PET was performed at screening, within 7 days before the start of lymphodepleting chemotherapy, as well as at 1, 3, 6, 9 and 12 months after the axi-cel infusion. Clinical examination (including ECOG performance status), blood sampling and laboratory tests (including complete blood count and serum chemistry) were also performed at screening, on the day of axi-cel infusion, daily between day 1 and day 10 after the axi-cel infusion, on day 14 and at 1, 3, 6, 9 and 12 months following the axi-cel infusion. Patients were followed-up for 3 years after the axi-cel infusion and could then consent to participate in a long-term follow-up for up to 15 years via DESCAR-T (ClinicalTrials.gov ID NCT04328298), a French register of patients with malignant hemopathies eligible for CAR-T therapy.
Safety was monitored continuously throughout the study. CAR-T cell toxicities such as CRS and ICANS were graded according to the American Society for Transplantation and Cellular Therapy grading system45. All other Adverse effects were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE v.5.0).
Outcomes
The primary end point was investigator-assessed CMR at 3 months from the axi-cel infusion according to the Lugano response criteria25.
Secondary efficacy end points included investigator-assessed ORR at 3 months from the axi-cel infusion, investigator-assessed CMR at 6 months from the axi-cel infusion and the best investigator-assessed ORR and CMR. CMR and ORR at 3 months from the axi-cel infusion were also assessed by a central review panel formed by two readers and an adjudicator. Other secondary efficacy outcomes included the best ORR and best CMR as assessed by the central review panel, DOR, EFS from leukapheresis, PFS from infusion and OS from infusion. DOR, EFS and PFS were investigator-assessed. DOR was defined as the time from attainment of PMR or CMR to the date of first documented disease progression/relapse or death from any cause. EFS was defined as the time between leukapheresis and any event preventing axi-cel infusion if axi-cel was never infused, or death, disease progression or instauration of a new lymphoma therapy for lymphoma progression after axi-cel infusion. PFS was defined as the time from axi-cel infusion to disease progression or death from any cause. OS was defined as the time from axi-cel infusion to death from any cause. The estimated rates of EFS, PFS and OS at 6 and 12 months were further evaluated.
Safety was evaluated as the incidence, nature and severity of adverse effects. Adverse effects of special interest were related to CAR-T cell toxicities and included CRS and ICANS. Mortality during the study was summarized by cause of death.
Statistical analysis
A one-sample binomial design was used to calculate the initial sample size. We hypothesized that axi-cel would yield a CMR at 3 months of 34% compared to 12% with a historical SOC estimated from a retrospective, real-world cohort6. On the basis of this assumption, the initial sample size was calculated to be 40 infused patients, with 96% power and a 0.05 α-level (one-sided). To enable a balanced comparison of the efficacy of axi-cel in different age subgroups (<70 and ≥70 years), with a power of 85% and considering potential dropouts, the required sample size was increased to 62 patients.
Efficacy and safety analyses were performed on the mFAS, which included all patients who signed an informed consent and were infused with axi-cel. A sensitivity analysis of the primary end point was also conducted on all patients who signed an informed consent and had leukapheresis (FAS). We calculated CMR with exact Clopper–Pearson CI values, without adjustment for multiplicity. Patients without response assessment, due to any reason, were considered as nonresponders. We used the Kaplan–Meier method to estimate medians and 95% CI values for PFS, EFS, OS and DOR. If patients did not have an event at the time of the PFS, EFS and DOR analysis, they were censored at the date of the last disease assessment. For assessment of OS, alive patients were censored at their last follow-up date. Safety end points were assessed using descriptive statistics (counts and percentages). A subgroup analysis of CMR at 3 months was conducted for prespecified covariates (such as sex) and a Forest plot was provided.
Collected data were entered using the Electronic Data Capture system from Ennov v.8.1 (Ennov). Sample size calculation was performed using EAST v.6.5 (Cytel). All statistical analyses were performed using SAS v.9.3 or higher (SAS Institute) and AdClin v.3.2.2 or higher (AdClin).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
This trial is currently ongoing. Requests for access to aggregate data and supporting clinical documents will be reviewed and approved by an independent review panel on the basis of scientific merit. The datasets generated and/or analyzed during the current study are not publicly available due to proprietary considerations. All data provided are anonymized to respect the privacy of patients who have participated in the trial, in line with applicable laws and regulations. Data requests pertaining to the manuscript may be made to the corresponding author (R.H.; roch.houot@chu-rennes.fr). Requests will be processed within 12 weeks.
Change history
09 October 2023
A Correction to this paper has been published: https://doi.org/10.1038/s41591-023-02624-w
References
Feugier, P. et al. Long-term results of the R-CHOP study in the treatment of elderly patients with diffuse large B-cell lymphoma: a study by the Groupe d’Etude des Lymphomes de l’Adulte. J. Clin. Oncol. 23, 4117–4126 (2005).
Pfreundschuh, M. et al. CHOP-like chemotherapy plus rituximab versus CHOP-like chemotherapy alone in young patients with good-prognosis diffuse large-B-cell lymphoma: a randomised controlled trial by the MabThera International Trial (MInT) Group. Lancet Oncol. 7, 379–391 (2006).
Sehn, L. H. & Salles, G. Diffuse large B-cell lymphoma. N. Engl. J. Med. 384, 842–858 (2021).
Gisselbrecht, C. et al. Salvage regimens with autologous transplantation for relapsed large B-cell lymphoma in the rituximab era. J. Clin. Oncol. 28, 4184–4190 (2010).
van Imhoff, G. W. et al. Ofatumumab versus rituximab salvage chemoimmunotherapy in relapsed or refractory diffuse large B-cell lymphoma: the ORCHARRD study. J. Clin. Oncol. 35, 544–551 (2017).
Cazelles, C. et al. Rituximab plus gemcitabine and oxaliplatin (R-GemOx) in refractory/relapsed diffuse large B-cell lymphoma: a real-life study in patients ineligible for autologous stem-cell transplantation. Leuk. Lymphoma 62, 2161–2168 (2021).
Kamdar, M. et al. Lisocabtagene maraleucel versus standard of care with salvage chemotherapy followed by autologous stem cell transplantation as second-line treatment in patients with relapsed or refractory large B-cell lymphoma (TRANSFORM): results from an interim analysis of an open-label, randomised, phase 3 trial. Lancet 399, 2294–2308 (2022).
Locke, F. L. et al. Axicabtagene ciloleucel as second-line therapy for large B-cell lymphoma. N. Engl. J. Med. 386, 640–654 (2022).
Westin, J. & Sehn, L. H. CAR T cells as a second-line therapy for large B-cell lymphoma: a paradigm shift? Blood 139, 2737–2746 (2022).
Abramson, J. S. et al. Lisocabtagene maraleucel as second-line therapy for large B-cell lymphoma: primary analysis of the phase 3 TRANSFORM study. Blood 141, 1675–1684 (2023).
Westin, J. R. et al. Survival with axicabtagene ciloleucel in large B-cell lymphoma. N. Engl. J. Med. https://doi.org/10.1056/nejmoa2301665 (2023).
Vic, S., Lemoine, J., Armand, P., Lemonnier, F. & Houot, R. Transplant-ineligible but chimeric antigen receptor T-cells eligible: a real and relevant population. Eur. J. Cancer 175, 246–253 (2022).
Salles, G. A. et al. Treatment of aggressive B-cell non-Hodgkin lymphoma beyond frontline therapy in patients not eligible for stem cell transplantation: a structured review. Leuk. Lymphoma 60, 1610–1625 (2019).
Mounier, N. et al. Rituximab plus gemcitabine and oxaliplatin in patients with refractory/relapsed diffuse large B-cell lymphoma who are not candidates for high-dose therapy. A phase II Lymphoma Study Association trial. Haematologica 98, 1726–1731 (2013).
Sehn, L. H. et al. Polatuzumab vedotin plus bendamustine and rituximab in relapsed/refractory DLBCL: survival update and new extension cohort data. Blood Adv. 6, 533–543 (2022).
Salles, G. et al. Tafasitamab plus lenalidomide in relapsed or refractory diffuse large B-cell lymphoma (L-MIND): a multicentre, prospective, single-arm, phase 2 study. Lancet Oncol. 21, 978–988 (2020).
Cazelles, C. et al. Rituximab plus gemcitabine and oxaliplatin (R-GemOx) in refractory/relapsed (R/R) DLBCL. A real life study in patients ineligible for autologous transplantation. Blood 134, 4115 (2019).
Neelapu, S. S. et al. Outcomes of older patients in ZUMA-1, a pivotal study of axicabtagene ciloleucel in refractory large B-cell lymphoma. Blood 135, 2106–2109 (2020).
Ram, R. et al. Toxicity and efficacy of chimeric antigen receptor T-cell therapy in patients with diffuse large B-cell lymphoma above the age of 70 years compared to younger patients - a matched control multicenter cohort study. Haematologica 107, 1111–1118 (2022).
Bethge, W. A. et al. GLA/DRST real-world outcome analysis of CAR T-cell therapies for large B-cell lymphoma in Germany. Blood 140, 349–358 (2022).
Bachy, E. et al. A real-world comparison of tisagenlecleucel and axicabtagene ciloleucel CAR T cells in relapsed or refractory diffuse large B cell lymphoma. Nat. Med. 28, 2145–2154 (2022).
Jacobson, C. A. et al. Real-world evidence of axicabtagene ciloleucel for the treatment of large B cell lymphoma in the United States. Transpl. Cell Ther. 28, e581–e588 (2022).
Kuhnl, A. et al. CAR T in patients with large B-cell lymphoma not fit for autologous transplant. Br. J. Haematol. https://doi.org/10.1111/bjh.18810 (2023).
Sorror, M. L. et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood 106, 2912–2919 (2005).
Cheson, B. D. et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J. Clin. Oncol. 32, 3059–3068 (2014).
Vercellino, L. et al. Predictive factors of early progression after CAR T-cell therapy in relapsed/refractory diffuse large B-cell lymphoma. Blood Adv. 4, 5607–5615 (2020).
Flowers, C. R. & Odejide, O. O. Sequencing therapy in relapsed DLBCL. Hematol. Am. Soc. Hematol. Educ. Program 2022, 146–154 (2022).
Voltin, C. A. et al. Outcome prediction in patients with large B-cell lymphoma undergoing chimeric antigen receptor T-cell therapy. Hemasphere 7, e817 (2023).
Lutfi, F. et al. Imaging biomarkers to predict outcomes in patients with large B-cell lymphoma with a day 28 partial response by 18F-FDG PET/CT imaging following CAR-T therapy. Clin. Lymphoma Myeloma Leuk. https://doi.org/10.1016/j.clml.2023.06.005 (2023).
Galtier, J. et al. Positron emission tomography-imaging assessment for guiding strategy in patients with relapsed/refractory large B-cell lymphoma receiving CAR T cells. Haematologica 108, 171–180 (2023).
Iacoboni, G. et al. Prognostic impact of total metabolic tumor volume in large B-cell lymphoma patients receiving CAR T-cell therapy. Ann. Hematol. 100, 2303–2310 (2021).
Nastoupil, L. J. et al. Standard-of-care axicabtagene ciloleucel for relapsed or refractory large B-cell lymphoma: results from the US Lymphoma CAR T Consortium. J. Clin. Oncol. 38, 3119–3128 (2020).
Sehgal, A. et al. Lisocabtagene maraleucel as second-line therapy in adults with relapsed or refractory large B-cell lymphoma who were not intended for haematopoietic stem cell transplantation (PILOT): an open-label, phase 2 study. Lancet Oncol. 23, 1066–1077 (2022).
Sorror, M. L. et al. Prospective validation of the predictive power of the hematopoietic cell transplantation comorbidity index: a CIBMTR® study. Biol. Blood Marrow Transplant. 21, 1479–1487 (2015).
Berro, M. et al. Hematopoietic cell transplantation-specific comorbidity index predicts morbidity and mortality in autologous stem cell transplantation. Biol. Blood Marrow Transplant. 23, 1646–1650 (2017).
Sehn, L. H. et al. Polatuzumab vedotin in relapsed or refractory diffuse large B-cell lymphoma. J. Clin. Oncol. 38, 155–165 (2020).
Hutchings, M. et al. Dose escalation of subcutaneous epcoritamab in patients with relapsed or refractory B-cell non-Hodgkin lymphoma: an open-label, phase 1/2 study. Lancet 398, 1157–1169 (2021).
Hutchings, M. et al. Glofitamab, a novel, bivalent CD20-targeting T-cell-engaging bispecific antibody, induces durable complete remissions in relapsed or refractory B-cell lymphoma: a phase I trial. J. Clin. Oncol. 39, 1959–1970 (2021).
Dickinson, M. J. et al. Glofitamab for relapsed or refractory diffuse large B-cell lymphoma. N. Engl. J. Med. 387, 2220–2231 (2022).
Bannerji, R. et al. Odronextamab, a human CD20×CD3 bispecific antibody in patients with CD20-positive B-cell malignancies (ELM-1): results from the relapsed or refractory non-Hodgkin lymphoma cohort in a single-arm, multicentre, phase 1 trial. Lancet Haematol. 9, e327–e339 (2022).
Budde, L. E. et al. Single-agent mosunetuzumab shows durable complete responses in patients with relapsed or refractory B-cell lymphomas: phase I dose-escalation study. J. Clin. Oncol. 40, 481–491 (2022).
Thieblemont, C. et al. Epcoritamab, a novel, subcutaneous CD3xCD20 bispecific T-cell-engaging antibody, in relapsed or refractory large B-cell lymphoma: dose expansion in a phase I/II trial. J. Clin. Oncol. 41, 2238–2247 (2023).
Bartlett, N.L. et al. Mosunetuzumab monotherapy is active and tolerable in patients with relapsed/refractory diffuse large B-cell lymphoma. Blood Adv. https://doi.org/10.1182/bloodadvances.2022009260 (2023).
Swerdlow, S. H. et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 127, 2375–2390 (2016).
Lee, D. W. et al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol. Blood Marrow Transplant. 25, 625–638 (2019).
Acknowledgements
This study was funded by Kite, a Gilead company. We thank the patients, their families and the study personnel involved in this trial. We also thank the ALYCANTE investigators, P. Cony-Makhoul, E. Chareyre and the LYSARC study team (Supplementary Table 1), including LYSA-IM and LYSA-P, for their precious contribution. In addition, we thank T. Rohban and M. El Hajj, from Partner 4 Health for providing medical writing support in accordance with Good Publication Practice (GPP3) guidelines.
Author information
Authors and Affiliations
Contributions
R.H. and F.L. contributed to the conception, design and planning of the study. All authors contributed to the acquisition and analysis of data. All authors contributed to the critical review and revision of the manuscript. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
R.H. has received honoraria from Kite/Gilead, Novartis, Incyte, Janssen, MSD, Takeda and Roche; and is a member on an entity’s Board of Directors or advisory committees of Kite/Gilead, Novartis, Bristol-Myers Squibb/Celgene, ADC Therapeutics, Incyte and Miltenyi. E.B. has received honoraria from Kite/Gilead, Bristol-Myers Squibb, Novartis, Pfizer, Incyte, ADC Therapeutics, Roche and Takeda; travel reimbursement from Kite/Gilead, Bristol-Myers Squibb, Novartis and Pfizer; and research funding from Amgen and Bristol-Myers Squibb. G.C. has received consulting fees from Roche, AbbVie, Bristol-Myers Squibb, MedXCell, Mabqi and Onward Therapeutics; honoraria from Jansen, Gilead, Novartis, Roche, Bristol-Myers Squibb and Incyte; and travel and accommodation expenses from Gilead, Roche and Jansen. F.X.G. has received consulting fees from Gilead, Bristol-Myers Squibb, Miltenyi and Novartis; and travel and accommodation expenses from Gilead and Novartis. F.M. has received consulting fees from Roche, Gilead, Novartis, Bristol-Myers Squibb, Genmab and AbbVie; and honoraria for advisory boards from Roche, Gilead and Miltenyi. L.O. has received consulting fees from Roche; honoraria from Bristol-Myers Squibb, Kite/Gilead and Incyte; and travel and accommodation expenses from Roche and AstraZeneca. T.G. has received consulting fees from Takeda and Kite/Gilead; honoraria from Kite/Gilead; and travel and accommodation expenses from Roche, Takeda and Kite/Gilead. P.F. has received consulting fees from Gilead, AstraZeneca, BeiGene, AbbVie and Janssen; honoraria from Gilead, AstraZeneca, BeiGene, AbbVie and Janssen; and travel and accommodation expenses from Gilead, AstraZeneca, BeiGene, AbbVie and Janssen. R.D. has received honoraria from Novartis and Takeda; research funding from Ligue contre le Cancer, Arthur Sachs, Monahan Foundation, Servier Foundation, Philippe Foundation and DCP AP-HP; and non-financial support from Kite/Gilead. C.T. has received institutional research funding from Kite/Gilead and Roche; honoraria for advisory boards from Roche, Novartis, AstraZeneca, BeiGene, AbbVie, Takeda, Roche, Novartis, Kite/Gilead, Bristol-Myers Squibb; and travel and accommodation expenses from Roche, Novartis, AbbVie, Takeda, Roche, Kite/Gilead and Bristol-Myers Squibb. F.J. has received honoraria from Roche, Gilead, Janssen and Bristol-Myers Squibb; honoraria for advisory boards from Roche; and travel and accommodation expenses from Roche and Gilead. S.C. has received consulting fees from Atara, Novartis, Kite/Gilead, Pierre Fabre, Takeda, AbbVie and AstraZeneca; honoraria for advisory boards from Kite/Gilead, Novartis, AbbVie, Takeda and Viatris; institutional funding from Janssen; and travel and accommodation expenses from Novartis, AbbVie and Pierre Fabre. O.C. has received honoraria from Roche, Takeda, Bristol-Myers Squibb, Merck, Kite/Gilead, AbbVie and ADC Therapeutics; and research funding from Roche, Takeda and Kite/Gilead. G.B. has received honoraria for advisory boards from Novartis, Kite/Gilead, Bristol-Myers Squibb and Incyte; and travel and accommodation expenses from Novartis, Kite/Gilead, Bristol-Myers Squibb and Incyte. M.C. has received institutional research funding from AP-HP, INSERM, INCA, Fondation ARC pour la Recherche sur le Cancer and CALYM; honoraria from Amgen and CSL Behring; research funding from Innate Pharma and Servier; and travel and accommodation expenses from Pfizer, Grifols, CSL Behring and Gilead. F.L.G. has received honoraria from Rennes University Hospital. C.M. has received research funding from Kite Pharmaceuticals. C.P. is a LYSARC employee. E.I. has received honoraria from Janssen-Cilag and Pfizer. C.L. has received research funding from Ligue contre le Cancer and Labex Toucan; and travel and accommodation expenses from Roche and Janssen. All other authors declare no competing interests.
Peer review
Peer review information
Nature Medicine thanks Jay Spiegel and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Saheli Sadanand, in collaboration with the Nature Medicine team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1
Study design and overview of study procedures.
Extended Data Fig. 2 Causes of autologous stem cell transplantation (ASCT)-ineligibility in the modified full analysis set (N = 62).
Abbreviation: HCT-CI, hematopoietic cell transplantation-specific comorbidity index.
Extended Data Fig. 3
Metabolic response over time in the modified full analysis set (mFAS) (N = 62).
Extended Data Fig. 4 Forest plot illustrating complete metabolic response (CMR) rates at 3 months by subgroup.
Blue squares denote CMR rates, and error bars indicate 95% two-sided 95% exact Clopper-Pearson confidence intervals (CIs). The overall study population is the modified full analysis set (N = 62), in which CMR at 3 months from the axi-cel infusion was 71.0% (95% CI, 58.1%–81.8%). Abbreviations: HCT-CI, hematopoietic cell transplantation-specific comorbidity index; IPI, international prognostic index; LDH, lactate dehydrogenase; PMD, progressive metabolic disease; PMR, partial metabolic response; SD, stable disease; TMTV, total metabolic tumor volume.
Supplementary information
Supplementary Information
Supplementary Table 1 and Study Protocol.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Houot, R., Bachy, E., Cartron, G. et al. Axicabtagene ciloleucel as second-line therapy in large B cell lymphoma ineligible for autologous stem cell transplantation: a phase 2 trial. Nat Med 29, 2593–2601 (2023). https://doi.org/10.1038/s41591-023-02572-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41591-023-02572-5