Abstract
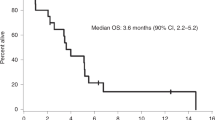
Brain metastases (BMs) are an emerging challenge in oncology due to increasing incidence and limited treatments. Here, we present results of a single-arm, open-label, phase 2 trial evaluating intracranial efficacy of pembrolizumab, a programmed cell death protein 1 inhibitor, in 9 patients with untreated BMs (cohort A) and 48 patients with recurrent and progressive BMs (cohort B) across different histologies. The primary endpoint was the proportion of patients achieving intracranial benefit, defined by complete response, partial response or stable disease. The primary endpoint was met with an intracranial benefit rate of 42.1% (90% confidence interval (CI): 31–54%). The median overall survival, a secondary endpoint, was 8.0 months (90% CI: 5.5–8.7 months) across both cohorts, 6.5 months (90% CI: 4.5–18.7 months) for cohort A and 8.1 months (90% CI: 5.3–9.6 months) for cohort B. Seven patients (12.3%), encompassing breast, melanoma and sarcoma histologies, had overall survival greater than 2 years. Thirty patients (52%; 90% CI: 41–64%) had one or more grade-3 or higher adverse events that were at least possibly treatment related. Two patients had grade-4 adverse events (cerebral edema) that were deemed at least possibly treatment related. These results suggest that programmed cell death protein 1 blockade may benefit a select group of patients with BMs, and support further studies to identify biomarkers and mechanisms of resistance. ClinicalTrials.gov identifier: NCT02886585
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The raw clinical and imaging data are protected due to patient privacy laws. Information is taken directly from the electronic medical record or original source generated by treating investigators (for example, email confirmations, AE logs). This is stored on a secured network drive to which only appropriately trained and delegated staff have access to. Lesion measurements are obtained from the Tumor Metrics Imaging Core online portal that uses a secure server to which only appropriately trained and delegated staff are granted access to. Any requests for raw and analyzed data should be sent in writing to P.B. and will be reviewed by the DF/HCC Institutional Review Board in an expeditious fashion (for example, approximately 6 months). Patient-related data not included in the paper were generated as part of a clinical trial and are subject to patient confidentiality. Any data and materials (for example, study protocol, clinical data or imaging data) that can be shared will require approval from the DF/HCC Institutional Review Board and a material transfer agreement. De-identified data then will be transferred to the inquiring investigator in an expeditious fashion over secure file transfer. The study protocol and statistical analysis plan are included with the submission.
References
Gradishar, W. J. et al. Clinical practice guidelines in oncology. J. Natl Compr. Cancer Netw. 16, 310–320 (2018).
Brastianos, P. K. et al. Genomic characterization of brain metastases reveals branched evolution and potential therapeutic targets. Cancer Discov. 5, 1164–1177 (2015).
Chen, G. et al. Molecular profiling of patient-matched brain and extracranial melanoma metastases implicates the PI3K pathway as a therapeutic target. Clin. Cancer Res. 20, 5537–5546 (2014).
Fischer, G. M. et al. Molecular profiling reveals unique immune and metabolic features of melanoma brain metastases. Cancer Discov. 9, 628–645 (2019).
Kim, N. et al. Single-cell RNA sequencing demonstrates the molecular and cellular reprogramming of metastatic lung adenocarcinoma. Nat. Commun. 11, 2285 (2020).
Tawbi, H. A. et al. Combined nivolumab and ipilimumab in melanoma metastatic to the brain. N. Engl. J. Med. 379, 722–730 (2018).
Goldberg, S. B. et al. Pembrolizumab for management of patients with NSCLC and brain metastases: long-term results and biomarker analysis from a non-randomised, open-label, phase 2 trial. Lancet Oncol. 21, 655–663 (2020).
Tawbi, H. A. et al. Long-term outcomes of patients with active melanoma brain metastases treated with combination nivolumab plus ipilimumab (CheckMate 204): final results of an open-label, multicentre, phase 2 study. Lancet Oncol. 22, 1692–1704 (2021).
Long, G. V. et al. Combination nivolumab and ipilimumab or nivolumab alone in melanoma brain metastases: a multicentre randomised phase 2 study. Lancet Oncol. 19, 672–681 (2018).
Sundermeyer, M. L., Meropol, N. J., Rogatko, A., Wang, H. & Cohen, S. J. Changing patterns of bone and brain metastases in patients with colorectal cancer. Clin. Colorectal Cancer 5, 108–113 (2005).
Powles, T. et al. Pembrolizumab plus axitinib versus sunitinib monotherapy as first-line treatment of advanced renal cell carcinoma (KEYNOTE-426): extended follow-up from a randomised, open-label, phase 3 trial. Lancet Oncol. 21, 1563–1573 (2020).
Brastianos, P. K. et al. Phase 2 study of pembrolizumab in patients with recurrent and residual high-grade meningiomas. Nat. Commun. 13, 1325 (2022).
Brastianos, P. K. et al. Single-arm, open-label phase 2 trial of pembrolizumab in patients with leptomeningeal carcinomatosis. Nat. Med. 26, 1280–1284 (2020).
Lin, N. U. et al. Response assessment criteria for brain metastases: proposal from the RANO group. Lancet Oncol. https://doi.org/10.1016/S1470-2045(15)70057-4 (2015).
Eisenhauer, E. A. et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur. J. Cancer https://doi.org/10.1016/j.ejca.2008.10.026 (2009).
Taggart, D. et al. Anti–PD-1/anti–CTLA-4 efficacy in melanoma brain metastases depends on extracranial disease and augmentation of CD8+T cell trafficking. Proc. Natl Acad. Sci. USA 115, E1540–E1549 (2018).
Thomas, D. L., Kranz, D. M. & Roy, E. J. Experimental manipulations of afferent immune responses influence efferent immune responses to brain tumors. Cancer Immunol. Immunother. 57, 1323–1333 (2008).
Sasaki, K. et al. Preferential expression of very late antigen-4 on type 1 CTL cells plays a critical role in trafficking into central nervous system tumors. Cancer Res. 67, 6451–6458 (2007).
Masson, F. et al. Brain microenvironment promotes the final functional maturation of tumor-specific effector CD8+T cells. J. Immunol. 179, 845–853 (2007).
Brastianos, P. K. et al. Palbociclib demonstrates intracranial activity in progressive brain metastases harboring cyclin-dependent kinase pathway alterations. Nat. Cancer 2, 498–502 (2021).
Brastianos, P. K., Curry, W. T. & Oh, K. S. Clinical discussion and review of the management of brain metastases. JNCCN J. Natl Compr. Canc. Netw. 11, 1153–1164 (2013).
Sperduto, P. W. et al. Summary report on the graded prognostic assessment: an accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J. Clin. Oncol. https://doi.org/10.1200/JCO.2011.38.0527 (2012).
Sperduto, P. W., Berkey, B., Gaspar, L. E., Mehta, M. & Curran, W. A new prognostic index and comparison to three other indices for patients with brain metastases: an analysis of 1,960 patients in the RTOG database. Int. J. Radiat. Oncol. Biol. Phys. https://doi.org/10.1016/j.ijrobp.2007.06.074 (2008).
Gaspar, L. et al. Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int. J. Radiat. Oncol. Biol. Phys. https://doi.org/10.1016/S0360-3016(96)00619-0 (1997).
Weber, J. et al. Adjuvant nivolumab versus ipilimumab in resected stage III or IV melanoma. N. Engl. J. Med. 377, 1824–1835 (2017).
Harrington, K. J. et al. Efficacy and safety of nivolumab plus ipilimumab vs nivolumab alone for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck: the phase 2 CheckMate 714 randomized clinical trial. JAMA Oncol. https://doi.org/10.1001/JAMAONCOL.2023.0147 (2023).
Wakelee, H. A. et al. IMpower010: primary results of a phase III global study of atezolizumab versus best supportive care after adjuvant chemotherapy in resected stage IB-IIIA non-small-cell lung cancer (NSCLC). J. Clin. Oncol. https://doi.org/10.1200/JCO.2021.39.15_suppl.8500 (2021).
Goldberg, S. B. et al. Pembrolizumab for patients with melanoma or non-small-cell lung cancer and untreated brain metastases: early analysis of a non-randomised, open-label, phase 2 trial. Lancet Oncol. 17, 976–983 (2016).
US Food and Drug Administration. Cancer clinical trial eligibility criteria: brain metastases. Guidance for industry. https://www.fda.gov/media/121317/download (2020).
Freedman, R. A. et al. TBCRC 022: a phase II trial of neratinib and capecitabine for patients with human epidermal growth factor receptor 2-positive breast cancer and brain metastases. J. Clin. Oncol. 37, 1081–1089 (2019).
Margolin, K. et al. Ipilimumab in patients with melanoma and brain metastases: an open-label, phase 2 trial. Lancet Oncol. 13, 459–465 (2012).
Lin, N. U. et al. Phase II trial of lapatinib for brain metastases in patients with human epidermal growth factor receptor 2-positive breast cancer. J. Clin. Oncol. 26, 1993–1999 (2008).
Lin, N. U. et al. Multicenter phase II study of lapatinib in patients with brain metastases from HER2-positive breast cancer. Clin. Cancer Res. 15, 1452–1459 (2009).
Acknowledgements
Funding for this trial was provided by Merck Sharp & Dohme, a subsidiary of Merck & Co. (to MGH), Damon Runyon Cancer Research Foundation (to P.K.B.), Ben and Catherine Ivy Foundation (to P.K.B.). P.K.B. also receives support from Breast Cancer Research Foundation, Hellenic Women’s Club Demetra Fund, Terry and Jean de Gunzburg MGH Research Scholar Fund and the National Institutes of Health (1R01CA227156-01 and 1R01CA244975-01). A.E.K. is supported by an American Brain Tumor Association Basic Research Fellowship In Honor of P. Fabbri, the William G. Kaelin, Jr., Physician-Scientist Award of the Damon Runyon Cancer Research Foundation, American Association for Cancer Research Breast Cancer Research Fellowship and the American Society of Clinical Oncology Young Investigator Award. E.R.G. is supported by the National Cancer Institute (R01CA211238 and R01CA244975). Most importantly, we thank the patients and their families for contributing to research efforts.
Author information
Authors and Affiliations
Contributions
P.K.B. and R.J.S. conceived the study and wrote the protocol with input from E.R.G., D.P.C., F.G.B. and A.G.-H. P.K.B., A.E.K., E.Q.L., N.U.L., B.O., P.Y.W., L.N., J.V.C., J.D., A.E., R.S.H., I.K., D.L., E.M., E.W., M.M., K.O., H.A.S., D.P.C., E.R.G., M.M. and R.J.S. supported the clinical trial, including recruitment and/or management of patients in the trial. A.G.-H. performed the statistical analysis. A.E.K., B.B., M.A.S., N.I., J.M.L., B.M., S.N., N.M., S.R. and E.J.S. helped collect data and samples. E.R.G. was the imaging chair of the study. A.E.K., P.K.B., E.R.G. and R.J.S. wrote the manuscript. All the authors interpreted the data, reviewed the manuscript and approved the final version.
Corresponding author
Ethics declarations
Competing interests
P.K.B. consulted for Tesaro, Angiochem, Genentech-Roche, ElevateBio, Eli Lilly, SK Life Sciences, Pfizer, Voyager Therapeutics, Sintetica, MPM, Advise Connect Inspire, Kazia and Dantari, received institutional research funding (to MGH) from Merck, Mirati, Eli Lilly, Kinnate, BMS and Pfizer and has received honoraria from Merck, Medscape, Pfizer, and Genentech-Roche. B.O. has received clinical trial support from Incyte and Eisai. J.D. has served as a consultant for Amgen, Blue Earth Diagnostics and Unum Therapeutics and received research support (to MGH) from Novartis and Eli Lilly. R.S.H. consulted for AbbVie, Daichii Sankyo, EMD Serono, Lilly, Novartis, Regeneron, and Sanofi; and received institutional research funding (to MGH) from Abbvie, Agios, Corvus, Daichii Sankyo, Erasca, Exelixis, Lilly, Mirati, Novartis and Turning Point. P.Y.W. received institutional research funding (to DF/HCC) from AstraZeneca/Medimmune, Beigene, Celgene, Chimerix, Eli Lilly, Erasca, Genentech/Roche, Kazia, MediciNova, Merck, Novartis, Nuvation Bio, Puma, Servier, Vascular Biogenics and VBI Vaccines, and has served on the scientific advisory board for AstraZeneca, Bayer, Black Diamond, Celularity, Chimerix, Day One Bio, Genenta, Mundipharma, Novartis, Novocure, Nuvation Bio, Prelude Therapeutics, Sapience, Servier, Sagimet, Vascular Biogenics and VBI Vaccines. H.A.S. has served on the scientific advisory board for Advanced Accelerator Applications, and received institutional research funding (to MGH) from AbbVie. R.J.S. consulted for Novartis, BMS and Pfizer, and received institutional research funding (to MGH) from Merck. The remaining authors declare no competing interests.
Peer review
Peer review information
Nature Medicine thanks Annette Molinaro, Carey Anders and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Saheli Sadanand, in collaboration with the Nature Medicine team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
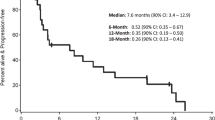
Extended Data Fig. 1 Kaplan-Meier Estimate for Intracranial Progression-free Survival, by Cohort.
The median intracranial PFS was 1.6 months for cohort A (blue line - 90% CI: 1.2-4.5 months) and 2.2 months for cohort B (red line - 90% CI: 1.4-3.1 months).
Extended Data Fig. 2 Kaplan-Meier Estimate for Extracranial Progression-Free Survival.
The median extracranial PFS was 4.5 months (90% CI: 2.7-8.0 months). Extracranial PFS was defined as the time of enrollment until the earlier of RECIST-defined disease progression or death. Patients who neither progressed nor died have follow-up that is censored at the date of last visit. CNS progression events are ignored. 53 of 57 patients (93%) experienced an extracranial PFS event. 18 patients experienced systemic progression and 35 additional died without systemic progression.
Extended Data Fig. 3 Kaplan-Meier Estimate for Extracranial Progression-Free Survival, by Cohort.
The median time to extracranial progression was 4.5 months (blue line - 90% CI: 1.2-6.7 months) for cohort A and 4.6 months (red line - 90% CI: 2.7-8.1 months) for cohort B.
Supplementary information
Supplementary Information
Clinical trial protocol.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Brastianos, P.K., Kim, A.E., Giobbie-Hurder, A. et al. Pembrolizumab in brain metastases of diverse histologies: phase 2 trial results. Nat Med 29, 1728–1737 (2023). https://doi.org/10.1038/s41591-023-02392-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41591-023-02392-7
This article is cited by
-
Predictive role of intracranial PD-L1 expression in a real-world cohort of NSCLC patients treated with immune checkpoint inhibition following brain metastasis resection
Journal of Neuro-Oncology (2024)
-
Neurotoxicity of Cancer Immunotherapies Including CAR T Cell Therapy
Current Neurology and Neuroscience Reports (2023)