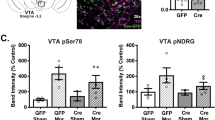
Abstract
Alcohol use disorder (AUD) exacts enormous personal, social and economic costs globally. Return to alcohol use in treatment-seeking patients with AUD is common, engendered by a cycle of repeated abstinence-relapse episodes even with use of currently available pharmacotherapies. Repeated ethanol use induces dopaminergic signaling neuroadaptations in ventral tegmental area (VTA) neurons of the mesolimbic reward pathway, and sustained dysfunction of reward circuitry is associated with return to drinking behavior. We tested this hypothesis by infusing adeno-associated virus serotype 2 vector encoding human glial-derived neurotrophic factor (AAV2-hGDNF), a growth factor that enhances dopaminergic neuron function, into the VTA of four male rhesus monkeys, with another four receiving vehicle, following induction of chronic alcohol drinking. GDNF expression ablated the return to alcohol drinking behavior over a 12-month period of repeated abstinence–alcohol reintroduction challenges. This behavioral change was accompanied by neurophysiological modulations to dopamine signaling in the nucleus accumbens that countered the hypodopaminergic signaling state associated with chronic alcohol use, indicative of a therapeutic modulation of limbic circuits countering the effects of alcohol. These preclinical findings suggest gene therapy targeting relapse prevention may be a potential therapeutic strategy for AUD.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The data that support the findings of this study, including behavioral, biochemical, and voltammetry analyses, are available on the public data repository Zenodo.org at https://zenodo.org/record/7236274.
References
Sacks, J. J., Gonzales, K. R., Bouchery, E. E., Tomedi, L. E. & Brewer, R. D. 2010 national and state costs of excessive alcohol consumption. Am. J. Prev. Med. 49, e73–e79 (2015).
Substance Abuse and Mental Health Services Administration. Key substance use and mental health indicators in the United States: Results from the 2017 national survey on drug use and health recommended citation substance abuse and mental health services administration. HHS Publication No. SMA 18-53 (2018).
Kanny, D., Naimi, T. S., Liu, Y. & Brewer, R. D. Trends in total binge drinks per adult who reported binge drinking—United States, 2011–2017. MMWR Morb. Mortal. Wkly. Rep. 69, 30–34 (2020).
Witkiewitz, K. et al. Advances in the science and treatment of alcohol use disorder. Sci. Adv. 5, eaax4043 (2019).
Gueorguieva, R., Wu, R., Krystal, J. H., Donovan, D. & O’Malley, S. S. Temporal patterns of adherence to medications and behavioral treatment and their relationship to patient characteristics and treatment response. Addict. Behav. 38, 2119–2127 (2013).
Koob, G. F. Theoretical frameworks and mechanistic aspects of alcohol addiction: alcohol addiction as a reward deficit disorder. Curr. Top. Behav. Neurosci. 13, 3–30 (2011).
Volkow, N. D., Wang, G. J., Fowler, J. S. & Tomasi, D. Addiction circuitry in the human brain. Annu. Rev. Pharmacol. Toxicol. 52, 321–336 (2012).
Charlet, K., Beck, A. & Heinz, A. The dopamine system in mediating alcohol effects in humans. Curr. Top. Behav. Neurosci. 13, 461–488 (2011).
Karkhanis, A. N., Rose, J. H., Huggins, K. N., Konstantopoulos, J. K. & Jones, S. R. Chronic intermittent ethanol exposure reduces presynaptic dopamine neurotransmission in the mouse nucleus accumbens. Drug Alcohol Depend. 150, 24–30 (2015).
Siciliano, C. A. et al. Increased presynaptic regulation of dopamine neurotransmission in the nucleus accumbens core following chronic ethanol self-administration in female macaques. Psychopharmacol. 233, 1435–1443 (2016).
Siciliano, C. A. et al. Chronic ethanol self-administration in macaques shifts dopamine feedback inhibition to predominantly D2 receptors in nucleus accumbens core. Drug Alcohol Depend. 158, 159–163 (2016).
Siciliano, C. A., Karkhanis, A. N., Holleran, K. M., Melchior, J. R. & Jones, S. R. Cross-species alterations in synaptic dopamine regulation after chronic alcohol exposure. Handb. Exp. Pharmacol. 248, 213–238 (2018).
Thanos, P. K. et al. Overexpression of dopamine D2 receptors reduces alcohol self-administration. J. Neurochem. 78, 1094–1103 (2001).
Airaksinen, M. S. & Saarma, M. The GDNF family: signalling, biological functions and therapeutic value. Nat. Rev. Neurosci. 3, 383–394 (2002).
Barak, S. et al. Positive autoregulation of GDNF levels in the ventral tegmental area mediates long-lasting inhibition of excessive alcohol consumption. Transl. Psychiatry 1, e60 (2011).
Barak, S., Carnicella, S., Yowell, Q. V. & Ron, D. Glial cell line-derived neurotrophic factor reverses alcohol-induced allostasis of the mesolimbic dopaminergic system: implications for alcohol reward and seeking. J. Neurosci. 31, 9885–9894 (2011).
Lin, L.-F., Doherty, D. H., Lile, J. D., Bektesh, S. & Collins, F. GDNF: A glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science 260, 1130–1132 (1993).
Ahmadiantehrani, S., Barak, S. & Ron, D. GDNF is a novel ethanol-responsive gene in the VTA: implications for the development and persistence of excessive drinking. Addict. Biol. 19, 623–633 (2014).
Heberlein, A. et al. BDNF and GDNF serum levels in alcohol-dependent patients during withdrawal. Prog. Neuropsychopharmacol. Biol. Psychiatry 34, 1060–1064 (2010).
Barak, S. et al. Glial cell line-derived neurotrophic factor (GDNF) is an endogenous protector in the mesolimbic system against excessive alcohol consumption and relapse. Addict. Biol. 20, 626–642 (2015).
Wang, J. et al. Nucleus accumbens-derived glial cell line-derived neurotrophic factor is a retrograde enhancer of dopaminergic tone in the mesocorticolimbic system. J. Neurosci. 30, 14502–14512 (2010).
Carnicella, S., Amamoto, R. & Ron, D. Excessive alcohol consumption is blocked by glial cell line-derived neurotrophic factor. Alcohol 43, 35–43 (2009).
Carnicella, S., Kharazia, V., Jeanblanc, J., Janak, P. H. & Ron, D. GDNF is a fast-acting potent inhibitor of alcohol consumption and relapse. Proc. Natl Acad. Sci. USA 105, 8114–8119 (2008).
Barak, S., Ahmadiantehrani, S., Logrip, M. L. & Ron, D. GDNF and alcohol use disorder. Addict. Biol. 24, 335–343 (2019).
Grant, K. A. et al. Drinking typography established by scheduled induction predicts chronic heavy drinking in a monkey model of ethanol self-administration. Alcohol Clin. Exp. Res. 32, 1824–1838 (2008).
Allen, D. C., Gonzales, S. W. & Grant, K. A. Effect of repeated abstinence on chronic ethanol self-administration in the rhesus monkey. Psychopharmacology 235, 109–120 (2018).
Cuzon Carlson, V. C. et al. Synaptic and morphological neuroadaptations in the putamen associated with long-term, relapsing alcohol drinking in primates. Neuropsychopharmacology 36, 2513–2528 (2011).
National Institute on Alcohol Abuse and Alcoholism NIAAA council approves definition of binge drinking. NIAAA Newsl. 3, 3 (2004).
Sudhakar, V. et al. Development of a novel frameless skull-mounted ball-joint guide array for use in image-guided neurosurgery. J. Neurosurg. 132, 595–604 (2020).
Sebastian, W. S. et al. Safety and tolerability of MRI-guided infusion of AAV2-hAADC into the mid-brain of nonhuman primate. Mol. Ther. Methods Clin. Dev. 3, 14049 (2014).
Ciesielska, A. et al. Anterograde axonal transport of AAV2-GDNF in rat basal ganglia. Mol. Ther. 19, 922–927 (2011).
Becker, H. C. Kindling in alcohol withdrawal. Alcohol Health Res. World 22, 25–33 (1998).
Summers, L., Clingerman, K. J. & Yang, X. Validation of a body condition scoring system in rhesus macaques (Macaca mulatta): assessment of body composition by using dual-energy X-ray absorptiometry. J. Am. Assoc. Lab Anim. Sci. 51, 88–93 (2012).
Labberton, L., Bakker, J., Klomp, R., Langermans, J. A. & van Geijlswijk, I. M. Challenges in oral administration of metronidazole dissolved in drinking water to rhesus monkeys (Macaca mulatta). Lab Anim. 42, 213–216 (2013).
Yamada, H., Louie, K. & Glimcher, P. W. Controlled water intake: a method for objectively evaluating thirst and hydration state in monkeys by the measurement of blood osmolality. J. Neurosci. Methods 191, 83–89 (2010).
Barnes, S. A., Der-Avakian, A. & Markou, A. Anhedonia, avolition, and anticipatory deficits: assessments in animals with relevance to the negative symptoms of schizophrenia. Eur. Neuropsychopharmacol. 24, 744–758 (2014).
Burns, R. S. et al. A primate model of parkinsonism: selective destruction of dopaminergic neurons in the pars compacta of the substantia nigra by N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Proc. Natl Acad. Sci. USA 80, 4546–4550 (1983).
Johnston, L. C. et al. Clinically relevant effects of convection-enhanced delivery of AAV2-GDNF on the dopaminergic nigrostriatal pathway in aged rhesus monkeys. Hum. Gene Ther. 20, 497–510 (2009).
Oiwa, Y. et al. Overlesioned hemiparkinsonian non human primate model: correlation between clinical, neurochemical and histochemical changes. Front. Biosci. 8, a155–a166 (2003).
Pifl, C., Bertel, O., Schingnitz, G. & Hornykiewicz, O. Extrastriatal dopamine in symptomatic and asymptomatic rhesus monkeys treated with 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP). Neurochem. Int. 17, 263–270 (1990).
Pifl, C., Schingnitz, G. & Hornykiewicz, O. The neurotoxin MPTP does not reproduce in the rhesus monkey the interregional pattern of striatal dopamine loss typical of human idiopathic Parkinson’s disease. Neurosci. Lett. 92, 228–233 (1988).
Sari, Y., Johnson, V. R. & Weedman, J. M. Role of the serotonergic system in alcohol dependence: from animal models to clinics. Prog. Mol. Biol. Transl. Sci. 98, 401–443 (2011).
Karkhanis, A., Holleran, K. M. & Jones, S. R. Dynorphin/Kappa Opioid Receptor Signaling in Preclinical Models of Alcohol, Drug, and Food Addiction 1st edn (Elsevier, 2017).
Schilaty, N. D. et al. Acute ethanol inhibits dopamine release in the nucleus accumbens via α6 nicotinic acetylcholine receptors. J. Pharmacol. Exp. Ther. 349, 559–567 (2014).
Yorgason, J. T., Rose, J. H., McIntosh, J. M., Ferris, M. J. & Jones, S. R. Greater ethanol inhibition of presynaptic dopamine release in C57BL/6J than DBA/2J mice: role of nicotinic acetylcholine receptors. Neuroscience 284, 854–864 (2015).
Martinez, D. et al. Alcohol dependence is associated with blunted dopamine transmission in the ventral striatum. Biol. Psychiatry 58, 779–786 (2005).
Volkow, N. D. et al. Profound decreases in dopamine release in striatum in detoxified alcoholics: possible orbitofrontal involvement. J. Neurosci. 27, 12700–12706 (2007).
Lu, L. et al. Role of ventral tegmental area glial cell line-derived neurotrophic factor in incubation of cocaine craving. Biol. Psychiatry 66, 137–145 (2009).
Carnicella, S. & Ron, D. GDNF – a potential target to treat addiction. Pharmacol. Ther. 122, 9–18 (2009).
Messer, C. J. et al. Role for GDNF in biochemical and behavioral adaptations to drugs of abuse. Neuron 26, 247–257 (2000).
Manfredsson, F. P. et al. Nigrostriatal rAAV-mediated GDNF overexpression induces robust weight loss in a rat model of age-related obesity. Mol. Ther. 17, 980–991 (2009).
Su, X. et al. Safety evaluation of AAV2-GDNF gene transfer into the dopaminergic nigrostriatal pathway in aged and parkinsonian rhesus monkeys. Hum. Gene Ther. 20, 1627–1640 (2009).
Tümer, N. et al. Hypothalamic rAAV-mediated GDNF gene delivery ameliorates age-related obesity. Neurobiol. Aging 27, 459–470 (2006).
Kells, A. P., Forsayeth, J. & Bankiewicz, K. S. Glial-derived neurotrophic factor gene transfer for Parkinson’s disease: anterograde distribution of AAV2 vectors in the primate brain. Neurobiol. Dis. 48, 228–235 (2012).
Nutt, J. G. et al. Randomized, double-blind trial of glial cell line-derived neurotrophic factor (GDNF) in PD. Neurology 60, 69–73 (2003).
Pearson, T. S. et al. Gene therapy for aromatic L-amino acid decarboxylase deficiency by MR-guided direct delivery of AAV2-AADC to midbrain dopaminergic neurons. Nat. Commun. 12, 4251 (2021).
AAV2-GDNF for Advanced Parkinson’s Disease (National Institute of Neurological Disorders and Stroke, 2023); www.clinicaltrials.gov
GDNF Gene Therapy for Parkinson’s Disease (Brain Neurotherapy Bio, Inc., 2023); www.clinicaltrials.gov
Richardson, R. M. et al. Interventional MRI-guided putaminal delivery of AAV2-GDNF for a planned clinical trial in Parkinson’s disease. Mol. Ther. 19, 1048–1057 (2011).
Terse, P. S., Kells, A. P., Noker, P., Wright, J. F. & Bankiewicz, K. S. Safety assessment of AAV2-hGDNF administered via intracerebral injection in rats for treatment of Parkinson’s disease. Int. J. Toxicol. 40, 4–14 (2021).
Helms, C. M. et al. The effects of age at the onset of drinking to intoxication and chronic ethanol self-administration in male rhesus macaques. Psychopharmacology 231, 1853–1861 (2014).
Green, K. L. et al. Comparison of ethanol metabolism in male and female cynomolgus macaques (Macaca fascicularis). Alcohol Clin. Exp. Res. 23, 611–616 (1999).
Welsh, J. P. et al. Bidirectional plasticity in the primate inferior olive induced by chronic ethanol intoxication and sustained abstinence. Proc. Natl Acad. Sci. USA 108, 10314–10319 (2011).
Kells, A. P. et al. Regeneration of the MPTP-lesioned dopaminergic system after convection-enhanced delivery of AAV2-GDNF. J. Neurosci. 30, 9567–9577 (2010).
Su, X. et al. Real-time MR imaging with gadoteridol predicts distribution of transgenes after convection-enhanced delivery of AAV2 vectors. Mol. Ther. 18, 1490–1495 (2010).
Breese, G. R., Sinha, R. & Heilig, M. Chronic alcohol neuroadaptation and stress contribute to susceptibility for alcohol craving and relapse. Pharmacol. Ther. 129, 149–171 (2011).
Koob, G. F. Alcoholism: allostasis and beyond. Alcohol Clin. Exp. Res. 27, 232–243 (2003).
Rodd, Z. A. et al. Effects of repeated alcohol deprivations on operant ethanol self-administration by alcohol-preferring (P) rats. Neuropsychopharmacology 28, 1614–1621 (2003).
Lopez, M. F. & Becker, H. C. Effect of pattern and number of chronic ethanol exposures on subsequent voluntary ethanol intake in C57BL/6J mice. Psychopharmacology 181, 688–696 (2005).
Baker, E. J., Farro, J., Gonzales, S., Helms, C. & Grant, K. A. Chronic alcohol self-administration in monkeys shows long-term quantity/frequency categorical stability. Alcohol Clin. Exp. Res. 38, 2835–2843 (2014).
Ford, M. M., Steele, A. M., McCracken, A. D., Finn, D. A. & Grant, K. A. The relationship between adjunctive drinking, blood ethanol concentration and plasma corticosterone across fixed-time intervals of food delivery in two inbred mouse strains. Psychoneuroendocrinology 38, 2598–2610 (2013).
Witkiewitz, K. & Tucker, J. A. Abstinence not required: Expanding the definition of recovery from alcohol use disorder. Alcohol Clin. Exp. Res. 44, 36–40 (2020).
Shabani, S. et al. A genetic animal model of differential sensitivity to methamphetamine reinforcement. Neuropharmacology 62, 2169–2177 (2012).
Naidoo, J. et al. Extensive transduction and enhanced spread of a modified AAV2 capsid in the non-human primate CNS. Mol. Ther. 26, 2418–2430 (2018).
Jimenez, V. A. et al. Synaptic adaptations in the central amygdala and hypothalamic paraventricular nucleus associated with protracted ethanol abstinence in male rhesus monkeys. Neuropsychopharmacology 44, 982–993 (2019).
Acknowledgements
We thank N. Newman, K. Diem, H. VanderJagt, C. Rudnicky, J. Schoen and J. Mootz for animal husbandry and data collection of daily drinking sessions; S. Gonzales for technical support with drinking session programming and equipment maintenance; and B. Park for consultation with statistical modeling and experimental design. We also acknowledge the contributions of C. Kroenke and M. Reusz for MRI support; L. Martin and T. Hobbs for surgical services support; A. Lewis, A. Johnson and L. Colgin, as well as W. Price and A. Beckman, for pathology services support; M. Travis and V. Sudhakar for histology support; the Electrophysiology Core overseen by V. Cuzon-Carlson for hosting and supporting the voltammetry experiments; the Molecular Virology Core at the Oregon National Primate Research Center (ONPRC) for performing viral serology assays; and the Clinical Pathology Laboratory (ONPRC) for conducting in-house assays for complete blood count and a comprehensive chemistry panel. This study was funded by National Institute on Alcohol Abuse and Alcoholism (NIAAA) grant R01 AA024757 (to M.M.F.). Additional support was provided by NIH grants U01 AA013510 (K.A.G.), U01 AA014091 (S.R.J.), P60 AA010760 (K.A.G. and M.M.F.), R24 AA019431 (K.A.G.) and P50 AA026117 (S.R.J.).
Author information
Authors and Affiliations
Contributions
M.F. supervised the research and oversaw coordination across laboratories, designed and performed experiments, analyzed and interpreted data and wrote and edited the manuscript. B.G. and K.H. performed voltammetry experiments, analyzed and interpreted data, assisted in drafting results section and edited the manuscript. V.V. performed tissue immunohistochemistry and microscopy, assisted on data analysis and interpretation, and assisted with manuscript writing and editing. J.N. assisted with coordination of intra-VTA infusions, analyzed GDNF ELISA and HPLC results, and assisted in drafting the results section. P.H. performed ELISA experiments, assisted with interpretation of viral serology results, and edited the manuscript. L.V. served as project lead of animal cohort, collected and analyzed data, coordinated institutional services associated with husbandry, surgery, MRI, and pathology, and edited the manuscript. E.P. and M.D. performed voltammetry experiments on housing control NHPs and assisted in collecting and interpreting voltammetry data. K.O. performed HPLC experiments and assisted with interpretation of HPLC results. J.B. assisted with surgeries and consulted on tissue collection and handling techniques. L.S. consulted on tissue collection and handling techniques and assisted with manuscript writing and editing. J.M. performed brain tissue processing and edited the manuscript. J.F. participated in project inception, and assisted with manuscript writing and editing. S.J. designed voltammetry experiments, interpreted data, supervised B.G., K.H., E.P., and M.D., and edited the manuscript. K.G. and K.B. conceived the project, provided feedback on data analyses, and edited the manuscript. K.G. supervised M.F. and L.V. whereas K.B. supervised J.N., P.H., J.B., L.S., V.V. and J.F. on their efforts. K.B. performed the MRI-guided infusions targeting the VTA.
Corresponding authors
Ethics declarations
Competing interests
K.S.B. is a consultant for Asklepios BioPharmaceutical, Scribe Therapeutics and Aviado Bio. The remaining authors declare no competing interests.
Peer review
Peer review information
Nature Medicine thanks Miguel Sena-Esteves, Alëna Balasanova and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Anna Maria Ranzoni, in collaboration with the Nature Medicine team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Chronic alcohol self-administration in rhesus macaques.
a) The relationship between blood ethanol concentrations (BECs) collected during the final week of the OAB (n = 2 per subject) and corresponding alcohol intakes recorded at timing of sampling for each subject as identified by drinking panel each was assigned (Panel 0–7), with the dashed horizontal line demarcating 80 mg/dl (the threshold for legal intoxication in humans). 6 of the 8 subjects exhibited alcohol consumption approaching or surpassing the 80 mg/dl threshold during the final week of OAB. b) The relationship between BECs collected during the final two months of the OAB and corresponding alcohol intakes recorded at timing of sampling divided between subjects destined for the Vehicle-treated control group and the AAV2-hGDNF-treated group (4 subjects per group, n = 20 BEC points per group), demonstrating the similarity of BEC-alcohol intake relationship between the two groups.
Extended Data Fig. 2 Pilot surgeries for AAV2 delivery to the VTA.
Prior to viral drug infusion of the experimental groups, a pilot study was conducted using two macaques to confirm surgical coordinates and trajectory planning, as well as infusate and transgene distribution within the VTA of macaques. AAV2-GFP spiked with gadolinium chelate (2 mM, ProHance, Bracco Diagnostics, Princeton, NJ, USA) was bilaterally infused into the VTA target via magnetic-resonance imaging-guided convection-enhanced delivery (MR-guided CED) followed by post-mortem immunohistochemical assessment of GFP protein expression. Below are representative images of MRI with gadoteridol contrast (a) and post-mortem immunohistochemical staining for GFP (b) from one of two preliminary macaque subjects used to confirm targeting and vector distribution in the VTA. AAV2 vector encoding green fluorescent protein (GFP) was delivered by CED as described in Methods. The white arrow (A) shows the cannula actively delivering the vector with contrast agent and the white arrowhead (A) shows the imaging detection of the contrast in the infusate. One month following infusion, subjects were sacrificed by intracardial perfusion, and brain tissue harvested for immunochemical analyses to address GFP expression and distribution (B). The black arrowhead (B) shows the positive immunochemical staining for the GFP antibody.
Extended Data Fig. 3 Individual Monthly Ethanol intakes and BECs, and Table of Day 1 Reintroduction Statistics.
a) Monthly individual subject means of daily alcohol intakes (g/kg/day) across each of the six alcohol reintroduction periods (R1–R6). b) Monthly individual subject means of BECs (mg/dl) across all six alcohol reintroduction periods (R1–R6). c) Table values and statistics of ethanol drinking patterns during each alcohol reintroduction onset (day 1) as a factor of AAV2-hGDNF treatment. All values represent the mean ± SEM of each treatment (n = 4/group). OAB measures reflect the grand mean from the final 7 days of access to ethanol prior to the first forced abstinence (A1) phase onset while measures for alcohol reintroduction phases (R1–R6) are representative of the initial day of alcohol reintroduction following abstinence. †Significant main effects of AAV2-hGDNF treatment; ‡significant treatment x phase interactions. (Mean ± SEM; n = 4/group are shown; *: p ≤ 0.05 and **: p ≤ 0.001 vs. respective Vehicle-treated control group value. Specific statistical tests and p-values are as provided in Fig. 3).
Extended Data Fig. 4 Effects of AAV2-hGDNF on food and water intake, weight, and sensorimotor tasks in macaques.
a) Monthly averages of subject weight (kg) shown by group (AAV2-hGDNF in orange; Vehicle in blue) from the last month of OAB and during alcohol reintroduction phases R1 through R6 (Mean ± SEM; n = 4/group; †: significant from within-group OAB value; *: significant from respective Vehicle group in phase [pairwise comparison using Students two-sided T-test]; R1 †, p = 0.0230, *, p = 0.0231; R2 †, p = 0.00938, *, p = 0.00334; R3 †, p = 0.00703, *, p = 0.00066; R4 †, p = 0.0103, *, p = 0.000103; R5 †, p = 0.0109, *, p = 0.00005; and R6 †, p = 0.0103, *, p = 0.000369). b) Monthly averages of subject water intake (ml/kg/day) shown by group (AAV2-hGDNF in orange; Vehicle in blue) from the last month of OAB and during alcohol reintroduction phases R1 through R6 (mean ± s.e.m.; n = 4/group; *: significant from respective Vehicle group in phase [pairwise comparison using Students two-sided T-test]; R2 *, p = 0.0303). c) The average daily caloric intake (kcal/kg/day) from ethanol (7 kcal/g) during the reintroduction phases for the Vehicle-treated (blue) and the AAV2-hGDNF-treated (orange) macaque groups (Mean ± SEM; n = 4/group; *: significant from respective Vehicle group in phase [pairwise comparison using Students two-sided T-test]; R1 *, p = 0.00968; R4 *, p = 0.00249; R5 *, p = 0.00806; and R6 *, p = 0.0182). d) The average group body weight in the final week of each phase in Phases A3-R6 for the Vehicle-treated and AAV2-hGDNF-treated groups. Linear regression lines represent the calculated rate-of-gain during this 8mo time period (0.100 kg/mo for Vehicle, 0.082 kg/mo for AAV2-hGDNF; (Mean ± SEM; n = 4/group). E-L) Panel Reversal Test in R3. Starting on day 12 of experimental phase R2, one GDNF-treated subject (panel 7) inexplicably developed an absolute side preference for the spout designated for alcohol access that was unrelated to any malfunction in apparatus. During the first week of phase R2 this subject consumed 0.94 g/kg/day of alcohol and exhibited an alcohol preference ratio (a measure of choosing alcohol versus water intake) of 0.13, but by the final week of phase R2 this drinking profile switched to 2.71 g/kg/day and a ratio of 0.98. To determine whether this shift in behavior was due to a side bias versus a change in motivation to consume alcohol, the spout designated for alcohol delivery for all subjects was reversed for the first 2 weeks of phase R3 and then returned to original position for the second 2 weeks of this alcohol reintroduction period (see ‘R’ in Fig. 1a). This evaluation clearly demonstrated that each subject, with the exception of the GDNF-treated subject on panel 7 (L), could track the location of the alcohol spout while maintaining a comparable preference ratio. Starting with phase R4, the alcohol spout for GDNF-treated subject on panel 7 was kept in the reversed position so that the subject had daily access to water and could still access ethanol for evaluation.
Extended Data Fig. 5 AAV2-hGDNF effects on 5HT concentrations and metabolism.
a) Concentrations of 5HT (VTA: **, p = 0.0359 vs. respective Vehicle-treated control group value via two-tailed Student’s T-test), and (b) its metabolite 5HIAA (SN: *, p = 0.0220; VTA: *, p = 0.0179 vs. respective Vehicle-treated control group value via two-tailed Student’s T-test) in ng/mg protein as determined by HPLC from tissue homogenates. c) Table of values detected for GDNF protein via ELISA (ng/mg total protein) and monoamine amounts via HPLC (ng/mg total protein). These values were used to generate the graphs shown in Fig. 5 and Extended Fig. 5. (Mean ± SEM; n = 4/group are shown; *: p ≤ 0.05 and **: p ≤ 0.001 vs. respective Vehicle-treated control group value. Specific statistical tests and p-values are as provided in Fig. 5 and above.).
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ford, M.M., George, B.E., Van Laar, V.S. et al. GDNF gene therapy for alcohol use disorder in male non-human primates. Nat Med 29, 2030–2040 (2023). https://doi.org/10.1038/s41591-023-02463-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41591-023-02463-9
This article is cited by
-
Rewinding reward
Nature Reviews Neuroscience (2023)