Abstract
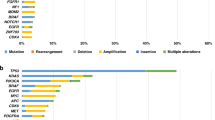
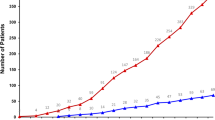
The NCI-MATCH (Molecular Analysis for Therapy Choice) trial (NCT02465060) was launched in 2015 as a genomically driven, signal-seeking precision medicine platform trial—largely for patients with treatment-refractory, malignant solid tumors. Having completed in 2023, it remains one of the largest tumor-agnostic, precision oncology trials undertaken to date. Nearly 6,000 patients underwent screening and molecular testing, with a total of 1,593 patients (inclusive of continued accrual from standard next-generation sequencing) being assigned to one of 38 substudies. Each substudy was a phase 2 trial of a therapy matched to a genomic alteration, with a primary endpoint of objective tumor response by RECIST criteria. In this Perspective, we summarize the outcomes of the initial 27 substudies in NCI-MATCH, which met its signal-seeking objective with 7/27 positive substudies (25.9%). We discuss key aspects of the design and operational conduct of the trial, highlighting important lessons for future precision medicine studies.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Jabbour, E. & Kantarjian, H. Chronic myelogenous leukemia: 2020 update on diagnosis, therapy and monitoring. Am. J. Hematol. 95, 691–709 (2020).
Waarts, M. R., Stonestrom, A. J., Park, Y. C. & Levine, R. L. Targeting mutations in cancer. J. Clin. Invest. 132, e154943 (2022).
Flaherty, K. T. et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N. Engl. J. Med. 363, 809–819 (2010).
Kopetz, S. et al. Phase II pilot study of vemurafenib in patients with metastatic BRAF-mutated colorectal cancer. J. Clin. Oncol. 33, 4032–4038 (2015).
Wheeler, D. A. et al. The complete genome of an individual by massively parallel DNA sequencing. Nature 452, 872–876 (2008).
Flaherty, K. T. et al. NCI-MATCH Team. The Molecular Analysis for Therapy Choice (NCI-MATCH) trial: lessons for genomic trial design. J. Natl. Cancer Inst. 112, 1021–1029 (2020).
Flaherty, K. T. et al. NCI-MATCH team. Molecular landscape and actionable alterations in a genomically guided gancer clinical trial: National Cancer Institute Molecular Analysis for Therapy Choice (NCI-MATCH). J. Clin. Oncol. 38, 3883–3894 (2020).
Rehm, H. L. et al. Working Group of the American College of Medical Genetics and Genomics Laboratory Quality Assurance Commitee ACMG clinical laboratory standards for next-generation sequencing. Genet Med. 15, 733–747 (2013).
Xuan, J., Yu, Y., Qing, T., Guo, L. & Shi, L. Next-generation sequencing in the clinic: promises and challenges. Cancer Lett. 340, 284–295 (2013).
Lih, C. J. et al. Analytical validation of the next-generation sequencing assay for a nationwide signal-finding clinical trial: Molecular Analysis for Therapy Choice Clinical Trial. J. Mol. Diagn. 19, 313–327 (2017).
Khoury, J. D. et al. Validation of immunohistochemical assays for integral biomarkers in the NCI-MATCH EAY131 clinical trial. Clin. Cancer Res. 24, 521–531 (2018).
Le Tourneau, C. et al. SHIVA investigators. Molecularly targeted therapy based on tumour molecular profiling versus conventional therapy for advanced cancer (SHIVA): a multicentre, open-label, proof-of-concept, randomised, controlled phase 2 trial. Lancet Oncol. 16, 1324–1334 (2015).
Massard, C. et al. High-throughput genomics and clinical outcome in hard-to-treat advanced cancers: results of the MOSCATO 01 trial. Cancer Discov. 7, 586–595 (2017).
Hyman, D. M. et al. HER kinase inhibition in patients with HER2- and HER3-mutant cancers. Nature 554, 189–194 (2018).
Schmoll, H. J. et al. MODUL-a multicenter randomized clinical trial of biomarker-driven maintenance therapy following first-line standard induction treatment of metastatic colorectal cancer: an adaptable signal-seeking approach. J. Cancer Res. Clin. Oncol. 144, 1197–1204 (2018).
Lee, J. et al. Tumor genomic profiling guides patients with metastatic gastric cancer to targeted treatment: The VIKTORY Umbrella Trial. Cancer Discov. 9, 1388–1405 (2019).
Salama, A. K. S. et al. Dabrafenib and trametinib in patients With tumors with BRAF V600E mutations: results of the NCI-MATCH trial subprotocol H. J. Clin. Oncol. 38, 3895–3904 (2020).
Azad, N. S. et al. Nivolumab is effective in mismatch repair-deficient noncolorectal cancers: results from arm Z1D-A subprotocol of the NCI-MATCH (EAY131) Study. J. Clin. Oncol. 38, 214–222 (2020).
Kalinsky, K. M. et al. Effect of capivasertib in patients with an AKT1 E17K-mutated tumor: NCI-MATCH subprotocol EAY131-Y nonrandomized trial. JAMA Oncol. 7, 271–278 (2020).
Kalinsky K. M. et al. Ipatasertib in patients with tumors with AKT mutations: results from the NCI-MATCH ECOG-ACRIN trial (EAY131) subprotocol Z1K. Presented at: 34th EORTC-NCI-AACR Symposium on Molecular Targets and Cancer Therapeutics, Barcelona, Spain; Abstract 11 (2021).
Damodaran, S. et al. Phase II study of copanlisib in patients with tumors with PIK3CA mutations: results from the NCI-MATCH ECOG-ACRIN trial (EAY131) subprotocol Z1F. J. Clin. Oncol. 40, 1552–1561 (2022).
Tuveson, D. A. et al. STI571 inactivation of the gastrointestinal stromal tumor c-KIT oncoprotein: biological and clinical implications. Oncogene 20, 5054–5058 (2001).
National Cancer Institute. NCI Dictionary of Cancer Terms. https://www.cancer.gov/publications/dictionaries/cancer-terms/def/rare-cancer/
Parsons, D. W. et al. NCI-COG Pediatric MATCH Team. Actionable tumor alterations and treatment protocol enrollment of pediatric and young adult patients with refractory cancers in the National Cancer Institute-Children’s Oncology Group Pediatric MATCH Trial. J. Clin. Oncol. 40, 2224–2234 (2022).
Chen, Y. & Chi, P. Basket trial of TRK inhibitors demonstrates efficacy in TRK fusion-positive cancers. J. Hematol. Oncol. 11, 78 (2018).
Gatta, G., RARECAREnet working group. et al. Burden and centralised treatment in Europe of rare tumours: results of RARECAREnet—a population-based study. Lancet Oncol. 18, 1022–1039 (2017).
Hoes, L. R. et al. Patients with rare cancers in the Drug Rediscovery Protocol (DRUP) benefit from genomics-guided treatment. Clin. Cancer Res. 28, 1402–1411 (2022).
Priestley, P. et al. Pan-cancer whole-genome analyses of metastatic solid tumours. Nature 575, 210–216 (2019).
Wheeler, D. A. et al. Molecular features of cancers exhibiting exceptional responses to treatment. Cancer Cell. 39, 38–53 (2021).
Adashek J. J. et al. Tissue agnostic activity of BRAF plus MEK inhibitor in BRAF V600 mutant tumors. Mol. Cancer Ther. https://doi.org/10.1158/1535-7163 (2022).
Howlader, N. et al. The effect of advances in lung-cancer treatment on population mortality. N. Engl. J. Med. 383, 640–649 (2020).
Kahlon, N. et al. Melanoma treatments and mortality rate trends in the US, 1975 to 2019. JAMA Netw. Open. 5, e2245269 (2022).
Long, G. V. et al. Adjuvant dabrafenib plus trametinib in stage III BRAF-mutated melanoma. N. Engl. J. Med. 377, 1813–1823 (2017).
Meric-Bernstam F, et al. National Cancer Institute Combination Therapy Platform Trial with Molecular Analysis for Therapy Choice (ComboMATCH). Clin. Cancer Res. https://doi.org/10.1158/1078-0432.CCR-22-3334 (2023).
Dentro, S. C. et al. PCAWG Evolution and Heterogeneity Working Group and the PCAWG Consortium. Characterizing genetic intra-tumor heterogeneity across 2,658 human cancer genomes. Cell 184, 2239–2254 (2021).
Hahn, W. C. et al. Cancer Target Discovery and Development Network. An expanded universe of cancer targets. Cell 184, 1142–1155 (2021).
Choueiri, T. K. et al.; CheckMate 9ER Investigators. Nivolumab plus cabozantinib versus sunitinib for advanced renal cell carcinoma. N. Engl. J. Med. 384, 829–841 (2021).
Tiacci, E. et al. Vemurafenib plus rituximab in refractory or relapsed hairy-cell leukemia. N. Engl. J. Med. 384, 1810–1823 (2021).
Mandelker, D. & Zhang, L. The emerging significance of secondary germline testing in cancer genomics. J. Pathol. 244, 610–615 (2018).
Reckamp, K. L. et al. Phase II trial of afatinib in patients with EGFR-mutated solid tumors excluding lung cancer: Results from the NCI-MATCH ECOG-ACRIN trial (EAY131) subprotocol A. Presented at: 34th EORTC-NCI-AACR Symposium on Molecular Targets and Cancer Therapeutics, Barcelona, Spain; Abstract 235 (2022).
Bedard, P. L. et al. Phase II study of Afatinib in patients with tumors with HER2-activating mutations: results from the NCI-MATCH ECOG-ACRIN trial (EAY131) subprotocol EAY131-B. JCO Precis. Oncol. 6, e2200165 (2022).
Mansfield, A. S. et al. Crizotinib in patients with tumors harboring ALK or ROS1 rearrangements: results from the NCI-MATCH trial (EAY131) subprotocols F and G. NPJ Precis. Oncol. 6, 13 (2022).
Krop, I. E. et al. Phase II study of taselisib in PIK3CA-mutated solid tumors other than breast and squamous lung cancer: results from the NCI-MATCH ECOG-ACRIN rial (EAY131) subprotocol I. JCO Precis. Oncol. 6, e2100424 (2022). PMC8865530.
Connolly, R. M. et al. Activity of trastuzumab and pertuzumab in patients with non-breast/gastroesophageal HER2 amplified tumors: results of the NCI-MATCH trial (EAY131) subprotocol. J. Ann. Oncol. 31, S479–S480 (2020).
Mita, A. C. et al. Erdafitinib in patients with tumors harboring FGFR gene mutations or fusions: results from the NCI-MATCH ECOG-ACRIN trial (EAY131) subprotocol K2. Mol. Cancer Ther. 20, Abstract LBA003 (2020).
Hays, J. L. et al. Results from the NCI-MATCH ECOG-ACRIN Trial (EAY131)—phase 2 study of MLN0128 (TAK-228) in patients with tumors with TSC1 or TSC2 mutations: subprotocol EAY131-M. Presented at: 34th EORTC-NCI-AACR Symposium on Molecular Targets and Cancer Therapeutics; Barcelona, Spain; Abstract 73 (2022)
Janku, F. et al. Phase II study of PI3K-beta inhibitor GSK2636771 in patients (pts) with cancers (ca) with PTEN mutation/deletion (mut/del) or PTEN protein loss. Ann Oncol. 29, Abstract 418PD (2018).
Jhaveri, K. et al. Ado-trastuzumab emtansine (T-DM1) in patients with HER2 amplified tumors excluding breast and gastric/gastro-esophageal junction (GEJ) adenocarcinomas: results from the NCI-MATCH Trial (EAY131) sub-protocol Q. Ann. Oncol. 30, 1821–1830 (2019).
Johnson, D. B. et al. Trametinib activity in patients with solid tumors and lymphomas harboring BRAF non-V600 mutations or fusions: results from NCI-MATCH (EAY131). Clin. Cancer Res. 26, 1812–1819 (2020).
Wisinski, K. B. et al. Trametinib in patients with NF1-, GNAQ- or GNA11-mutant tumors: results from the NCI-MATCH ECOG-ACRIN Trial (EAY131) subprotocols S1 and S2. Ann. Oncol. 7, e2200421 (2023).
Tsao, A. S. et al. Phase II study of vismodegib in patients with SMO- or PTCH1-mutated tumors: results from NCI-MATCH ECOG-ACRIN trial (EAY131) subprotocol T. J. Clin. Oncol. 40, Abstract 3010 (2022).
Jackman, D. M. et al. A phase 2 study of defactinib (VS-6063) in patients with NF2 altered tumors: results from NCI-MATCH (EAY131) subprotocol U. J. Clin. Oncol. 39, Abstract 3087 (2021).
Gien, L. T. et al. Phase II study of Sunitinib in tumors with c-KIT mutations: results from the NCI-MATCH ECOG-ACRIN trial (EAY131) subprotocol V. Presented at: 34th EORTC-NCI-AACR Symposium on Molecular Targets and Cancer Therapeutics; Barcelona, Spain; Abstract 238 (2022).
Chae, Y. et al. Phase II study of AZD4547 in patients with tumors harboring aberrations in the FGFR pathway: results from the NCI-MATCH trial (EAY131) subprotocol W. J. Clin. Oncol. 38, 2407–2417 (2020).
Cleary, J. M. et al. Differential outcomes in codon 12/13 and codon 61 NRAS-mutated cancers in the phase 2 NCI-MATCH trial of binimetinib in patients with NRAS-mutated tumors. Clin. Cancer Res. 27, 2996–3004 (2021).
Clark, A. S. et al. Molecular analysis for therapy choice (NCI-MATCH, EAY131) arm Z1B: phase II trial of palbociclib for CCND1, 2 or 3 amplified tumors. Cancer Res. 79, Abstract LB-010 (2020).
Janku, F. et al. Phase II study of PI3K inhibitor copanlisib in patients with cancers with deleterious PTEN sequencing results and retained PTEN protein expression: results from the NCI-MATCH Trial (EAY131) subprotocol Z1H. Ann. Oncol. 32, S595–S596 (2021).
Subbiah, V. et al. BVD-523FB (Ulixertinib) in patients with tumors with BRAF fusions, or with non-V600E, non-V600K BRAF mutations: results from the NCI-MATCH ECOG-ACRIN trial (EAY131) subprotocol EAY131-Z1L. Proc. Am. Assoc. Cancer Res. 82, Abstract CT160 (2022).
Eisenhauer, E. A. et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur. J. Cancer 45, 228–247 (2009).
Author information
Authors and Affiliations
Contributions
All authors made substantial contributions to conception and design, or acquisition of data, or analysis and interpretation of data; assisted in drafting the article and/or revising it critically for important intellectual content; and approved the final version to be published.
Corresponding author
Ethics declarations
Competing interests
P.J.O.’D. received research support from Pfizer, Genentech, BMS, AstraZeneca, GSK, Five Prime, FortySeven, Merck, Syndax, BBI, Novartis, Celgene, Incyte, Lilly/Imclone, array, h3biomedicine, Taiho, Minneamrata, pharmacyclics/abbvie and Mirati; and provided expert testimony for Dai-ichi Sankyo. K.T.F. has served on the board of directors of Loxo Oncology, Clovis Oncology, Strata Oncology, Checkmate Pharmaceuticals, Kinnate Pharmaceuticals and Scorpion Therapeutics; on the scientific advisory boards of PIC Therapeutics, Apricity, Oncoceutics, Fog Pharma, Tvardi, xCures, Monopteros, Vibliome, ALX Oncology, OMRx, Soley Therapeutics and Quanta Therapeutics; as a consultant to Lilly, Novartis, Genentech and Takeda; and received research funding from Novartis and Sanofi. A.J.I. holds stock options in, and has served on the scientific advisory boards of, PAIGE.AI, Kinnate Biopharma, Repare Therapeutics and SequreDx, and has received research support from Invitae. C.A. serves or has served as a scientific advisor to Novartis, Lilly, Merck, AstraZeneca, Daiichi Sankyo, Sanofi, TAIHO Oncology, PUMA Biotechnology, OrigiMed, Arvinas and the Komen Foundation; received grant support from Pfizer, Lilly and Takeda; and holds minor stock options in Provista Diagnostics. The remaining authors report no competing interests.
Peer review
Peer review information
Nature Medicine thanks Shumei Kato, Birgit Geoerger and Maud Kamal for their contribution to the peer review of this work. Primary Handling Editor: Karen O’Leary, in collaboration with the Nature Medicine team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
O’Dwyer, P.J., Gray, R.J., Flaherty, K.T. et al. The NCI-MATCH trial: lessons for precision oncology. Nat Med 29, 1349–1357 (2023). https://doi.org/10.1038/s41591-023-02379-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41591-023-02379-4
This article is cited by
-
The future of precision cancer therapy might be to try everything
Nature (2024)
-
New clinical trial design in precision medicine: discovery, development and direction
Signal Transduction and Targeted Therapy (2024)
-
Statistical classification of treatment responses in mouse clinical trials for stratified medicine in oncology drug discovery
Scientific Reports (2024)
-
Identification of potentially actionable genetic variants in epithelial ovarian cancer: a retrospective cohort study
npj Precision Oncology (2024)
-
Personalisierte Medizin in der Onkologie
Die Pathologie (2024)