Abstract
The emergence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants in the Omicron lineage has resulted in diminished Coronavirus Disease 2019 (COVID-19) vaccine efficacy and persistent transmission. In this study, we evaluated the immunogenicity and protective efficacy of two, recently authorized, bivalent COVID-19 vaccines that contain two mRNAs encoding Wuhan-1 and either BA.1 (mRNA-1273.214) or BA.4/5 (mRNA-1273.222) spike proteins. As a primary two-dose immunization series in mice, both bivalent vaccines induced greater neutralizing antibody responses against Omicron variants than the parental, monovalent mRNA-1273 vaccine. When administered to mice as a booster at 7 months after the primary vaccination series with mRNA-1273, the bivalent vaccines induced broadly neutralizing antibody responses. Whereas most anti-Omicron receptor binding domain antibodies in serum induced by mRNA-1273, mRNA-1273.214 and mRNA-1273.222 boosters cross-reacted with the antecedent Wuhan-1 spike antigen, the mRNA-1273.214 and mRNA-1273.222 bivalent vaccine boosters also induced unique BA.1-specific and BA.4/5-specific responses, respectively. Although boosting with parental or bivalent mRNA vaccines substantially improved protection against BA.5 compared to mice receiving two vaccine doses, the levels of infection, inflammation and pathology in the lung were lowest in animals administered the bivalent mRNA vaccines. Thus, boosting with bivalent Omicron-based mRNA-1273.214 or mRNA-1273.222 vaccines enhances immunogenicity and confers protection in mice against a currently circulating SARS-CoV-2 strain.
Similar content being viewed by others
Main
The SARS-CoV-2 pandemic has caused more than 600 million infections and 6.6 million deaths (https://covid19.who.int). In response to the global public health challenge, multiple companies rapidly developed vaccines that have been deployed in billions of people, resulting in reduced numbers of infections, hospitalizations and COVID-19-related deaths. The target antigen for most of these SARS-CoV-2 vaccines is the viral spike protein derived from historical strains that circulated in early 2020. However, the continuing evolution of SARS-CoV-2, resulting in amino acid changes in the spike protein amidst successive waves of infection, has jeopardized the global vaccination campaigns and the control of virus transmission1.
The SARS-CoV-2 spike protein binds to angiotensin-converting enzyme 2 (ACE2) on human cells to facilitate viral entry and infection2. The S1 fragment of the spike protein contains the receptor binding domain (RBD), which is the primary target of neutralizing antibodies elicited by vaccination or produced after natural infection3,4,5. In late 2021, the first Omicron variants (BA.1 and BA.1.1) emerged, with more than 30 amino acid substitutions, deletions or insertions in the spike protein. Since then, the Omicron lineage has continued to evolve (that is, BA.2, BA.4, BA.5, BA.2.75 and BA.4.6) with additional or different sets of spike mutations that facilitate escape from neutralizing antibodies6,7. These changes in the spike protein of Omicron strains are associated with symptomatic breakthrough infections in vaccinated and/or previously infected individuals8,9,10.
To overcome the loss in efficacy of authorized two-dose vaccines against Omicron strains, third and even fourth doses (herein called boosters) of vaccines encoding the historical (Wuhan-1) spike protein were recommended, and vaccines with Omicron variant-matched spikes were designed and tested. In humans, a booster dose of mRNA-1273 vaccine, encoding the historical spike protein, was associated with neutralizing antibody titers against BA.1 that were approximately 20-fold higher than after the second dose of vaccine11. In both mice and non-human primates, boosting with either mRNA-1273 or an Omicron BA.1-matched (mRNA-1273.529) vaccine increased neutralizing titers and protection against BA.1 infection compared to animals given a primary (two-dose) vaccination series of mRNA-1273 (refs. 12,13). Moreover, neutralizing antibody titers were higher, and BA.1 viral burden in the lung was lower, in mice boosted with mRNA-1273.529 compared to the mRNA-1273 vaccine, highlighting the potential clinical benefit of variant-specific boosters.
Bivalent vaccines are one strategy to increase protection against currently circulating variants as well as broaden neutralization to previous and potentially yet-to-emerge variants14,15. Bivalent vaccine boosters also may show greater induction of immune responses against earlier variants (which, in theory, could re-emerge) than monovalent variant-matched boosters. When administered as a booster dose, the bivalent vaccine mRNA-1273.211 encoding for the Wuhan-1 and Beta (B.1.351) spike proteins induced neutralizing antibody responses in humans against B.1.351, Delta (B.1.617.2) and Omicron (BA.1) that were greater than those achieved by boosting with the parental mRNA-1273 vaccine16,17. Similarly, in interim data from other human studies, boosting with a bivalent mRNA-1273.214 vaccine targeting the Wuhan-1 and BA.1 strains elicited higher neutralizing antibody responses against BA.1, BA.2 and BA.4/5 than the mRNA-1273 booster, with neutralization of BA.4 and BA.5 assessed together, as the spike proteins of these two sublineages are the same14,18. Despite a lack of published data on the efficacy of bivalent Omicron-matched vaccines or boosters against infection by Omicron variants in humans, bivalent mRNA vaccine boosters that include Wuhan-1 and either BA.1 or BA.4/5 components recently were authorized in Europe and the United States, in part due to the urgent need to broaden protection against circulating SARS-CoV-2 variants.
In this study, we evaluated in mice the antibody responses and protective activity against the prevailing circulating Omicron variant, BA.5, after a primary vaccination series or boosting with either of two Moderna bivalent vaccines, mRNA-1273.214 (containing 1:1 mix of mRNAs encoding Wuhan-1 and BA.1 spike proteins) and mRNA-1273.222 (1:1 mix of mRNAs encoding the Wuhan-1 and BA.4/5 spike proteins) and compared the results to monovalent vaccines that contain mRNAs encoding for a single spike antigen (Wuhan-1 (mRNA-1273), BA.1 (mRNA-1273.529) or BA.4/5 (mRNA1273.045)). In immunogenicity studies in mice, performed in the context of a primary vaccination series, robust anti-spike antibody responses were detected with all mRNA vaccines, as measured against Wuhan-1, BA.1 and BA.4/5 spike proteins. Both bivalent vaccines induced greater neutralizing antibody responses against Omicron variants than the parental mRNA-1273 vaccine. In immunogenicity studies in mice performed 7 months after a primary vaccination series with mRNA-1273, animals boosted with mRNA-1273.214 or mRNA-1273.222 had higher neutralization titers against authentic BA.1 and BA.5 viruses, as well as similar neutralization titers against Wuhan-1 and B.1.617.2 viruses, compared to animals boosted with mRNA-1273. The serum anti-RBD antibody response against BA.1 or BA.4/5 elicited by mRNA-1273, mRNA-1273.214 and mRNA-1273.222 boosters was shaped by immune memory of the Wuhan-1 spike developed from the primary vaccination series. However, for mRNA-1273.214 and mRNA-1273.222 boosters, which target BA.1 and BA.4/5, respectively, BA.1-specific and BA.4/5-specific antibody responses as well as Wuhan-1-specific antibody responses were apparent. Boosting was associated with increased protection 1 month later against challenge with BA.5, with the lowest levels of viral RNA, pro-inflammatory cytokines and pathology in the lung observed in mice administered mRNA-1273.214 or mRNA-1273.222 boosters. Thus, bivalent mRNA vaccine boosters that include mRNAs for Wuhan-1 and Omicron spike proteins induce immunity against historical and current SARS-CoV-2 variant strains, which is capable of protecting against infection with Omicron strain BA.5.
Results
Preclinical bivalent mRNA vaccines induce robust antibody responses
Immunization with bivalent vaccines that include components targeting an Omicron spike and the original Wuhan-1 spikes could broaden immunity against variants of SARS-CoV-2. To begin to address this question, we generated two lipid nanoparticle (LNP)-encapsulated mRNA vaccines (mRNA-1273.529 and mRNA-1273.045) encoding a proline-stabilized SARS-CoV-2 spike from BA.1 and BA.4/5 viruses, respectively. The mRNA-LNPs then were combined with mRNA-1273 in a 1:1 ratio to generate benchside mixed versions of mRNA-1273.214 and mRNA-1273.222. As a first test of their activity, we immunized BALB/c mice twice at a 3-week interval with 1 μg (total dose) of preclinical versions of mRNA-1273, mRNA-1273.529, mRNA-1273.045, mRNA-1273.214 or mRNA-1273.222 vaccines (Fig. 1a). Three weeks after the first dose (day 21) and 2 weeks after the second dose (day 35), we analyzed serum for binding to Wuhan-1, BA.1 and BA.4/5 spike proteins by ELISA (Fig. 1b).
Six-to-eight-week-old female BALB/c mice were immunized twice over a 3-week interval with PBS or 1 μg total dose of preclinical versions of mRNA-1273 (Wuhan-1 spike), mRNA-1273.529 (BA.1 spike), mRNA-1273.045 (BA.4/5 spike), mRNA-1273.214 (benchside 1:1 mixture of mRNA-1273 + mRNA-1273.529) or mRNA-1273.222 (benchside 1:1 mixture of mRNA-1273 + mRNA-1273.045). Immediately before (day 21) or 2 weeks after (day 35) the second vaccine dose, serum was collected. a, Scheme of immunization and blood draws. b, Serum antibody binding to Wuhan-1, BA.1 or BA.4/5 spike proteins by ELISA at day 21 and day 35 (n = 8 mice per group, one experiment; tops of boxes show GMTs, which are indicated above each column and dotted lines show LOD). c, Neutralizing activity of serum at day 35 against VSV pseudoviruses displaying the spike proteins of Wuhan-1 D614G, BA.1, BA.2.75 or BA.4/5 (n = 8 mice per group, one experiment; tops of boxes show GMTs, which are indicated above each column and dotted lines show LOD). d, Neutralizing activity of serum at day 35 against pseudotyped lentiviruses displaying the spike proteins of Wuhan-1, BA.1 or BA.4/5 (n = 8 mice per group, one experiment; tops of boxes show GMTs, which are indicated above each column, and dotted lines show LOD). Statistical analysis. b, One-way ANOVA with Tukey’s multiple comparison post test; comparisons are between all groups except for the PBS control, which is shown for reference. c,d, One-way Kruskal–Wallis ANOVA with Dunn’s multiple comparison post test; comparisons are between all groups. Exact P values are indicated, and only significant comparisons are shown. Primary data are provided as a Source Data file.
Robust serum IgG binding was observed against Wuhan-1, BA.1 and BA.4/5 spike proteins after a two-dose primary series with monovalent mRNA-1273, mRNA-1273.529 and mRNA-1273.045 vaccines as well as bivalent mRNA-1273.214 and mRNA-1273.222 vaccines, compared to immunizing with PBS only. On day 21, geometric mean titers (GMTs) against Wuhan-1 spike ranged from 907 to 3,229 and increased by approximately 23-fold to 52-fold on day 35, with values ranging from 27,001 to 111,514 across the vaccine groups (Fig. 1b). On day 35, mice vaccinated with mRNA-1273, mRNA-1273.214 or mRNA-1273.222 achieved higher GMTs than mice vaccinated with mRNA-1273.529 or mRNA-1273.045. Serum binding GMTs against BA.1 spike at day 21 ranged from 147 to 527 and increased by approximately 61-fold to 90-fold on day 35, with values ranging from 9,372 to 34,494 across the vaccine groups (Fig. 1b). There were equivalent binding titers against BA.1 spike across most groups on day 35, although serum from mice vaccinated with mRNA-1273.045 showed reduced binding compared to other vaccinated groups. At day 21, serum IgG-binding GMTs against BA.5 spike ranged from 1,545 to 3,421 and increased by approximately 17-fold to 52-fold on day 35, with values ranging from 45,495 to 83,142 (Fig. 1b). On day 35, robust IgG GMTs against BA.5 spike were observed for all mRNA vaccinated groups with no substantive differences noted.
To test the inhibitory activity of serum antibodies obtained at day 35 from BALB/c mice that received two doses of the different preclinical mRNA vaccines, we used a vesicular stomatitis virus (VSV)-based neutralization assay19 with pseudoviruses displaying spike proteins of Wuhan-1 D614G, BA.1, BA.2.75 or BA.4/5 (Fig. 1c and Extended Data Fig. 1). The mRNA-1273 vaccine showed a strong inhibitory response against Wuhan-1 D614G (GMT: 16,997) but induced lower levels of neutralizing antibody against BA.1 (GMT: 1,024), BA.2.75 (GMT: 232) or BA.4/5 (GMT:157). Mice vaccinated with mRNA-1273.045 or mRNA-1273.222 vaccines showed greater neutralizing responses (GMT: 19,036 and 13,804, respectively) against BA.4/5 pseudoviruses than mRNA-1273 vaccinated mice. Serum from mRNA-1273.214 and mRNA-1273.529 vaccinated animals also effectively inhibited infection of BA.1 pseudoviruses. Finally, the bivalent mRNA-1273.214 and mRNA-1273.222 vaccines induced greater serum neutralizing activity against Wuhan-1 D614G (GMT: 8,444 and 3,035, respectively) than the Omicron-matched monovalent mRNA-1273.529 (GMT: 254) and mRNA-1273.045 (GMT: 143) vaccines. All of the Omicron-matched vaccines induced slightly greater (3.5-fold to 6.4-fold) neutralizing responses than mRNA-1273 against BA.2.75, although these differences did not attain statistical significance.
We also measured serum neutralizing antibody capacity using a lentivirus-based pseudovirus assay with virions displaying Wuhan-1, BA.1 or BA.4/5 spike proteins (Fig. 1d and Extended Data Fig. 2). On day 35, serum from animals immunized with mRNA-1273 vaccine efficiently neutralized Wuhan-1, but the responses against BA.1 and BA.4/5 were substantially lower. Mice immunized with the mRNA-1273.045 or mRNA-1273.222 vaccines had robust responses against BA.4/5, although the titers against Wuhan-1 and BA.1 were lower. The mRNA-1273.529 and mRNA-1273.214 vaccines induced neutralizing antibody responses against BA.1 but had less activity against Wuhan-1 and BA.4/5. Overall, based on data from the VSV and lentivirus pseudovirus assays, both bivalent mRNA-1273.222 and mRNA-1273.214 vaccines induced stronger neutralizing antibody responses against Omicron variants than the mRNA-1273 vaccine and generally retained activity against pseudoviruses with Wuhan-1 spike proteins.
Clinically representative bivalent mRNA vaccines induce robust neutralizing antibody responses
Using larger cohorts of mice (n = 16) to gain increased statistical power, we evaluated the immunogenicity of clinically representative versions of mRNA-1273.214 and mRNA-1273.222, where two monovalent mRNAs were separately formulated into LNPs and then mixed in a 1:1 ratio in the vial, a process that is representative of the commercial drug product (Methods). These versions were compared to responses obtained with similarly prepared versions of mRNA-1273. BALB/c mice were immunized twice at a 3-week interval with 1 μg (total dose) of mRNA-1273, mRNA-1273.214 or mRNA-1273.222 vaccines (Fig. 2a). Two weeks after the second dose (day 35), serum was collected and analyzed for neutralizing activity using VSV-based pseudoviruses displaying Wuhan-1 D614G, BA.1, BA.2.75 or BA.4/5 spike proteins (Fig. 2b and Extended Data Fig. 3). Whereas mRNA-1273 induced a robust neutralizing antibody response against Wuhan-1 D614G (GMT: 28,920), 30-fold to 194-fold less activity was measured against pseudoviruses displaying BA.1, BA.2.75 and BA.4/5. The neutralizing activity seen with the bivalent mRNA-1273.214 vaccine was better than with mRNA-1273, with the greatest responses against the matched BA.1 (GMT: 13,183) and 1.7-fold to 52-fold reductions against Wuhan-1 D614G, BA.2.75 and BA.4/5, with the lowest potency against BA.4/5 (GMT: 293). The mRNA-1273.222 vaccine achieved the broadest inhibitory activity with the highest neutralizing titers against the matched BA.4/5 (GMT: 15,561) and only 1.7-fold to 6.7-fold reductions in activity against Wuhan-1 D614G, BA.1 and BA.2.75.
Six-to-eight-week-old female BALB/c mice were immunized twice over a 3-week interval with PBS or 1 μg total dose of clinically representative versions of mRNA-1273, mRNA-1273.214 (1:1 mixture in the vial of separately formulated mRNA-1273 and mRNA-1273.529) or mRNA-1273.222 (1:1 mixture in the vial of separately formulated mRNA-1273 and mRNA-1273.045). Two weeks after (day 35) the second vaccine dose, serum was collected. a, Scheme of immunization and blood draws. b, Neutralizing activity of serum at day 35 against VSV pseudoviruses displaying the spike proteins of Wuhan-1 D614G, BA.1, BA.2.75 or BA.4/5 (n = 16 mice per group, one experiment; tops of boxes show GMTs, which are indicated above each column, and dotted lines show LOD). c, Neutralizing activity of serum at day 35 against pseudotyped lentiviruses displaying the spike proteins of Wuhan-1 DG14G, BA.1 or BA.4/5 (n = 15 for mRNA-1273 and mRNA-1273.214 and n = 16 for mRNA-1273.222, one experiment; tops of boxes shows GMTs, which are indicated above each column, and dotted lines show LOD). Statistical analysis. b,c, One-way Kruskal–Wallis ANOVA with Dunn’s multiple comparison post test; comparisons are between all groups. Exact P values are indicated, and only significant comparisons are shown. Primary data are provided as a Source Data file.
We also evaluated serum neutralizing antibody activity using a lentivirus-based pseudovirus assay with virions displaying Wuhan-1 D614G, BA.1 or BA.4/5 spike proteins (Fig. 2c and Extended Data Fig. 4). Serum from animals immunized with mRNA-1273 vaccine efficiently neutralized Wuhan-1 pseudoviruses, but the responses against BA.1 and BA.4/5 were decreased. The mRNA-1273.214 vaccine induced greater neutralizing antibody responses against BA.1 compared to mRNA-1273 and mRNA-1273.222 vaccinated mice but elicited less inhibitory activity against Wuhan-1 and BA.4/5. Mice immunized with mRNA-1273.222 vaccine had higher responses against BA.4/5 compared to mRNA-1273 or mRNA-1273.214 vaccinated mice, although the responses against Wuhan-1 and BA.1 were lower. In aggregate, both clinically representative bivalent mRNA vaccines offered greater neutralization of Omicron variants.
Boosting with bivalent mRNA vaccines enhances antibody responses against Omicron variants
We next evaluated the bivalent vaccines as booster injections. We took advantage of an existing cohort of female K18-hACE2 transgenic C57BL/6 mice that had received two 0.25-μg doses of mRNA-1273 or control mRNA vaccine over a 3-week interval and were rested for 31 weeks (Fig. 3a). The 0.25-μg dose of mRNA vaccine was used because the B and T cell responses generated in C57BL/6 mice with this dose approximate those observed in humans receiving 100-μg doses12,20. Blood was collected (pre-boost sample), and groups of animals were boosted with either PBS (sham control) or 0.25 μg of control mRNA, mRNA-1273, mRNA-1273.214 or mRNA-1273.222 vaccines. Four weeks later, a post-boost blood sample was collected (Fig. 3a), and the neutralizing activity of pre-boost and post-boost serum antibodies was determined using authentic SARS-CoV-2 viruses. At 31 weeks after completion of the primary mRNA-1273 vaccination series, pre-boost neutralizing antibody levels against WA1/2020 D614G (GMT: 454) and B.1.617.2 (GMT: 277) were above the expected threshold (~1/60) of protection21 (Fig. 3b and Extended Data Figs. 5 and 6). However, these samples showed reduced or no neutralizing activity (GMT: 63) against BA.1 or BA.5 at the lowest dilution tested (Fig. 3b and Extended Data Figs. 5 and 6), consistent with reductions reported in serum from humans immunized with mRNA vaccines targeting ancestral SARS-CoV-2 strains6,7.
Seven-week-old female K18-hACE2 mice were immunized with 0.25 μg of control mRNA or mRNA-1273 vaccine and then boosted 31 weeks later with PBS, 0.25 μg of control mRNA or 0.25 μg of clinically representative versions of mRNA-1273, mRNA-1273.214 or mRNA-1273.222 vaccines. a, Scheme of immunizations, blood draws and virus challenge. b,c, Serum neutralizing antibody responses immediately before (b, pre-boost) and 4 weeks after (c, post-boost) receiving the indicated mRNA boosters or PBS as judged by focus reduction neutralization test (FRNT) with authentic WA1/2020 D614G, B.1.617.2, BA.1 and BA.5 viruses (n = 9 for mRNA-1273 and n = 10 for other groups, two experiments; tops of boxes show GMT values, which are indicated above each column, and dotted lines show LOD based on a 1/60 (WA1/2020 D614G and B.1.617.2) or 1/30 serum dilution (BA.1 and BA.5)). d, Scheme of serum depletion of anti-RBD antibodies. e, Serum (1/150 dilution) from mice boosted with mRNA-1273, mRNA-1273.214 or mRNA-1273.222 was incubated with empty, Wuhan-1 RBD-loaded or BA.4/5 RBD-loaded magnetic beads. After separation of pre-clearing beads, supernatants were diluted serially and added to ELISA plates coated with Wuhan-1 or BA.4/5 RBD. Representative depletion curves performed in technical duplicate corresponding to individual mice are shown for the indicated vaccines. f, Bar graphs derived from AUC analysis of the data in e showing the relative proportions of Wuhan-1-specific, Wuhan-1/BA.4/5 cross-reactive and BA.4/5-specific RBD responses (n = 9 for mRNA-1273 and n = 10 for mRNA-1273.214 and mRNA1273.222, two experiments; right edge of boxes illustrate mean values, and error bars indicate standard deviations). Statistical analysis. c, One-way Kruskal–Wallis ANOVA with Dunn’s multiple comparisons post test; comparisons are between all groups except for the PBS control, which is shown for reference. Exact P values are indicated, and only significant comparisons are shown. Primary data are provided as a Source Data file. Abs, antibodies.
Four weeks after boosting with mRNA-1273, mRNA-1273.214 or mRNA-1273.222, neutralizing titers against WA1/2020 D614G and B.1.617.2 were approximately 4.2-fold to 6.0-fold and 4.8-fold to 5.5-fold higher, respectively, than before boosting (Fig. 3c and Extended Data Figs. 5–7). Boosting with mRNA-1273.214 or mRNA-1273.222 resulted in increased neutralizing titers against BA.1 (5.5-fold to 10.3-fold) and BA.5 (5.6-fold to 8.9-fold), respectively (Fig. 3c and Extended Data Figs. 5–7), whereas mRNA-1273 boosted titers by a lesser degree (2.4-fold to 2.6-fold). Thus, although all three boosters increased neutralizing titers against WA1/2020 D614G and B.1.617.2 compared to the two-dose mRNA-1273 series, only the Omicron-matched bivalent boosters substantially augmented serum neutralizing activity against the Omicron variants.
To determine whether the response to Omicron variants after boosting with mRNA-1273.214 or mRNA-1273.222 was due to antibodies that were specifically induced by the Omicron spike or imprinted by the mRNA-1273 primary immunization series, we designed an RBD-based antibody depletion assay (Methods). We focused on anti-RBD antibodies, because most potently neutralizing antibodies bind this region on both ancestral and variant SARS-CoV-2 spike proteins4. Serum harvested from K18-hACE2 mice 1 month after boosting with mRNA-1273, mRNA-1273.214 or mRNA-1273.222 was pre-cleared with Wuhan-1 or BA.4/5 RBD protein and then tested for binding to Wuhan-1 or BA.4/5 RBD by ELISA (Fig. 3d). As expected, Wuhan-1 RBD beads pre-cleared RBD-specific antibodies from the serum of mice immunized and boosted with mRNA-1273, and residual binding was not observed to either Wuhan-1 or BA.4/5 RBD by ELISA (Fig. 3e). However, when the serum of mice immunized and boosted with mRNA-1273 was pre-cleared with BA.4/5 RBD beads, a large fraction (79%) of the response still bound to Wuhan-1 RBD, indicating that most anti-RBD antibodies do not cross-react with BA.4/5 (Fig. 3f). For the serum from mRNA-1273.214 or mRNA-1273.222 boosted mice, pre-clearing with BA.4/5 RBD beads abrogated the binding to BA.4/5 RBD and left substantial (68% and 73%, respectively) residual binding to Wuhan-1 RBD (Fig. 3e,f). Although the pre-clearing of serum from mRNA-1273.214 boosted mice with Wuhan-1 RBD beads abrogated binding to BA.4/5 RBD, approximately 37% of the total anti-BA.4/5 RBD response in serum from mRNA-1273.222 boosted mice was not pre-cleared by Wuhan-1 RBD beads (Fig. 3f). Analogous results showing type-specific responses were observed from serum of mRNA-1273.214 boosted mice after pre-clearing with Wuhan-1 RBD and binding to BA.1 RBD (Extended Data Fig. 8). These results suggest that the anti-RBD antibody response in serum generated after boosting is shaped both by immune memory against Wuhan-1 spike and by variant-specific responses against the matched spike protein. Pre-existing memory B cell clones likely are reactivated to proliferate, differentiate and secrete antibodies, and, for mRNA-1273.214 and mRNA-1273.222, additional BA.1-specific or BA.4/5-specific memory B cell clones are formed.
Boosting with bivalent mRNA vaccines protects against BA.5 infection
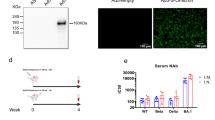
One or two days after the post-boost bleed, K18-hACE2 transgenic mice were challenged with BA.5 administered by an intranasal route (Fig. 3a), and, at 4 days post-infection (dpi), viral RNA levels were measured in the nasal wash, nasal turbinate and lung (Fig. 4a). Although Omicron strains are less pathogenic in mice22,23, viral replication occurs, allowing for evaluation of vaccine protection. In the upper respiratory tract (nasal wash and nasal turbinate), mice that had received a primary two-dose immunization series and were boosted with mRNA-1273, mRNA-1273-214 or mRNA-1273.222 vaccines showed reduced levels of BA.5 viral RNA at 4 dpi compared to animals administered three doses of the control mRNA vaccine (Fig. 4a). Although not reaching statistical significance, mice immunized with two doses of mRNA-1273 and boosted with PBS sustained levels of viral RNA in tissues that were intermediate between the control mRNA vaccine series and mice boosted with any of the spike-targeted vaccines, suggesting that a primary mRNA-1273 vaccination series provides some limited protection against infection by BA.5. In contrast, boosting with mRNA-1273, mRNA-1273-214 or mRNA-1273.222 vaccines conferred greater protection against BA.5 infection in the lung with 1,374-fold to 28,436-fold reductions in viral RNA (Fig. 4a). Moreover, boosting with the bivalent vaccines resulted in lower (7-fold to 21-fold) viral RNA levels in the lungs than boosting with the mRNA-1273 vaccine. Analysis of infectious virus in the lung at 4 dpi using plaque assays showed substantial reductions in viral burden in animals boosted with mRNA-1273, mRNA-1273-214 or mRNA-1273.222 vaccines compared to those receiving a control vaccine or immunized with two doses of mRNA-1273 and boosted with PBS (Fig. 4b).
Seven-week-old female K18-hACE2 mice were immunized with 0.25 μg of control mRNA or mRNA-1273; boosted 31 weeks later with PBS, 0.25 μg of control mRNA or 0.25 μg of clinically representative versions of mRNA-1273, mRNA-1273.214 or mRNA-1273.222 vaccines; and then, 1 month later, challenged via intranasal route with 104 FFU of BA.5. a, Viral RNA levels at 4 dpi in the nasal washes, nasal turbinates and lungs. b, Infectious viral load at 4 dpi in the lungs after BA.5 challenge of vaccinated and boosted mice as determined by plaque assay (a,b: n = 8 for mRNA-1273.214, n = 9 for control mRNA and mRNA-1273 and n = 10 for PBS and mRNA-1273.222, two experiments; tops of boxes illustrate mean values, and dotted lines show LOD based on tissue weight or volume). c, Cytokine and chemokine levels in lung homogenates at 4 dpi. Data are expressed as fold change relative to naive mice, and log2 values are plotted (n = 8 for mRNA-1273.214, n = 9 for control mRNA and mRNA-1273, n = 10 for PBS and mRNA-1273.222 and n = 4 for naive, two experiments; lines illustrate median values, and dotted lines indicate LOD for each respective analyte based on a standard curve). Measurements are also shown in Supplementary Table 1. d, Hematoxylin and eosin staining of lung sections harvested at 4 dpi from mice immunized with control mRNA (CTRL-mRNA) or mRNA-1273 (primary two dose series) and then boosted with PBS, mRNA-1273, mRNA-1273.214 or mRNA-1273.222 vaccines. An uninfected (naive) animal is shown for comparison. Low (top: scale bars, 1 mm), moderate (middle: scale bars, 200 µm) and high (bottom: scale bars, 50 µm) power images are shown. Representative images of multiple lung sections from n = 2 for each group, two experiments. Statistical analysis. a–c, One-way Kruskal–Wallis ANOVA with Dunn’s multiple comparisons post test; comparisons are between all groups. Exact P values are indicated, and only significant comparisons are shown. Primary data are provided as a Source Data file.
As another gauge of vaccine-induced protection, we measured cytokine and chemokine levels in the lung of the BA.5-challenged K18-hACE2 mice at 4 dpi using a multiplexed assay. Mice immunized with the control vaccine or those receiving a primary vaccination series with mRNA-1273 and a booster dose of PBS showed higher levels of many inflammatory cytokines and chemokines in lung homogenates than unvaccinated, unchallenged (naive) animals (Fig. 4c and Supplementary Table 1). In comparison, the lungs of BA.5-challenged mice that were boosted with mRNA-1273, mRNA-1273-214 or mRNA-1273.222 vaccines had substantially lower or undetectable levels of most pro-inflammatory cytokines and chemokines that we measured. For several cytokines and chemokines (for example, IFN-γ, IL-6, CXCL9 and CXCL10), lower levels were observed after boosting with mRNA-1273-214 and mRNA-1273.222, but not mRNA-1273, compared to boosting with PBS. Thus, and consistent with the virological data, protection against BA.5-induced lung inflammation was increased by boosting with mRNA vaccines, with a modest, albeit not statistically significant, improvement after administration of bivalent vaccines compared to the parental monovalent mRNA vaccine.
We also performed histological analysis of lung tissues as an additional measure of vaccine protection. Lung sections obtained at 4 dpi from BA.5-challenged mice immunized with the control vaccine or those receiving a primary mRNA-1273 vaccination series with a booster of PBS showed patchy, bridging immune cell infiltration, airway space thickening and alveolar congestion (Fig. 4d). The pathology seen after BA.5 infection of K18-hACE2 mice, however, was less than with historical or other variant SARS-CoV-2 strains24,25, as reported for other Omicron strains22,26,27. BA.5-challenged mice that had been boosted with mRNA-1273 had more limited, focal immune cell infiltration in airspaces, whereas those boosted with mRNA-1273.214 or mRNA-1273.222 vaccines showed virtually no lung pathology, similarly to uninfected control mice. Overall, these findings are consistent with the viral burden and cytokine and chemokine data and demonstrate protection against SARS-CoV-2 infection and lung injury after boosting with bivalent mRNA vaccines.
Discussion
Vaccine-induced immunity against SARS-CoV-2 has reduced human disease and curtailed the COVID-19 pandemic. However, the emergence of SARS-CoV-2 variants with constellations of amino acid changes in regions of the spike protein that bind neutralizing antibodies jeopardizes immunity derived from vaccines designed against the historical Wuhan-1 SARS-CoV-2 strain. The objective of this study was to evaluate in mice the activity of preclinical and clinically representative Omicron-matched (BA.1 or BA.4/5) bivalent mRNA vaccines when administered as a primary vaccination series or booster dose and to assess the breadth of neutralizing antibody responses and ability to protect against one prevalent, currently circulating Omicron variant. We first compared the immunogenicity of the bivalent and constituent monovalent mRNA vaccines in mice in the context of a two-dose primary immunization series. Although bivalent mRNA vaccines are conceived principally as boosters because most of the global population has been previously infected or vaccinated, the analysis after a primary immunization series provides insight into the potential antibody breadth. Robust serum antibody binding responses were detected against Wuhan-1, BA.1 and BA.5 spike proteins by all vaccines, although the mRNA-1273.529 and mRNA-1273.045 vaccines had lower titers against non-matched spike antigens. Serum generated from the bivalent mRNA-1273.222 and mRNA-1273.214 vaccines potently neutralized infection of both Omicron (BA.1 and BA.4/5) pseudoviruses, as well as those displaying the historical Wuhan-1 D614G spike, demonstrating the greatest neutralization breadth. These results are consistent with studies showing that monovalent vaccines that match the spike protein generate greater inhibitory responses against specific variants relative to historical viruses12,28,29. In the context of boosting, the bivalent BA.1-matched and BA.4/5-matched vaccines induced serum antibody responses that effectively neutralized WA1/2020 D614G, B.1.617.2, BA.1 and BA.5 authentic viruses. These results are consistent with human serum data obtained after immunization with bivalent mRNA vaccines targeting B.1.351 (refs. 16,17) or BA.1 (refs. 14,18). Increases in neutralizing antibody breadth with bivalent vaccine formulations or boosters also have been reported in the context of inactivated30,31 and spike-protein-based32,33,34 SARS-CoV-2 vaccine candidates.
Seven months after completion of a primary mRNA-1273 vaccination series, K18-hACE2 mice were boosted with PBS, mRNA-1273, mRNA-1273.214 or mRNA-1273.222 and then, 1 month later, challenged with BA.5. Both bivalent mRNA-1273.214 and mRNA-1273.222 vaccine boosters induced robust neutralizing antibody responses against BA.1 and BA.5, and these correlated with slightly increased protection against infection, inflammation and pathology in the lung after intranasal challenge with BA.5 compared to the parental mRNA-1273 boost. In contrast, animals that received a primary two-dose mRNA-1273 series, and were boosted with PBS, showed marginal protection against lung infection. These results showing benefit of matched bivalent vaccine boosters targeting Omicron variants are consistent with studies in mice immunized with mRNA-1273, boosted with a monovalent mRNA-1273.529 vaccine and challenged with BA.1 (ref. 12), and with predictive models in humans35.
Notwithstanding the immunogenicity and protection generated by the bivalent vaccines, boosting with the parental mRNA-1273 vaccine alone conferred protection against infection (119-fold reduction in viral RNA levels and 142-fold reduction in infectious virus compared to boosting with PBS) and reduced both inflammation and pathology in the lung against BA.5, despite inducing rather limited levels of serum neutralizing antibodies against this variant. These findings are consistent with studies in non-human primates13 and could reflect effects of neutralizing antibodies below the limit of detection (LOD) of our assay (<1/30); non-neutralizing, cross-reactive antibodies against BA.5 that promote clearance through Fc effector function activities36,37; cross-reactive T cell responses38,39; or anamnestic B cell responses that rapidly generate cross-reactive neutralizing antibodies.
A key question in the field is whether booster vaccines that target specific emerging SARS-CoV-2 strains induce de novo B cell responses in addition to imprinted, cross-reactive antibodies40. Studies in monkeys and humans so far have suggested that booster responses against SARS-CoV-2 principally are shaped by the recall of memory B cells elicited by the priming vaccine or previous infection11,13,41, although longitudinal analysis has not yet been performed. Our serum depletion experiments with Wuhan-1, BA.1 or BA.4/5 RBD-loaded beads are consistent with the idea that the antibody response generated shortly after boosting is shaped by memory against the Wuhan-1 spike developed in response to the primary vaccination series and favors use of pre-existing B cell clones42. Most anti-BA.1 and anti-BA.4/5 RBD antibodies in serum generated after boosting with mRNA-1273.214 and mRNA-1273.222, respectively, cross-reacted with the original Wuhan-1 RBD protein. Although this response can be maladaptive, as described for some sequences of influenza virus vaccination43, the imprinted response that we observed after mRNA-1273.214 boosting resulted in more cross-neutralization of BA.5 than after mRNA-1273 boosting. Possibly, the BA.1-matched vaccine component (mRNA-1273.529) of the bivalent mRNA-1273.214 booster expands cross-reactive B cell clones with antigen specificities that bind and neutralize BA.5 better than those recalled after the mRNA-1273 booster. For both mRNA-1273.214 and mRNA-1273.222 boosters, both BA.1 and BA.4/5 RBD-specific and cross-reactive responses, respectively, were detected using depletion studies with Wuhan-1 RBD. Thus, boosting with variant-matched bivalent vaccines may expand both cross-reactive and type-specific clones, and, from each population, neutralizing antibodies likely are produced against the variant spike protein.
Study limitations
We note several limitations in our study. (1) Female BALB/c and K18-hACE2 mice were used to allow for group caging. Follow-up experiments with male mice and larger cohorts are needed to extend these results and detect significant differences between the mRNA-1273.214 and mRNA-1273.222 boosters. (2) We challenged K18-hACE2 mice with a BA.5 isolate. Although BA.5 is currently the dominant circulating strain (reaching up to ~68% in the United States (https://covid.cdc.gov/covid-data-tracker/#variant-proportions) for the week ending 15 October 2022), future infection experiments using BA.2.75, BA.2.75.2, BA.4.6, BQ.1, BQ.1.1, BF.7 or other emerging strains may be informative to determine the breadth of protection. (3) Our analysis did not account for non-neutralizing antibody or cross-reactive T cell responses, both of which could impact protective immunity. (4) Experiments were performed in mice to allow for rapid testing and multiple comparison groups. Vaccination, boosting and BA.5 challenge in other animal models and in humans is required for corroboration. (5) We did not have sufficient group sizes available to conduct head-to-head efficacy analysis against BA.5 challenge after boosting with monovalent (mRNA-1273.529 and mRNA-1273.045) and bivalent (mRNA-1273.214 and mRNA-1273.222) mRNA vaccines.
In summary, our data showing that both bivalent vaccine boosters elicited neutralizing activity against BA.5 as well as reduced viral loads, inflammation and pathology in the lungs of BA.5-infected mice supports the decision for roll-out of BA.1-based or BA.4/5-based bivalent boosters in Europe and the United States. Nonetheless, as boosting with the parental mRNA-1273 also conferred protection compared to boosting with PBS (that is, effectively non-boosted), vaccines that target the historical SARS-CoV-2 spike may have utility as boosters in regions where roll-out of the bivalent boosters is delayed.
Methods
Cells
African green monkey Vero-TMPRSS2 (ref. 44) and Vero-hACE2-TMPRRS2 (ref. 45) cells were cultured at 37 °C in DMEM supplemented with 10% FBS, 10 mM HEPES pH 7.3, 1 mM sodium pyruvate, 1× non-essential amino acids and 100 U ml−1 of penicillin–streptomycin. Vero-TMPRSS2 cells were supplemented with 5 µg ml−1 of blasticidin. Vero-hACE2-TMPRSS2 cells were supplemented with 10 µg ml−1 of puromycin. BHK-21/WI-2 cells and HEK293T/17 cells were obtained from the American Type Culture Collection (ATCC) and cultured in DMEM supplemented with 10% FBS. All cells routinely tested negative for mycoplasma using a polymerase chain reaction (PCR)-based assay.
Viruses
The WA1/2020 D614G and B.1.617.2 strains were described previously20,46. The BA.1 isolate (hCoV-19/USA/WI-WSLH-221686/2021) was obtained from an individual in Wisconsin as a mid-turbinate nasal swab22. The BA.5 isolate was isolated in California (hCoV-19/USA/CA-Stanford-79_S31/2022) and was a gift from M. Suthar (Emory University). All viruses were passaged once on Vero-TMPRSS2 cells and subjected to next-generation sequencing45 to confirm the introduction and stability of substitutions. All virus experiments were performed in an approved biosafety level 3 (BSL-3) facility at Washington University School of Medicine.
Mice
Animal studies were carried out in accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. For studies (K18-hACE2 mice) at Washington University School of Medicine, the protocols were approved by the Institutional Animal Care and Use Committee at Washington University School of Medicine (assurance no. A3381–01). Virus inoculations were performed under anesthesia that was induced and maintained with ketamine hydrochloride and xylazine, and all efforts were made to minimize animal suffering. For studies with BALB/c mice, animal experiments were carried out in compliance with approval from the Animal Care and Use Committee of Moderna, Inc. Sample size for animal experiments was determined on the basis of criteria set by the institutional Animal Care and Use Committee. Experiments were neither randomized nor blinded.
Heterozygous K18-hACE2 C57BL/6J mice (strain: 2B6.Cg-Tg(K18-ACE2)2Prlmn/J, cat. no. 34860) were obtained from The Jackson Laboratory. BALB/c mice (strain: BALB/cAnNCrl, cat. no. 028) were obtained from Charles River Laboratories. Animals were housed in groups of 4–5, fed standard chow diets, subjected to a photoperiod of 12 hours on, 12 hours off dark/light cycle and kept at an ambient animal room temperature of 70° ± 2° F with a room humidity of 50% ± 5%.
mRNA vaccine and LNP production process
A sequence-optimized mRNA encoding prefusion-stabilized Wuhan-Hu-1 (mRNA-1273), BA.1 (mRNA-1273.529, the Omicron gene component in mRNA-1273.214) and BA.5 (mRNA-1273.045, the Omicron gene component in mRNA-1273.222) was designed. The genes of SARS-CoV-2 S2P proteins were synthesized in vitro using an optimized T7 RNA polymerase-mediated transcription reaction with complete replacement of uridine by N1m-pseudouridine47. In addition to the two-proline substitution, the BA.1 spike gene in the mRNA-1273.529 and mRNA-1273.214 vaccines encoded the following substitutions: A67V, Δ69–70, T95I, G142D, Δ143–145, Δ211, L212I, ins214EPE, G339D, S371L, S373P, S375F, K417N, N440K, G446S, S477N, T478K, E484A, Q493R, G496S, Q498R, N501Y, Y505H, T547K, D614G, H655Y, N679K, P681H, N764K, D796Y, N856K, Q954H, N969K and L981F. The BA.5 gene in mRNA-1273.045 and mRNA-1273.222 vaccines encoded the following substitutions: T19I, Δ24–26, A27S, Δ69–70, G142D, V213G, G339D, S371F, S373P, S375F, T376A, D405N, R408S, K417N, N440K, L452R, S477N, T478K, E484A, F486V, Q498R, N501Y, Y505H, D614G, H655Y, N679K, P681H, N764K, D796Y, Q954H and N969K.
A non-translating control mRNA was synthesized and formulated into LNPs as previously described48. The reaction included a DNA template containing the immunogen open reading frame flanked by 5′ untranslated region (UTR) and 3′ UTR sequences and was terminated by an encoded polyA tail. After RNA transcription, the cap-1 structure was added using the vaccinia virus capping enzyme and 2′-O-methyltransferase (New England Biolabs). The mRNA was purified by oligo-dT affinity purification, buffer-exchanged by tangential flow filtration into sodium acetate, pH 5.0, sterile filtered and kept frozen at –20 °C until further use.
The mRNA was encapsulated in an LNP through a modified ethanol-drop nanoprecipitation process described previously49. Ionizable, structural, helper and polyethylene glycol lipids were briefly mixed with mRNA in an acetate buffer, pH 5.0, at a ratio of 2.5:1 (lipid:mRNA). The mixture was neutralized with Tris-HCl, pH 7.5; sucrose was added as a cryoprotectant; and the final solution was sterile-filtered. Vials were filled with formulated LNP and stored frozen at –20 °C until further use. The vaccine product underwent analytical characterization, which included the determination of particle size and polydispersity, encapsulation, mRNA purity, double-stranded RNA content, osmolality, pH, endotoxin and bioburden, and the material was deemed acceptable for in vivo study. The preclinical materials used in this study were: (1) monovalent mRNA-1273 vaccine that contains a single mRNA encoding the SARS-CoV-2 S2P antigen; (2) monovalent mRNA-1273.529 vaccine that contains a single mRNA encoding the SARS-CoV-2 S2P antigen for BA.1; (3) monovalent mRNA-1273.045 vaccine that contains a single mRNA encoding the SARS-CoV-2 S2P antigen of the BA.4/BA.5 subvariants of Omicron; (4) research-grade bivalent mRNA-1273.214 vaccine, which is a 1:1 benchside mix of separately formulated mRNA-1273 and mRNA-1273.529 vaccines; (5) research-grade bivalent mRNA-1273.222 vaccine, which is a 1:1 benchside mix of separately formulated mRNA-1273 and mRNA-1273.045 vaccines; (6) clinically representative bivalent mRNA-1273.214 vaccine, which is a 1:1 mix in the vial of separately formulated mRNA-1273 and mRNA-1273.529; and (7) clinically representative bivalent mRNA-1273.222 vaccine, which is a 1:1 mix in the vial of separately formulated mRNA-1273 and mRNA-1273.045. All mRNAs are formulated into a mixture of four lipids: SM-102, cholesterol, DSPC and PEG2000-DMG.
Clinically representative material is made in the process development laboratory using a proportionally scaled-down version of the commercial manufacturing process, whereas the research-grade materials are prepared in an automated high-throughput, small-scale process. The main difference between the two, beyond scale, is when the vaccine components are mixed and the sourcing of raw materials. In the case of research grade, the materials are separately prepared and vialed and then mixed 1:1 immediately before injection. For the clinically representative material, each mRNA is separately formulated into LNPs and then mixed, before vialing so that both mRNA/LNPs are present in the vial. The preclinical process involves encapsulating capped, full-length mRNA in LNP, although the exact series of unit operations and set points have been adapted to maximize batch success. Thus, there are batch-to-batch differences in the research-grade material. Example Certificate of Analyses for both types of materials can be supplied upon request. All vaccines were prepared with the same method as the Good Manufacturing Practice for clinical vaccines. Recombinant soluble S and RBD proteins from Wuhan-1, BA.1 and BA.4/5 SARS-CoV-2 strains were expressed as described50,51.
Recombinant spike and RBD proteins
Recombinant proteins were produced in Expi293F cells (Thermo Fisher Scientific) by transfection of DNA using the ExpiFectamine 293 Transfection Kit (Thermo Fisher Scientific). Supernatants were harvested at 3 dpi, and recombinant proteins were purified using Ni-NTA agarose (Thermo Fisher Scientific) and then buffer exchanged into PBS and concentrated using Amicon Ultracel centrifugal filters (EMD Millipore). SARS-CoV-2 B.1.617.2 RBD protein was purchased from Sino Biological (40592-V08H90).
ELISA
Assays were performed in 96-well microtiter plates (Thermo Fisher Scientific) coated with 100 μl of recombinant Wuhan-1, BA.1 or BA.4/BA.5 spike proteins. Plates were incubated at 4 °C overnight and then blocked for 1 hour at 37 °C using SuperBlock (Thermo Fisher Scientific, 37516) and then washed four times with PBS 0.05% + Tween 20 (PBST). Serum samples were serially diluted in 5% BSA in TBS (Boston BioProducts, IBB-187), added to plates, incubated for 1 hour at 37 °C and then washed four times with PBST. Goat anti-mouse IgG-HRP (SouthernBiotech, 1030-05) was diluted in 5% BSA in TBS before adding to the wells and incubating for 1 hour at 37 °C. Plates were washed four times with PBST before the addition of TMB substrate (Thermo Fisher Scientific, 34029). Reactions were stopped by the addition of TMB stop solution (Invitrogen, SS04). The optical density (OD) measurements were taken at 450 nm, and titers were determined using a four-parameter logistic curve fit in GraphPad Prism version 9.
Focus reduction neutralization test with authentic SARS-CoV-2 strains
Serial dilutions of sera were incubated with 102 focus-forming units (FFU) of WA1/2020 D614G, B.1.617.2, BA.1 or BA.5 for 1 hour at 37 °C. Antibody–virus complexes were added to Vero-TMPRSS2 cell monolayers in 96-well plates and incubated at 37 °C for 1 hour. Subsequently, cells were overlaid with 1% (w/v) methylcellulose in MEM. Plates were harvested 30 hours (WA1/2020 D614G and B.1.617.2) or 70 hours (BA.1 and BA.5) later by removing overlays and fixed with 4% paraformaldehyde (PFA) in PBS for 20 minutes at room temperature. Plates were washed and sequentially incubated with a pool (SARS2–02, −08, −09, −10, −11, −13, −14, −17, −20, −26, −27, −28, −31, −38, −41, −42, −44, −49, −57, −62, −64, −65, −67 and −71 (ref. 52)) of anti-S murine antibodies (including cross-reactive monoclonal antibodies to SARS-CoV) and HRP-conjugated goat anti-mouse IgG (Sigma-Aldrich, A8924, RRID: AB_258426) in PBS supplemented with 0.1% saponin and 0.1% BSA. SARS-CoV-2-infected cell foci were visualized using TrueBlue Peroxidase Substrate (KPL) and quantitated on an ImmunoSpot microanalyzer (Cellular Technologies).
VSV pseudovirus neutralization assay
Codon-optimized full-length spike genes (Wuhan-1 with D614G, BA.2.75, BA.1 and BA.5) were cloned into a pCAGGS vector. Spike genes contained the following mutations: (a) BA.2.75: T19I, Δ24–26, A27S, G142D, K147E, W152R, F157L, I210V, V213G, G257S, G339H, S371F, S373P, S375F, T376A, D405N, R408S, K417N, N440K, G446S, N460K, S477N, T478K, E484A, Q498R, N501Y, Y505H, D614G, H655Y, N679K, P681H, N764K, D796Y, Q954H and N969K; (b) BA.1: A67V, Δ69–70, T95I, G142D/ΔVYY143–145, ΔN211/L212I, ins214EPE, G339D, S371L, S373P, S375F, K417N, N440K, G446S, S477N, T478K, E484A, Q493R, G496S, Q498R, N501Y, Y505H, T547K, D614G, H655Y, N679K, P681H, N764K, D796Y, N856K, Q954H, N969K and L981F; and (c) BA.4/5: T19I, Δ24–26, A27S, Δ69–70, G142D, V213G, G339D, S371F, S373P, S375F, T376A, D405N, R408S, K417N, N440K, L452R, S477N, T478K, E484A, F486V, Q498R, N501Y, Y505H, D614G, H655Y, N679K, P681H, N764K, D796Y, Q954H and N969K. To generate VSVΔG-based SARS-CoV-2 pseudovirus, BHK-21/WI-2 cells were transfected with the spike expression plasmid and infected by VSVΔG-firefly-luciferase as previously described53. Vero E6 cells were used as target cells for the neutralization assay and maintained in DMEM supplemented with 10% FBS. To perform neutralization assay, mouse serum samples were heat-inactivated for 45 minutes at 56 °C, and serial dilutions were made in DMEM supplemented with 10% FBS. The diluted serum samples or culture medium (serving as virus-only control) were mixed with VSVΔG-based SARS-CoV-2 pseudovirus and incubated at 37 °C for 45 minutes. The inoculum virus or virus–serum mix was subsequently used to infect Vero E6 cells (ATCC, CRL-1586) for 18 hours at 37 °C. At 18 hours after infection, an equal volume of One-Glo reagent (Promega, E6120) was added to culture medium for readout using a BMG PHERastar-FSX plate reader. The percentage of neutralization was calculated based on relative light units (RLUs) of the virus control and subsequently analyzed using four-parameter logistic curve (GraphPad Prism 8.0).
Lentivirus-based pseudovirus neutralization assay
Neutralization of SARS-CoV-2 also was measured in a single-round-of-infection assay with lentivirus-based pseudovirus assay as previously described54. To produce SARS-CoV-2 pseudoviruses, an expression plasmid bearing codon-optimized SARS-CoV-2 full-length spike plasmid was co-transfected into HEK293T/17 cells (ATCC, CRL-11268) with packaging plasmid pCMVDR8.2, luciferase reporter plasmid pHR′CMV-Luc and a TMPRSS2 plasmid. Mutant spike plasmids were produced by GenScript. Pseudoviruses were mixed with eight serial four-fold dilutions of sera or antibodies in duplicate and then added to monolayers of 293T-hACE2 cells in duplicate. Three days after infection, cells were lysed; luciferase was activated with the Luciferase Assay System (Promega); and RLUs were measured at 570 nm on a SpectraMax L luminometer (Molecular Devices). After subtraction of background RLU (uninfected cells), % neutralization was calculated as 100 × ((virus-only control) − (virus + antibody)) / (virus-only control). Dose–response curves were generated with a five-parameter non-linear function, and titers were reported as the serum dilution or antibody concentration required to achieve ID50 neutralization. The input dilution of serum was 1:50; thus, 20 was the lower LOD. Samples that did not neutralize at the LOD at 50% were plotted at 25, and that value was used for geometric mean calculations. Each assay included duplicates. In addition, the reported values were the geometric mean of two independent assays.
Anti-RBD depletion assay
Hexahistidine-tagged Wuhan-1, BA.1 and BA.4/5 RBD proteins were produced recombinantly in Escherichia coli and purified as described previously12. After buffer-exchange into PBS or TBS, RBD proteins (15 μg) or no protein (negative control) were incubated with 0.5 mg of Ni-NTA Magnetic Beads (HisPur, 88832) overnight at 4 °C. After unbound proteins were washed away with 50 mM Tris (pH 8.0) supplemented with 200 mM NaCl, 50 mM imidazole and 0.05% Tween 20, beads were mixed with diluted serum (1/150 in 50 mM Tris (pH 8.0) supplemented with 200 mM NaCl, 30 mM imidazole and 0.05% Tween 20) obtained from K18-hACE2 mice 1 month after boosting with mRNA-1273, mRNA-1273.214 or mRNA-1273.222 vaccines and incubated with agitation at room temperature for 4 hours. Subsequently, beads were removed with magnetic separation on a KingFisher Flex robot (Thermo Fisher Scientific), and serial dilutions of the supernatant (in 2% BSA in PBST) were added to 96-well microtiter plates coated with Wuhan-1 RBD, BA.1 or BA.4/5 RBD (0.1–0.3 μg per well) for 1 hour at 37 °C. After washing four times with PBST, goat anti-mouse IgG-HRP (Sigma-Aldrich, A5278) was diluted in 2% BSA in PBST before adding to the wells and incubating for 1 hour at 37 °C. Plates were washed four times with PBST before the addition of TMB substrate. Reactions were stopped by the addition of TMB stop solution. The OD measurements were taken at 450 nm, and area under the curve (AUC) analysis was performed using GraphPad Prism 9.4.1.
Mouse experiments
K18hACE2 transgenic mice
Seven-week-old female K18-hACE2 mice were immunized 3 weeks apart with 0.25 µg of mRNA vaccines (control or mRNA-1273) in 50 µl of PBS via intramuscular injection in the hind leg. Animals were bled 31 weeks after the second vaccine dose for immunogenicity analysis and then boosted with PBS (no vaccine) or 0.25 µg of mRNA-1273, mRNA-1273.214 or mRNA-1273.222 vaccines. Four weeks later, K18-hACE2 mice were challenged with 104 FFU of BA.5 by the intranasal route. Animals were euthanized at 4 dpi, and tissues were harvested for virological analyses.
BALB/c mice
Six-to-eight-week-old female BALB/c mice were immunized 3 weeks apart with 1 µg of mRNA vaccines (mRNA-1273, mRNA-1273.529, mRNA-1273.045, mRNA-1273.214 or mRNA-1273.222) or PBS (in 50 µl) via intramuscular injection in the quadriceps muscle of the hind leg under isoflurane anesthesia. Blood was sampled 3 weeks after the first immunization and 2 weeks after the second immunization, and anti-spike and neutralizing antibody levels were measured by ELISA and VSV-based or lentivirus-based pseudovirus neutralization assays.
Measurement of viral burden
Tissues were weighed and homogenized with zirconia beads in a MagNA Lyser instrument (Roche Life Science) in 1 ml of DMEM medium supplemented with 2% heat-inactivated FBS. Tissue homogenates were clarified by centrifugation at 10,000g for 5 minutes and stored at −80 °C.
Viral RNA measurement
RNA was extracted using the MagMAX mirVana Total RNA Isolation Kit (Thermo Fisher Scientific) on the KingFisher Flex robot (Thermo Fisher Scientific). RNA was reverse transcribed and amplified using the TaqMan RNA-to-CT 1-Step Kit (Thermo Fisher Scientific). Reverse transcription was carried out at 48 °C for 15 minutes, followed by 2 minutes at 95 °C. Amplification was accomplished over 50 cycles as follows: 95 °C for 15 seconds and 60 °C for 1 minute. Copies of SARS-CoV-2 N gene RNA in samples were determined using a published assay55. The following primers and probe series were used: SARS-CoV-2 N Forward: 5′-ATGCTGCAATCGTGCTACAA-3′; SARS-CoV-2 N Reverse: 5′-GACTGCCGCCTCTGCTC-3′; and SARS-CoV-2 N Probe: 5′-/56-FAM/TCAAGGAAC/ZEN/AACATTGCCAA/3IABkFQ/−3′.
Viral plaque assay
Vero-TMPRSS2-hACE2 cells were seeded at a density of 1 × 105 cells per well in 24-well tissue culture plates. The next day, medium was removed and replaced with 200 μl of clarified lung homogenate that was diluted serially in DMEM supplemented with 2% FBS. One hour later, 1 ml of methylcellulose overlay was added. Plates were incubated for 96 hours and then fixed with 4% PFA (final concentration) in PBS for 20 minutes. Plates were stained with 0.05% (w/v) crystal violet in 20% methanol and washed twice with distilled, deionized water.
Cytokine and chemokine protein measurements
Lung homogenates were incubated with Triton X-100 (1% final concentration) for 1 hour at room temperature to inactivate SARS-CoV-2. Homogenates were analyzed for cytokines and chemokines by Eve Technologies Corporation using their Mouse Cytokine Array/Chemokine Array 31-Plex (MD31) platform.
Lung histology
Lungs of euthanized mice were inflated with ∼2 ml of 10% neutral buffered formalin using a 3-ml syringe and catheter inserted into the trachea and kept in fixative for 7 days. Tissues were embedded in paraffin, and sections were stained with hematoxylin and eosin. Images were captured using the NanoZoomer (Hamamatsu) at the Alafi Neuroimaging Core at Washington University.
Materials availability
All requests for resources and reagents should be directed to the corresponding authors. This includes viruses, vaccines and primer-probe sets. All reagents will be made available upon reasonable request after completion of a materials transfer agreement (MTA). All mRNA vaccines can be obtained under an MTA with Moderna (contact: Darin Edwards, darin.edwards@modernatx.com).
Statistical analysis
No statistical methods were used to predetermine sample sizes, but our sample sizes are similar to those reported in previous publications12,20. Significance was assigned when P values were less than 0.05 using GraphPad Prism version 9.3. Tests, number of animals, mean or median values and statistical comparison groups are indicated in the figure legends. Changes in infectious virus titer, viral RNA levels or serum antibody responses were compared to mRNA-control immunized and/or PBS-boosted animals and were analyzed by one-way Kruskal–Wallis or one-way ANOVA with multiple comparisons tests or two-tailed Wilcoxon signed-rank test depending on the type of results, number of comparisons and distribution of the data. No animals or data points were excluded from the analyses.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All data supporting the findings of this study are available within the paper, its Extended Data or Source Data files. Any additional information related to the study also is available from the corresponding author upon reasonable request. Source data are provided with this paper.
Code availability
No code was used in the course of the data acquisition or analysis.
References
Krause, P. R. et al. SARS-CoV-2 variants and vaccines. N. Engl. J. Med. 385, 179–186 (2021).
Letko, M., Marzi, A. & Munster, V. Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nat. Microbiol. 5, 562–569 (2020).
Rathe, J. A. et al. SARS-CoV-2 serologic assays in control and unknown populations demonstrate the necessity of virus neutralization testing. J. Infect. Dis. 223, 1120–1131 (2020).
Errico, J. M., Adams, L. J. & Fremont, D. H. Antibody-mediated immunity to SARS-CoV-2 spike. Adv. Immunol. 154, 1–69 (2022).
Qi, H., Liu, B., Wang, X. & Zhang, L. The humoral response and antibodies against SARS-CoV-2 infection. Nat. Immunol. 23, 1008–1020 (2022).
Wang, Q. et al. Antibody evasion by SARS-CoV-2 Omicron subvariants BA.2.12.1, BA.4 and BA.5. Nature 608, 603–608 (2022).
Iketani, S. et al. Antibody evasion properties of SARS-CoV-2 Omicron sublineages. Nature 604, 553–556 (2022).
Andrews, N. et al. Covid-19 vaccine effectiveness against the Omicron (B.1.1.529) variant. N. Engl. J. Med. 386, 1532–1546 (2022).
Tseng, H. F. et al. Effectiveness of mRNA-1273 against SARS-CoV-2 Omicron and Delta variants. Nat. Med. 28, 1063–1071 (2022).
Elliott, P. et al. Rapid increase in Omicron infections in England during December 2021: REACT-1 study. Science 375, 1406–1411 (2022).
Pajon, R. et al. SARS-CoV-2 Omicron variant neutralization after mRNA-1273 booster vaccination. N. Engl. J. Med. 386, 1088–1091 (2022).
Ying, B. et al. Boosting with variant-matched or historical mRNA vaccines protects against Omicron infection in mice. Cell 185, 1572–1587 (2022).
Gagne, M. et al. mRNA-1273 or mRNA-Omicron boost in vaccinated macaques elicits similar B cell expansion, neutralizing responses, and protection from Omicron. Cell 185, 1556–1571 (2022).
Chalkias, S. et al. A bivalent Omicron-containing booster vaccine against Covid-19. N Engl. J. Med. 387, 1279–1291 (2022).
Caserta, L. C. et al. White-tailed deer (Odocoileus virginianus) may serve as a wildlife reservoir for nearly extinct SARS-CoV-2 variants of concern. Preprint at https://www.biorxiv.org/content/10.1101/2022.09.02.506368v1 (2022).
Choi, A. et al. Safety and immunogenicity of SARS-CoV-2 variant mRNA vaccine boosters in healthy adults: an interim analysis. Nat. Med. 27, 2025–2031 (2021).
Chalkias, S. et al. Safety, immunogenicity and antibody persistence of a bivalent Beta-containing booster vaccine against COVID-19: a phase 2/3 trial. Nat. Med. 28, 2388–2397 (2022).
Branche, A. R. et al. SARS-CoV-2 variant vaccine boosters trial: preliminary analyses. Preprint at https://www.medrxiv.org/content/10.1101/2022.07.12.22277336v1 (2022).
Case, J. B. et al. Neutralizing antibody and soluble ACE2 inhibition of a replication-competent VSV-SARS-CoV-2 and a clinical isolate of SARS-CoV-2. Cell Host Microbe 28, 475–485 (2020).
Ying, B. et al. Protective activity of mRNA vaccines against ancestral and variant SARS-CoV-2 strains. Sci. Transl. Med. 14, eabm3302 (2021).
Khoury, D. S. et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat. Med. 27, 1205–1211 (2021).
Halfmann, P. J. et al. SARS-CoV-2 Omicron virus causes attenuated disease in mice and hamsters. Nature 603, 687–692 (2022).
Uraki, R. et al. Characterization and antiviral susceptibility of SARS-CoV-2 Omicron BA.2. Nature 607, 119–127 (2022).
Winkler, E. S. et al. SARS-CoV-2 infection of human ACE2-transgenic mice causes severe lung inflammation and impaired function. Nat. Immunol. 21, 1327–1335 (2020).
Chen, R. E. et al. In vivo monoclonal antibody efficacy against SARS-CoV-2 variant strains. Nature 596, 103–108 (2021).
Bentley, E. G. et al. SARS-CoV-2 Omicron-B.1.1.529 variant leads to less severe disease than Pango B and Delta variants strains in a mouse model of severe COVID-19. Preprint at https://www.biorxiv.org/content/10.1101/2021.12.26.474085v2 (2021).
Shuai, H. et al. Attenuated replication and pathogenicity of SARS-CoV-2 B.1.1.529 Omicron. Nature 603, 693–699 (2022).
Lee, I.-J. et al. Omicron-specific mRNA vaccine induced potent neutralizing antibody against Omicron but not other SARS-CoV-2 variants. Preprint at https://www.biorxiv.org/content/10.1101/2022.01.31.478406v1 (2022).
Rössler, A., Knabl, L., Laer, D.v. & Kimpel, J. Reduced sensitivity of antibody tests after omicron infection. Lancet Microbe S2666-5247(22)00222-1 (2022).
Zhang, Z. et al. A heterologous V-01 or variant-matched bivalent V-01D-351 booster following primary series of inactivated vaccine enhances the neutralizing capacity against SARS-CoV-2 Delta and Omicron strains. J. Clin. Med. 11, 4164 (2022).
Deng, W. et al. Sequential immunizations confer cross-protection against variants of SARS-CoV-2, including Omicron in Rhesus macaques. Signal Transduct. Target. Ther. 7, 124 (2022).
Ding, C. et al. Evaluation of humoral immune responses induced by different SARS-CoV-2 spike trimers from wild-type and emerging variants with individual, sequential, and combinational delivered strategies. J. Med. Virol. 94, 5841–5849 (2022).
Du, P. et al. A bivalent vaccine containing D614G and BA.1 spike trimer proteins or a BA.1 spike trimer protein booster shows broad neutralizing immunity. J. Med Virol. 94, 4287–4293 (2022).
Pavot, V. et al. Protein-based SARS-CoV-2 spike vaccine booster increases cross-neutralization against SARS-CoV-2 variants of concern in non-human primates. Nat. Commun. 13, 1699 (2022).
Khoury, D. S. et al. Predicting the efficacy of variant-modified COVID-19 vaccine boosters. Preprint at https://www.medrxiv.org/content/10.1101/2022.08.25.22279237v1 (2022).
Kaplonek, P. et al. mRNA-1273 vaccine-induced antibodies maintain Fc effector functions across SARS-CoV-2 variants of concern. Immunity 55, 355–365 (2022).
Gorman, M. J. et al. Fab and Fc contribute to maximal protection against SARS-CoV-2 following NVX-CoV2373 subunit vaccine with Matrix-M vaccination. Cell Rep. Med. 2, 100405 (2021).
Flemming, A. Cross reactive T cells hold up against Omicron. Nat. Rev. Immunol. 22, 146 (2022).
Tarke, A. et al. SARS-CoV-2 vaccination induces immunological T cell memory able to cross-recognize variants from Alpha to Omicron. Cell 185, 847–859 (2022).
Reincke, S. M., Prüss, H., Wilson, I. A. & Kreye, J. Antigenic imprinting in SARS-CoV-2. Clin. Transl. Med. 12, e923 (2022).
Park, Y.-J. et al. Imprinted antibody responses against SARS-CoV-2 Omicron sublineages. Science 378, 619–627 (2022).
Schiepers, A. et al. Molecular fate-mapping of serum antibodies reveals the effects of antigenic imprinting on repeated immunization. Preprint at https://www.biorxiv.org/content/10.1101/2022.08.29.505743v1 (2022).
Webster, R. G. Original antigenic sin in ferrets: the response to sequential infections with influenza viruses. J. Immunol. 97, 177–183 (1966).
Zang, R. et al. TMPRSS2 and TMPRSS4 promote SARS-CoV-2 infection of human small intestinal enterocytes. Sci. Immunol. 5, eabc3582 (2020).
Chen, R. E. et al. Resistance of SARS-CoV-2 variants to neutralization by monoclonal and serum-derived polyclonal antibodies. Nat. Med. 27, 717–726 (2021).
Plante, J. A. et al. Spike mutation D614G alters SARS-CoV-2 fitness. Nature 592, 116–121 (2020).
Nelson, J. et al. Impact of mRNA chemistry and manufacturing process on innate immune activation. Sci. Adv. 6, eaaz6893 (2020).
Corbett, K. S. et al. SARS-CoV-2 mRNA vaccine design enabled by prototype pathogen preparedness. Nature 586, 567–571 (2020).
Hassett, K. J. et al. Optimization of lipid nanoparticles for intramuscular administration of mRNA vaccines. Mol. Ther. Nucleic Acids 15, 1–11 (2019).
Stadlbauer, D. et al. SARS-CoV-2 seroconversion in humans: a detailed protocol for a serological assay, antigen production, and test setup. Curr. Protoc. Microbiol. 57, e100 (2020).
Amanat, F. et al. SARS-CoV-2 mRNA vaccination induces functionally diverse antibodies to NTD, RBD, and S2. Cell 184, 3936–3948 (2021).
VanBlargan, L. A. et al. A potently neutralizing SARS-CoV-2 antibody inhibits variants of concern by utilizing unique binding residues in a highly conserved epitope. Immunity 54, 2399–2416 (2021).
Whitt, M. A. Generation of VSV pseudotypes using recombinant ΔG-VSV for studies on virus entry, identification of entry inhibitors, and immune responses to vaccines. J. Virol. Methods 169, 365–374 (2010).
Jackson, L. A. et al. An mRNA vaccine against SARS-CoV-2—preliminary report. N. Engl. J. Med. 383, 1920–1931 (2020).
Case, J. B., Bailey, A. L., Kim, A. S., Chen, R. E. & Diamond, M. S. Growth, detection, quantification, and inactivation of SARS-CoV-2. Virology 548, 39–48 (2020).
Acknowledgements
This study was supported by the National Institutes of Health (R01 AI157155, NIAID Centers of Excellence for Influenza Research and Response (CEIRR) contracts HHSN272201400008C, 75N93021C00014 and 75N93019C00051, all to M.S.D.). We thank M. Suthar for the BA.5 isolate used in this study. We also acknowledge and thank M. Whitt for support on VSV-based pseudovirus production.
Author information
Authors and Affiliations
Contributions
G.-Y.C., G.S.-J. and A.N. performed variant monitoring and Omicron variant vaccine design and quality control. S.M.S. performed and analyzed authentic virus neutralization assays. S.M.S., B.W. and B.Y. performed mouse experiments. B.W. and B.Y. performed and analyzed viral burden analyses. B.Y. analyzed chemokine, cytokine and histology data. H.J., P.M. and N.J.A. performed ELISA binding experiments and analysis. C.-Y.L. performed the anti-RBD depletion assays. K.W., D.L., D.M.B. and L.A. performed VSV pseudovirus neutralization assays and analysis. S.D.S., S.O., R.A.K. and N.D.-R. performed lentivirus pseudovirus neutralization assays and analysis. A.C., S.E. and D.K.E. provided mRNA vaccines and helped to design experiments. L.B.T. and M.S.D. designed studies and supervised the research. M.S.D., L.B.T. and D.K.E. wrote the initial draft, with the other authors providing editorial comments.
Corresponding authors
Ethics declarations
Competing interests
M.S.D. is a consultant for Inbios, Vir Biotechnology, Senda Biosciences, Moderna and Immunome. The Diamond laboratory has received unrelated funding support in sponsored research agreements from Vir Biotechnology, Emergent BioSolutions and Moderna. G.-Y.C., G.S.-J., A.N., K.W., D.L., D.M.B., L.A., H.J., P.M., N.J.A., A.C., S.E. and D.K.E. are employees of and shareholders in Moderna, Inc. All other authors declare no competing interests.
Peer review
Peer review information
Nature Medicine thanks Malik Peiris and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary handling editor: Alison Farrell, in collaboration with the Nature Medicine team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Comparison of serum neutralization using VSV pseudoviruses expressing Wuhan-1 D614G, BA.1, BA.2.75, or BA.4/5 spike proteins.
BALB/c mice were immunized with two 1 μg doses of preclinical versions of mRNA-1273, mRNA-1273.529, mRNA-1273.045, mRNA-1273.214 or mRNA-1273.222 vaccines. Serum neutralizing antibody responses against Wuhan-1 D614G, BA.1, and BA.4/5 were assessed two weeks after the second dose using VSV pseudoviruses. Representative neutralization curves corresponding to individual mice are shown for the indicated vaccines. Primary data are provided as a Source Data file.
Extended Data Fig. 2 Comparison of serum neutralization using pseudotyped lentiviruses expressing Wuhan-1, BA.1, or BA.4/5 spike proteins.
BALB/c mice were immunized with two 1 μg doses of preclinical versions of mRNA-1273, mRNA-1273.529, mRNA-1273.045, mRNA-1273.214 or mRNA-1273.222 vaccines. Serum neutralizing antibody responses against Wuhan-1, BA.1, and BA.4/5 were assessed two weeks after the second dose using pseudotyped lentiviruses. Average neutralization curves (n = 2) corresponding to individual mice are shown for the indicated vaccines. Primary data are provided as a Source Data file.
Extended Data Fig. 3 Comparison of serum neutralization using VSV pseudoviruses expressing Wuhan-1 D614G, BA.1, BA.2.75, or BA.4/5 spike proteins.
BALB/c mice were immunized with two 1 μg doses of clinically representative versions of mRNA-1273, mRNA-1273.214 or mRNA-1273.222 vaccines. Serum neutralizing antibody responses against Wuhan-1 D614G, BA.1, BA.2.75, and BA.4/5 were assessed two weeks after the second dose using VSV pseudoviruses. Representative neutralization curves corresponding to individual mice are shown for the indicated vaccines. Primary data are provided as a Source Data file.
Extended Data Fig. 4 Comparison of serum neutralization using pseudotyped lentiviruses expressing Wuhan-1 D614G, BA.1, or BA.4/5 spike proteins.
BALB/c mice were immunized with two sequential 1 μg doses of clinically representative versions of mRNA-1273, mRNA-1273.214 or mRNA-1273.222 vaccines. Serum neutralizing antibody responses against Wuhan-1 D614G, BA.1, and BA.4/5 were assessed two weeks after the second dose using pseudotyped lentiviruses. Average neutralization curves (n = 2) corresponding to individual mice are shown for the indicated vaccines. Primary data are provided as a Source Data file.
Extended Data Fig. 5 Comparison of serum neutralization of authentic WA1/2020 D614G, B.1.617.2, BA.1, and BA.5 viruses before and after boosting.
Seven-week-old female K18-hACE2 mice were immunized with two sequential 0.25 μg doses of control mRNA or mRNA-1273 and then boosted 31 weeks later with PBS, 0.25 μg of control mRNA, or 0.25 μg mRNA-1273, mRNA-1273.214, or mRNA-1273.222. Paired analysis of pre- and post-boost serum neutralizing titers against WA1/2020 D614G, B.1.617.2, BA.1 and BA.5 from samples obtained from animals (data from Fig. 3) (n = 8 for mRNA-1273.214 booster, n = 9 for control mRNA and mRNA-1273 booster, n = 10 for PBS and mRNA-1273.222 booster, two experiments. GMT values are indicated at the top of the graphs, dotted lines show the LOD based on a 1/60 (WA1/2020 D614G, B.1.617.2) or 1/30 serum dilution (BA.1, BA.5). Statistical analysis: two-tailed Wilcoxon signed-rank test (exact P values are indicated except for P > 0.05, not significant (ns)). Primary data are provided as a Source Data file.
Extended Data Fig. 6 Pre-boost serum neutralization of authentic WA1/2020 D614G, B.1.617.2, BA.1, and BA.5 viruses.
Seven-week-old female K18-hACE2 mice were immunized with two sequential 0.25 μg doses of control mRNA or mRNA-1273 and then boosted 31 weeks later with PBS, 0.25 μg of control mRNA, or 0.25 μg mRNA-1273, mRNA-1273.214, or mRNA-1273.222. Neutralizing antibody responses against WA1/2020 D614G, B.1.617.2, BA.1, and BA.5 from serum immediately before boosting with the indicated vaccines. Neutralization curves (FRNT analysis) corresponding to individual mice are shown for the indicated immunizations. Serum are from two experiments, and each point represents the mean of two technical replicates. Primary data are provided as a Source Data file.
Extended Data Fig. 7 Post-boost serum neutralization of authentic WA1/2020 D614G, B.1.617.2, BA.1, and BA.5 viruses.
Seven-week-old female K18-hACE2 mice were immunized with two sequential 0.25 μg doses of control mRNA or mRNA-1273 and then boosted 31 weeks later with PBS, 0.25 μg of control mRNA, or 0.25 μg mRNA-1273, mRNA-1273.214, or mRNA-1273.222. Neutralizing antibody responses against WA1/2020 D614G, B.1.617.2, BA.1, and BA.5 from serum one month after boosting with the indicated vaccines. Neutralization curves (FRNT analysis) corresponding to individual mice are shown for the indicated immunizations. Serum are from two experiments, and each point represents the mean of two technical replicates. Primary data are provided as a Source Data file.
Extended Data Fig. 8 Type-specific and cross-reactive antibodies detected in serum after boosting with mRNA-1273, mRNA-1273.214, and mRNA-1273.222.
a. Scheme of serum depletion of anti-RBD antibodies. b. Serum (1/150 dilution) from mice boosted with mRNA-1273, mRNA-1273.214, or mRNA-1273.222 was incubated with empty, Wuhan-1 RBD-loaded, or BA.1 RBD-loaded magnetic beads. After separation of pre-clearing beads, supernatants were diluted serially and added to ELISA plates coated with Wuhan-1 or BA.1 RBD. Representative depletion curves performed in technical duplicate corresponding to individual mice are shown for the indicated vaccines. c. Bar graphs derived from area under the curve analysis of data in panel b showing the relative proportions of Wuhan-1-specific, Wuhan-1/BA.1 cross-reactive, and BA.1-specific RBD responses (n = 9 for mRNA-1273, n = 10 for mRNA-1273.214 and mRNA1273.222, two experiments, right edge of boxes illustrate mean values and error bars indicate standard deviations). Primary data are provided as a Source Data file.
Supplementary information
Supplementary Information
List of Supplementary Information
Source data
Source Data Fig. 1
Excel file with source data for graphs
Source Data Fig. 2
Excel file with source data for graphs
Source Data Fig. 3
Excel file with source data for graphs
Source Data Fig. 4
Excel file with source data for graphs
Source Data Extended Data Fig. 1
Excel file with source data for graphs
Source Data Extended Data Fig. 2
Excel file with source data for graphs
Source Data Extended Data Fig. 3
Excel file with source data for graphs
Source Data Extended Data Fig. 4
Excel file with source data for graphs
Source Data Extended Data Fig. 5
Excel file with source data for graphs
Source Data Extended Data Fig. 6
Excel file with source data for graphs
Source Data Extended Data Fig. 7
Excel file with source data for graphs
Source Data Extended Data Fig. 8
Excel file with source data for graphs
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Scheaffer, S.M., Lee, D., Whitener, B. et al. Bivalent SARS-CoV-2 mRNA vaccines increase breadth of neutralization and protect against the BA.5 Omicron variant in mice. Nat Med 29, 247–257 (2023). https://doi.org/10.1038/s41591-022-02092-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41591-022-02092-8
This article is cited by
-
An ancestral SARS-CoV-2 vaccine induces anti-Omicron variants antibodies by hypermutation
Nature Communications (2024)
-
Humoral and cellular immune responses to COVID-19 mRNA vaccines in immunosuppressed liver transplant recipients
Communications Medicine (2024)
-
Omicron-specific and bivalent omicron-containing vaccine candidates elicit potent virus neutralisation in the animal model
Scientific Reports (2024)
-
Repeated Omicron exposures override ancestral SARS-CoV-2 immune imprinting
Nature (2024)
-
Mucosal vaccine-induced cross-reactive CD8+ T cells protect against SARS-CoV-2 XBB.1.5 respiratory tract infection
Nature Immunology (2024)