Abstract
The CONSORT 2010 statement provides minimum guidelines for reporting randomized trials. Its widespread use has been instrumental in ensuring transparency in the evaluation of new interventions. More recently, there has been a growing recognition that interventions involving artificial intelligence (AI) need to undergo rigorous, prospective evaluation to demonstrate impact on health outcomes. The CONSORT-AI (Consolidated Standards of Reporting Trials–Artificial Intelligence) extension is a new reporting guideline for clinical trials evaluating interventions with an AI component. It was developed in parallel with its companion statement for clinical trial protocols: SPIRIT-AI (Standard Protocol Items: Recommendations for Interventional Trials–Artificial Intelligence). Both guidelines were developed through a staged consensus process involving literature review and expert consultation to generate 29 candidate items, which were assessed by an international multi-stakeholder group in a two-stage Delphi survey (103 stakeholders), agreed upon in a two-day consensus meeting (31 stakeholders) and refined through a checklist pilot (34 participants). The CONSORT-AI extension includes 14 new items that were considered sufficiently important for AI interventions that they should be routinely reported in addition to the core CONSORT 2010 items. CONSORT-AI recommends that investigators provide clear descriptions of the AI intervention, including instructions and skills required for use, the setting in which the AI intervention is integrated, the handling of inputs and outputs of the AI intervention, the human–AI interaction and provision of an analysis of error cases. CONSORT-AI will help promote transparency and completeness in reporting clinical trials for AI interventions. It will assist editors and peer reviewers, as well as the general readership, to understand, interpret and critically appraise the quality of clinical trial design and risk of bias in the reported outcomes.
Similar content being viewed by others
Main
Randomized controlled trials (RCTs) are considered the gold-standard experimental design for providing evidence of the safety and efficacy of an intervention1,2. Trial results, if adequately reported, have the potential to inform regulatory decisions, clinical guidelines and health policy. It is therefore crucial that RCTs are reported with transparency and completeness so that readers can critically appraise the trial methods and findings and assess the presence of bias in the results3,4,5.
The CONSORT statement provides evidence-based recommendations to improve the completeness of the reporting of RCTs. The statement was first introduced in 1996 and has since been widely endorsed by medical journals internationally5. Over the past two decades, it has undergone two updates and has demonstrated a substantial positive impact on the quality of RCT reports6,7. The most recent CONSORT 2010 statement provides a 25-item checklist of the minimum reporting content applicable to all RCTs, but it recognizes that certain interventions may require extension or elaboration of these items. Several such extensions exist8,9,10,11,12,13.
AI is an area of enormous interest with strong drivers to accelerate new interventions through to publication, implementation and market14. While AI systems have been researched for some time, recent advances in deep learning and neural networks have gained considerable interest for their potential in health applications. Examples of such applications are wide ranging and include AI systems for screening and triage15,16, diagnosis17,18,19,20,prognostication21,22, decision support23 and treatment recommendation24. However, in the most recent cases, published evidence has consisted of in silico, early-phase validation. It has been recognized that most recent AI studies are inadequately reported and existing reporting guidelines do not fully cover potential sources of bias specific to AI systems25. The welcome emergence of RCTs seeking to evaluate newer interventions based on, or including, an AI component (called ‘AI interventions’ here)23,26,27,28,29,30,31 has similarly been met with concerns about the design and reporting25,32,33,34. This has highlighted the need to provide reporting guidance that is ‘fit for purpose’ in this domain.
CONSORT-AI (as part of the SPIRIT-AI and CONSORT-AI initiative) is an international initiative supported by CONSORT and the EQUATOR (Enhancing the Quality and Transparency of Health Research) Network to evaluate the existing CONSORT 2010 statement and to extend or elaborate this guidance where necessary, to support the reporting of clinical trials for AI interventions35,36. It is complementary to the SPIRIT-AI statement, which aims to promote high-quality protocol reporting for AI trials. This Consensus Statement describes the methods used to identify and evaluate candidate items and gain consensus. In addition, it also provides the CONSORT-AI checklist, which includes the new extension items and their accompanying explanations.
Methods
The SPIRIT-AI and CONSORT-AI extensions were simultaneously developed for clinical trial protocols and trial reports. An announcement for the SPIRIT-AI and CONSORT-AI initiative was published in October 2019 (ref. 35), and the two guidelines were registered as reporting guidelines under development on the EQUATOR library of reporting guidelines in May 2019. Both guidelines were developed in accordance with the EQUATOR Network’s methodological framework37. The SPIRIT-AI and CONSORT-AI Steering Group, consisting of 15 international experts, was formed to oversee the conduct and methodology of the study. Definitions of key terms are provided in the glossary (Box 1).
Ethical approval
This study was approved by the ethical review committee at the University of Birmingham, UK (ERN_19-1100). Participant information was provided to Delphi participants electronically before survey completion and before the consensus meeting. Delphi participants provided electronic informed consent, and written consent was obtained from consensus meeting participants.
Literature review and candidate item generation
An initial list of candidate items for the SPIRIT-AI and CONSORT-AI checklists was generated through review of the published literature and consultation with the Steering Group and known international experts. A search was performed on 13 May 2019 using the terms ‘artificial intelligence’, ‘machine learning’ and ‘deep learning’ to identify existing clinical trials for AI interventions listed within the US National Library of Medicine’s clinical trial registry (ClinicalTrials.gov). There were 316 registered trials, of which 62 were completed and 7 had published results30,38,39,40,41,42,43. Two studies were reported with reference to the CONSORT statement30,42, and one study provided an unpublished trial protocol42. The Operations Team (X.L., S.C.R., M.J.C. and A.K.D.) identified AI-specific considerations from these studies and reframed them as candidate reporting items. The candidate items were also informed by findings from a previous systematic review that evaluated the diagnostic accuracy of deep-learning systems for medical imaging25. After consultation with the Steering Group and additional international experts (n = 19), 29 candidate items were generated, 26 of which were relevant for both SPIRIT-AI and CONSORT-AI and 3 of which were relevant only for CONSORT-AI. The Operations Team mapped these items to the corresponding SPIRIT and CONSORT items, revising the wording and providing explanatory text as required to contextualize the items. These items were included in subsequent Delphi surveys.
Delphi consensus process
In September 2019, 169 key international experts were invited to participate in the online Delphi survey to vote upon the candidate items and suggest additional items. Experts were identified and contacted via the Steering Group and were allowed one round of ‘snowball’ recruitment in which contacted experts could suggest additional experts. In addition, individuals who made contact following publication of the announcement were included35. The Steering Group agreed that individuals with expertise in clinical trials and AI and machine learning (ML), as well as key users of the technology, should be well represented in the consultation. Stakeholders included healthcare professionals, methodologists, statisticians, computer scientists, industry representatives, journal editors, policy makers, health ‘informaticists’, experts in law and ethics, regulators, patients and funders. Participant characteristics are described in Supplementary Table 1. Two online Delphi surveys were conducted. DelphiManager software (version 4.0), developed and maintained by the COMET (Core Outcome Measures in Effectiveness Trials) initiative, was used to undertake the e-Delphi survey. Participants were given written information about the study and were asked to provide their level of expertise within the fields of (i) AI/ML, and (ii) clinical trials. Each item was presented for consideration (26 for SPIRIT-AI and 29 for CONSORT-AI). Participants were asked to vote on each item using a 9-point scale, as follows: 1–3, not important; 4–6, important but not critical; and 7–9, important and critical. Respondents provided separate ratings for SPIRIT-AI and CONSORT-AI. There was an option to opt out of voting for each item, and each item included space for free text comments. At the end of the Delphi survey, participants had the opportunity to suggest new items. 103 responses were received for the first Delphi round, and 91 responses (88% of participants from round one) were received for the second round. The results of the Delphi survey informed the subsequent international consensus meeting. 12 new items were proposed by the Delphi study participants and were added for discussion at the consensus meeting. Data collected during the Delphi survey were anonymized, and item-level results were presented at the consensus meeting for discussion and voting.
The two-day consensus meeting took place in January 2020 and was hosted by the University of Birmingham, UK, to seek consensus on the content of SPIRIT-AI and CONSORT-AI. 31 international stakeholders from among the Delphi survey participants were invited to discuss the items and vote on their inclusion. Participants were selected to achieve adequate representation from all the stakeholder groups. 41 items were discussed in turn, comprising the 29 items generated in the initial literature review and item-generation phase (26 items relevant to both SPIRIT-AI and CONSORT-AI; 3 items relevant only to CONSORT-AI) and the 12 new items proposed by participants during the Delphi surveys. Each item was presented to the consensus group, alongside its score from the Delphi exercise (median and interquartile ranges) and any comments made by Delphi participants related to that item. Consensus-meeting participants were invited to comment on the importance of each item and whether the item should be included in the AI extension. In addition, participants were invited to comment on the wording of the explanatory text accompanying each item and the position of each item relative to the SPIRIT 2013 and CONSORT 2010 checklists. After open discussion of each item and the option to adjust wording, an electronic vote took place, with the option to include or exclude the item. An 80% threshold for inclusion was pre-specified and deemed reasonable by the Steering Group to demonstrate majority consensus. Each stakeholder voted anonymously using Turning Point voting pads (Turning Technologies, version 8.7.2.14).
Checklist pilot
Following the consensus meeting, attendees were given the opportunity to make final comments on the wording and agree that the updated SPIRIT-AI and CONSORT-AI items reflected discussions from the meeting. The Operations Team assigned each item as an extension or elaboration item on the basis of a decision tree and produced a penultimate draft of the SPIRIT-AI and CONSORT-AI checklists (Supplementary Fig. 1). A pilot of the penultimate checklists was conducted with 34 participants to ensure clarity of wording. Experts participating in the pilot included the following: (a) Delphi participants who did not attend the consensus meeting, and (b) external experts who had not taken part in the development process but who had reached out to the Steering Group after the Delphi study commenced. Final changes were made on wording only to improve clarity for readers, by the Operations Team (Supplementary Fig. 2).
Recommendations
CONSORT-AI checklist items and explanation
The CONSORT-AI extension recommends that 14 new checklists items be added to the existing CONSORT 2010 statement (11 extensions and 3 elaborations). These items were considered sufficiently important for clinical-trial reports for AI interventions that they should be routinely reported in addition to the core CONSORT 2010 checklist items. Table 1 lists the CONSORT-AI items.
The 14 items below passed the threshold of 80% for inclusion at the consensus meeting. CONSORT-AI 2a, CONSORT-AI 5 (ii) and CONSORT-AI 19 each resulted from the merging of two items after discussion with the consensus group. CONSORT-AI 4a (i) and (ii) was split into two items for clarity and was voted upon separately. CONSORT-AI 5(iii) did not fulfill the criteria for inclusion on the basis of its initial wording (77% vote to include); however, after extensive discussion and rewording, the consensus group unanimously supported a re-vote, at which point it passed the inclusion threshold (97% to include). The Delphi and voting results for each included and excluded item are described in Supplementary Table 2.
Title and abstract
CONSORT-AI 1a,b (i) Elaboration: Indicate that the intervention involves artificial intelligence/machine learning in the title and/or abstract and specify the type of model
Explanation
Indicating in the title and/or abstract of the trial report that the intervention involves a form of AI is encouraged, as it immediately identifies the intervention as an AI/ML intervention and also serves to facilitate indexing and searching of the trial report. The title should be understandable by a wide audience; therefore, a broader umbrella term such as ‘artificial intelligence’ or ‘machine learning’ is encouraged. More-precise terms should be used in the abstract, rather than the title, unless they are broadly recognized as being a form of AI/ML. Specific terminology relating to the model type and architecture should be detailed in the abstract.
CONSORT-AI 1a,b (ii) Elaboration: State the intended use of the AI intervention within the trial in the title and/or abstract
Explanation
Describe the intended use of the AI intervention in the trial report title and/or abstract. This should describe the purpose of the AI intervention and the disease context26,44. Some AI interventions may have multiple intended uses, or the intended use may evolve over time. Therefore, documenting this allows readers to understand the intended use of the algorithm at the time of the trial.
Introduction
CONSORT-AI 2a (i) Extension: Explain the intended use for the AI intervention in the context of the clinical pathway, including its purpose and its intended users (for example, healthcare professionals, patients, public)
Explanation
In order to clarify how the AI intervention is intended to fit into a clinical pathway, a detailed description of its role should be included in the background of the trial report. AI interventions may be designed to interact with different users, including healthcare professionals, patients and the public, and their roles can be wide-ranging (for example, the same AI intervention could theoretically be replacing, augmenting or adjudicating components of clinical decision-making). Clarifying the intended use of the AI intervention and its intended user helps readers understand the purpose for which the AI intervention was evaluated in the trial.
Methods
CONSORT-AI 4a (i) Elaboration: State the inclusion and exclusion criteria at the level of participants
Explanation
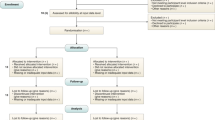
The inclusion and exclusion criteria should be defined at the participant level as per usual practice in non-AI interventional trial reports (Fig. 1). This is distinct from the inclusion and exclusion criteria made at the input-data level, which is addressed in item 4a (ii).
CONSORT-AI 4a (i): State the inclusion and exclusion criteria at the level of participants. CONSORT-AI 4a (ii): State the inclusion and exclusion criteria at the level of the input data. CONSORT 13b (core CONSORT item): For each group, losses and exclusions after randomization, together with reasons.
CONSORT-AI 4a (ii) Extension: State the inclusion and exclusion criteria at the level of the input data
Explanation
‘Input data’ refers to the data required by the AI intervention to serve its purpose (for example, for a breast-cancer diagnostic system, the input data could be the unprocessed or vendor-specific post-processing mammography scan upon which a diagnosis is being made; for an early-warning system, the input data could be physiological measurements or laboratory results from the electronic health record). The trial report should pre-specify if there were minimum requirements for the input data (such as image resolution, quality metrics or data format) that determined pre-randomization eligibility. It should specify when, how and by whom this was assessed. For example, if a participant met the eligibility criteria for lying flat for a CT scan as per item 4a (i), but the scan quality was compromised (for any given reason) to such a level that it was deemed unfit for use by the AI system, this should be reported as an exclusion criterion at the input-data level. Note that where input data are acquired after randomization, any exclusion is considered to be from the analysis, not from enrollment (CONSORT item 13b and Fig. 1).
CONSORT-AI 4b Extension: Describe how the AI intervention was integrated into the trial setting, including any onsite or offsite requirements
Explanation
There are limitations to the generalizability of AI algorithms, one of which is when they are used outside of their development environment45,46. AI systems are dependent on their operational environment, and the report should provide details of the hardware and software requirements to allow technical integration of the AI intervention at each study site. For example, it should be stated if the AI intervention required vendor-specific devices, if there was specialized computing hardware at each site, or if the site had to support cloud integration, particularly if this was vendor specific. If any changes to the algorithm were required at each study site as part of the implementation procedure (such as fine-tuning the algorithm on local data), then this process should also be clearly described.
CONSORT-AI 5 (i) Extension: State which version of the AI algorithm was used
Explanation
Similar to other forms of software as a medical device, AI systems are likely to undergo multiple iterations and updates during their lifespan. It is therefore important to specify which version of the AI system was used in the clinical trial, whether this is the same as the version evaluated in previous studies that have been used to justify the study rationale, and whether the version changed during the conduct of the trial. If applicable, the report should describe what has changed between the relevant versions and the rationales for the changes. Where available, the report should include a regulatory marking reference, such as an unique device identifier, that requires a new identifier for updated versions of the device47.
CONSORT-AI 5 (ii) Extension: Describe how the input data were acquired and selected for the AI intervention
Explanation
The measured performance of any AI system may be critically dependent on the nature and quality of the input data48. A description of the input-data handling, including acquisition, selection and pre-processing before analysis by the AI system, should be provided. Completeness and transparency of this description is integral to the replicability of the intervention beyond the clinical trial in real-world settings. It also helps readers identify whether input-data-handling procedures were standardized across trial sites.
CONSORT-AI 5 (iii) Extension: Describe how poor-quality or unavailable input data were assessed and handled
Explanation
As with CONSORT-AI 4a (ii), ‘input data’ refers to the data required by the AI intervention to serve its purpose. As discussed in item 4a (ii), the performance of AI systems may be compromised as a result of poor quality or missing input data49 (for example, excessive movement artifact on an electrocardiogram). The trial report should report the amount of missing data, as well as how this was identified and handled. The report should also specify if there was a minimum standard required for the input data and, where this standard was not achieved, how this was handled (including the impact on, or any changes to, the participant care pathway).
Poor quality or unavailable data can also affect non-AI interventions. For example, sub-optimal quality of a scan could affect a radiologist’s ability to interpret it and make a diagnosis. It is therefore important that this information is reported equally in the control intervention, where relevant. If this minimum quality standard was different from the inclusion criteria for input data used to assess eligibility pre-randomization, this should be stated.
CONSORT-AI 5 (iv) Extension: Specify whether there was human–AI interaction in the handling of the input data, and what level of expertise was required of users
Explanation
A description of the human–AI interface and the requirements for successful interaction when input data are handled should be provided — for example, clinician-led selection of regions of interest from a histology slide that is then interpreted by an AI diagnostic system50, or an endoscopist’s selection of a colonoscopy video clips as input data for an algorithm designed to detect polyps28. A description of any user training provided and instructions for how users should handle the input data provides transparency and replicability of trial procedures. Poor clarity on the human–AI interface may lead to lack of a standard approach and may carry ethical implications, particularly in the event of harm51,52. For example, it may become unclear whether an error case occurred due to human deviation from the instructed procedure, or if it was an error made by the AI system.
CONSORT-AI 5 (v) Extension: Specify the output of the AI intervention
Explanation
The output of the AI intervention should be clearly specified in the trial report. For example, an AI system may output a diagnostic classification or probability, a recommended action, an alarm alerting to an event, an instigated action in a closed-loop system (such as titration of drug infusions) or another output. The nature of the AI intervention’s output has direct implications on its usability and how it may lead to downstream actions and outcomes.
CONSORT-AI 5 (vi) Extension: Explain how the AI intervention’s outputs contributed to decision-making or other elements of clinical practice
Explanation
Since health outcomes may also critically depend on how humans interact with the AI intervention, the report should explain how the outputs of the AI system were used to contribute to decision-making or other elements of clinical practice. This should include adequate description of downstream interventions that can affect outcomes. As with CONSORT-AI 5 (iv), any effects of human–AI interaction on the outputs should be described in detail, including the level of expertise required to understand the outputs and any training and/or instructions provided for this purpose. For example, a skin cancer detection system that produced a percentage likelihood as its output should be accompanied by an explanation of how this output was interpreted and acted upon by the user, specifying both the intended pathways (for example, skin lesion excision if the diagnosis is positive) and the thresholds for entry to these pathways (for example, skin lesion excision if the diagnosis is positive and the probability is >80%). The information produced by comparator interventions should be similarly described, alongside an explanation of how such information was used to arrive at clinical decisions on patient management, where relevant. Any discrepancy in how decision-making occurred versus how it was intended to occur (that is, as specified in the trial protocol) should be reported.
Results
CONSORT-AI 19 Extension: Describe results of any analysis of performance errors and how errors were identified, where applicable. If no such analysis was planned or done, explain why not
Explanation
Reporting performance errors and failure case analysis is especially important for AI interventions. AI systems can make errors that may be hard to foresee but that, if allowed to be deployed at scale, could have catastrophic consequences53. Therefore, reporting cases of error and defining risk-mitigation strategies are important for informing when, and for which populations, the intervention can be safely implemented. The results of any performance-error analysis should be reported and the implications of the results should be discussed.
Other information
CONSORT-AI 25 Extension: State whether and how the AI intervention and/or its code can be accessed, including any restrictions to access or re-use
Explanation
The trial report should make it clear whether and how the AI intervention and/or its code can be accessed or re-used. This should include details about the license and any restrictions to access.
Discussion
CONSORT-AI is a new reporting-guideline extension developed through international multi-stakeholder consensus. It aims to promote transparent reporting of AI intervention trials and is intended to facilitate critical appraisal and evidence synthesis. The extension items added in CONSORT-AI address a number of issues specific to the implementation and evaluation of AI interventions, which should be considered alongside the core CONSORT 2010 checklist and other CONSORT extensions54. It is important to note that these are minimum requirements and there may be value in including additional items not included in the checklists in the report or in supplementary materials (Supplementary Table 2).
In both CONSORT-AI and its companion project SPIRIT-AI, a major emphasis was the addition of several new items related to the intervention itself and its application in the clinical context. Items 5 (i)–5 (vi) were added to address AI-specific considerations in descriptions of the intervention. Specific recommendations were made pertinent to AI systems related to algorithm version, input and output data, integration into trial settings, expertise of the users and protocol for acting upon the AI system’s recommendations. It was agreed that these details are critical for independent evaluation or replication of the trial. Journal editors reported that despite the importance of these items, they are currently often missing from trial reports at the time of submission for publication, which provides further weight for their inclusion as specifically listed extension items.
A recurrent focus of the Delphi comments and consensus group discussion was the safety of AI systems. This was in recognition that AI systems, unlike other health interventions, can unpredictably yield errors that are not easily detectable or explainable by human judgement. For example, changes to medical imaging that are invisible, or appear random, to the human eye may change the likelihood of the diagnostic output entirely55,56. The concern is that given the theoretical ease with which AI systems could be deployed at scale, any unintended harmful consequences could be catastrophic. CONSORT-AI item 19, which requires specification of any plans to analyze performance errors, was added to emphasize the importance of anticipating systematic errors made by the algorithm and their consequences. Beyond this, investigators should also be encouraged to explore differences in performance and error rates across population subgroups. It has been shown that AI systems may be systematically biased toward different outputs, which may lead to different or even unfair treatment, on the basis of extant features53,57,58,59.
The topic of ‘continuously evolving’ AI systems (also known as ‘continuously adapting’ or ‘continuously learning’ AI systems) was discussed at length during the consensus meeting, but it was agreed that this be excluded from CONSORT-AI. These are AI systems with the ability to continuously train on new data, which may cause changes in performance over time. The group noted that, while of interest, this field is relatively early in its development without tangible examples in healthcare applications, and that it would not be appropriate for it to be included in CONSORT-AI at this stage60. This topic will be monitored and will be revisited in future iterations of CONSORT-AI. It is worth noting that incremental software changes, whether continuous or iterative, intentional or unintentional, could have serious consequences on safety performance after deployment. It is therefore of vital importance that such changes be documented and identified by software version and that a robust post-deployment surveillance plan is in place.
This study is set in the current context of AI in health; therefore, several limitations should be noted. First, there are relatively few published interventional trials in the field of AI for healthcare; therefore, the discussions and decisions made during this study were not always supported by existing examples of completed trials. This arises from our stated aim of addressing the issues of poor reporting in this field as early as possible, recognizing the strong drivers in the field and the specific challenges of study design and reporting for AI. As the science and study of AI evolves, we welcome collaboration with investigators to co-evolve these reporting standards to ensure their continued relevance. Second, the literature search of AI RCTs used terminology such as ‘artificial intelligence’, ‘machine learning’ and ‘deep learning’, but not terms such as ‘clinical decision support systems’ or ‘expert systems’, which were more commonly used in the 1990s for technologies underpinned by AI systems and share risks similar to those of recent examples61. It is likely that such systems, if published today, would be indexed under ‘artificial intelligence’ or ‘machine learning’; however, clinical decision support systems were not actively discussed during this consensus process. Third, the initial candidate items list was generated by a relatively small group of experts consisting of SteeringGroup members and additional international experts; however, additional items from the wider Delphi group were taken forward for consideration by the consensus group, and no new items were suggested during the consensus meeting or post-meeting evaluation.
As with the CONSORT statement, the CONSORT-AI extension is intended as a minimum reporting guidance, and there are additional AI-specific considerations for trial reports that may warrant consideration (Supplementary Table 2). This extension is aimed particularly at investigators and readers reporting or appraising clinical trials; however, it may also serve as useful guidance for developers of AI interventions in earlier validation stages of an AI system. Investigators seeking to report studies developing and validating the diagnostic and predictive properties of AI models should refer to TRIPOD-ML (Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis–Machine Learning) and STARD-AI (Standards for Reporting Diagnostic Accuracy Studies–Artificial Intelligence), both of which are currently under development32,62. Other potentially relevant guidelines, which are agnostic to study design, are registered with the EQUATOR Network63. The CONSORT-AI extension is expected to encourage careful early planning of AI interventions for clinical trials and this, in conjunction with SPIRIT-AI, should help to improve the quality of trials for AI interventions. The development of the CONSORT-AI guidance does not include additional items within the discussion section of trial reports. The guidance provided by CONSORT 2010 on trial limitations, generalizability and interpretation were deemed to be translatable to trials for AI interventions.
There is also recognition that AI is a rapidly evolving field, and there will be the need to update CONSORT-AI as the technology, and newer applications for it, develop. Currently, most applications of AI involve disease detection, diagnosis and triage, and this is likely to have influenced the nature and prioritization of items within CONSORT-AI. As wider applications that utilize ‘AI as therapy’ emerge, it will be important to continue to evaluate CONSORT-AI in the light of such studies. Additionally, advances in computational techniques and the ability to integrate them into clinical workflows will bring new opportunities for innovation that benefits patients. However, they may be accompanied by new challenges around study design and reporting. In order to ensure transparency, minimize potential biases and promote the trustworthiness of the results and the extent to which they may be generalizable, the SPIRIT-AI and CONSORT-AI Steering Group will continue to monitor the need for updates.
Data availability
Data requests should be made to the corresponding author and release will be subject to consideration by the SPIRIT-AI and CONSORT-AI Steering Group.
References
Sibbald, B. & Roland, M. Understanding controlled trials. Why are randomised controlled trials important? Br. Med. J. 316, 201 (1998).
Peto, R., Collins, R. & Gray, R. Large-scale randomized evidence: large, simple trials and overviews of trials. J. Clin. Epidemiol. 48, 23–40 (1995).
Jüni, P., Altman, D. G. & Egger, M. Systematic reviews in health care: assessing the quality of controlled clinical trials. Br. Med. J. 323, 42–46 (2001).
Schulz, K. F., Chalmers, I., Hayes, R. J. & Altman, D. G. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. J. Am. Med. Assoc. 273, 408–412 (1995).
Moher, D. et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. Br. Med. J. 340, c869 (2010).
Moher, D., Jones, A. & Lepage, L. CONSORT Group (Consolidated Standards for Reporting of Trials) Use of the CONSORT statement and quality of reports of randomized trials: a comparative before-and-after evaluation. J. Am. Med. Assoc. 285, 1992–1995 (2001).
Glasziou, P. et al. Reducing waste from incomplete or unusable reports of biomedical research. Lancet 383, 267–276 (2014).
Boutron, I., Altman, D. G., Moher, D., Schulz, K. F. & Ravaud, P. CONSORT statement for randomized trials of nonpharmacologic treatments: a 2017 update and a CONSORT extension for nonpharmacologic trial abstracts. Ann. Intern. Med. 167, 40–47 (2017).
Hopewell, S. et al. CONSORT for reporting randomised trials in journal and conference abstracts. Lancet 371, 281–283 (2008).
MacPherson, H. et al. Revised STandards for Reporting Interventions in Clinical Trials of Acupuncture (STRICTA): extending the CONSORT statement. PLoS Med. 7, e1000261 (2010).
Gagnier, J. J. et al. Reporting randomized, controlled trials of herbal interventions: an elaborated CONSORT statement. Ann. Intern. Med. 144, 364–367 (2006).
Cheng, C.-W. et al. CONSORT extension for Chinese herbal medicine formulas 2017: recommendations, explanation, and elaboration. Ann. Intern. Med. 167, 112–121 (2017).
Calvert, M. et al. Reporting of patient-reported outcomes in randomized trials: the CONSORT PRO extension. J. Am. Med. Assoc. 309, 814–822 (2013).
He, J. et al. The practical implementation of artificial intelligence technologies in medicine. Nat. Med. 25, 30–36 (2019).
McKinney, S. M. et al. International evaluation of an AI system for breast cancer screening. Nature 577, 89–94 (2020).
Abràmoff, M. D. et al. Improved automated detection of diabetic retinopathy on a publicly available dataset through integration of deep learning. Invest. Ophthalmol. Vis. Sci. 57, 5200–5206 (2016).
De Fauw, J. et al. Clinically applicable deep learning for diagnosis and referral in retinal disease. Nat. Med. 24, 1342–1350 (2018).
Esteva, A. et al. Dermatologist-level classification of skin cancer with deep neural networks. Nature 542, 115–118 (2017).
Rajpurkar, P. et al. Deep learning for chest radiograph diagnosis: A retrospective comparison of the CheXNeXt algorithm to practicing radiologists. PLoS Med. 15, e1002686 (2018).
Fleuren, L. M. et al. Machine learning for the prediction of sepsis: a systematic review and meta-analysis of diagnostic test accuracy. Intensive Care Med. 46, 383–400 (2020).
Yim, J. et al. Predicting conversion to wet age-related macular degeneration using deep learning. Nat. Med. 26, 892–899 (2020).
Kim, H., Goo, J. M., Lee, K. H., Kim, Y. T. & Park, C. M. Preoperative CT-based deep learning model for predicting disease-free survival in patients with lung adenocarcinomas. Radiology 296, 216–224 (2020).
Wang, P. et al. Real-time automatic detection system increases colonoscopic polyp and adenoma detection rates: a prospective randomised controlled study. Gut 68, 1813–1819 (2019).
Tyler, N. S. et al. An artificial intelligence decision support system for the management of type 1 diabetes. Nat. Metab. 2, 612–619 (2020).
Liu, X. et al. A comparison of deep learning performance against health-care professionals in detecting diseases from medical imaging: a systematic review and meta-analysis. Lancet Digit. Health 1, e271–e297 (2019).
Wijnberge, M. et al. Effect of a machine learning-derived early warning system for intraoperative hypotension vs standard care on depth and duration of intraoperative hypotension during elective noncardiac surgery: the HYPE randomized clinical trial. J. Am. Med. Assoc. 323, 1052–1060 (2020).
Gong, D. et al. Detection of colorectal adenomas with a real-time computer-aided system (ENDOANGEL): a randomised controlled study. Lancet Gastroenterol. Hepatol. 5, 352–361 (2020).
Wang, P. et al. Effect of a deep-learning computer-aided detection system on adenoma detection during colonoscopy (CADe-DB trial): a double-blind randomised study. Lancet Gastroenterol. Hepatol. 5, 343–351 (2020).
Wu, L. et al. Randomised controlled trial of WISENSE, a real-time quality improving system for monitoring blind spots during esophagogastroduodenoscopy. Gut 68, 2161–2169 (2019).
Lin, H. et al. Diagnostic efficacy and therapeutic decision-making capacity of an artificial intelligence platform for childhood cataracts in eye clinics: a multicentre randomized controlled trial. EClinicalMedicine 9, 52–59 (2019).
Su, J.-R. et al. Impact of a real-time automatic quality control system on colorectal polyp and adenoma detection: a prospective randomized controlled study (with videos). Gastrointest. Endosc. 91, 415–424.e4 (2020).
Collins, G. S. & Moons, K. G. M. Reporting of artificial intelligence prediction models. Lancet 393, 1577–1579 (2019).
Gregory, J., Welliver, S. & Chong, J. Top 10 reviewer critiques of radiology artificial intelligence (AI) articles: qualitative thematic analysis of reviewer critiques of machine learning/deep learning manuscripts submitted to JMRI. J. Magn. Reson. Imaging 52, 248–254 (2020).
Nagendran, M. et al. Artificial intelligence versus clinicians: systematic review of design, reporting standards, and claims of deep learning studies. Br. Med. J. 368, m689 (2020).
CONSORT-AI and SPIRIT-AI Steering Group. Reporting guidelines for clinical trials evaluating artificial intelligence interventions are needed. Nat. Med. 25, 1467–1468 (2019).
Liu, X., Faes, L., Calvert, M. J. & Denniston, A. K. Extension of the CONSORT and SPIRIT statements. Lancet 394, 1225 (2019).
Moher, D., Schulz, K. F., Simera, I. & Altman, D. G. Guidance for developers of health research reporting guidelines. PLoS Med. 7, e1000217 (2010).
Caballero-Ruiz, E. et al. A web-based clinical decision support system for gestational diabetes: Automatic diet prescription and detection of insulin needs. Int. J. Med. Inform. 102, 35–49 (2017).
Kim, T. W. B., Gay, N., Khemka, A. & Garino, J. Internet-based exercise therapy using algorithms for conservative treatment of anterior knee pain: a pragmatic randomized controlled trial. JMIR Rehabil. Assist. Technol. 3, e12 (2016).
Labovitz, D. L., Shafner, L., Reyes Gil, M., Virmani, D. & Hanina, A. Using artificial intelligence to reduce the risk of nonadherence in patients on anticoagulation therapy. Stroke 48, 1416–1419 (2017).
Nicolae, A. et al. Evaluation of a machine-learning algorithm for treatment planning in prostate low-dose-rate brachytherapy. Int. J. Radiat. Oncol. Biol. Phys. 97, 822–829 (2017).
Voss, C. et al. Effect of wearable digital intervention for improving socialization in children with autism spectrum disorder: a randomized clinical trial. JAMA Pediatr. 173, 446–454 (2019).
Mendes-Soares, H. et al. Assessment of a personalized approach to predicting postprandial glycemic responses to food among individuals without diabetes. JAMA Netw. Open 2, e188102 (2019).
Choi, K. J. et al. Development and validation of a deep learning system for staging liver fibrosis by using contrast agent-enhanced CT images in the liver. Radiology 289, 688–697 (2018).
Kelly, C. J., Karthikesalingam, A., Suleyman, M., Corrado, G. & King, D. Key challenges for delivering clinical impact with artificial intelligence. BMC Med. 17, 195 (2019).
Pooch, E. H. P., Ballester, P. L. & Barros, R. C. Can we trust deep learning models diagnosis? The impact of domain shift in chest radiograph classification. arXiv http://arxiv.org/abs/1909.01940 (2019).
International Medical Device Regulators Forum. Unique Device Identification System (UDI System) Application Guide. http://www.imdrf.org/documents/documents.asp (2019).
Sabottke, C. F. & Spieler, B. M. The effect of image resolution on deep learning in radiography. Radiology: Artif. Intell. 2, e190015 (2020).
Heaven, D. Why deep-learning AIs are so easy to fool. Nature 574, 163–166 (2019).
Kiani, A. et al. Impact of a deep learning assistant on the histopathologic classification of liver cancer. NPJ Digit. Med 3, 23 (2020).
Wiens, J. et al. Do no harm: a roadmap for responsible machine learning for health care. Nat. Med. 25, 1337–1340 (2019).
Habli, I., Lawton, T. & Porter, Z. Artificial intelligence in health care: accountability and safety. Bull. World Health Organ. https://www.who.int/bulletin/online_first/BLT.19.237487.pdf (2020).
Oakden-Rayner, L., Dunnmon, J., Carneiro, G. & Ré, C. Hidden stratification causes clinically meaningful failures in machine learning for medical imaging. arXiv http://arxiv.org/abs/1909.12475 (2019).
CONSORT. Extensions of the CONSORT Statement. http://www.consort-statement.org/extensions (accessed 24 March 2020).
Zech, J. R. et al. Confounding variables can degrade generalization performance of radiological deep learning models. arXiv http://arxiv.org/abs/1807.00431 (2018).
Finlayson, S. G. et al. Adversarial attacks on medical machine learning. Science 363, 1287–1289 (2019).
Adamson, A. S. & Smith, A. Machine learning and health care disparities in dermatology. JAMA Dermatol. 154, 1247–1248 (2018).
Zou, J. & Schiebinger, L. AI can be sexist and racist - it’s time to make it fair. Nature 559, 324–326 (2018).
Chen, I. Y., Joshi, S. & Ghassemi, M. Treating health disparities with artificial intelligence. Nat. Med. 26, 16–17 (2020).
Lee, C. S. & Lee, A. Y. Clinical applications of continual learning machine learning. Lancet Digit. Health 2, e279–e281 (2020).
Sutton, R. T. et al. An overview of clinical decision support systems: benefits, risks, and strategies for success. NPJ Digit. Med. 3, 17 (2020).
Sounderajah, V. et al. Developing specific reporting guidelines for diagnostic accuracy studies assessing AI interventions: The STARD-AI Steering Group. Nat. Med. 26, 807–808 (2020).
Talmon, J. et al. STARE-HI-Statement on reporting of evaluation studies in Health Informatics. Int. J. Med. Inform. 78, 1–9 (2009).
Acknowledgements
We thank the participants who were involved in the Delphi study and Pilot study (Supplementary Note); E. Marston (University of Birmingham, UK) for strategic support; and C. Radovanovic (University Hospitals Birmingham NHS Foundation Trust, UK) and A. Walker (University of Birmingham, UK) for administrative support. The views expressed in this publication are those of the authors, Delphi participants and stakeholder participants and may not represent the views of the broader stakeholder group or host institution. This work was funded by a Wellcome Trust Institutional Strategic Support Fund: Digital Health Pilot Grant, Research England (part of UK Research and Innovation), Health Data Research UK and the Alan Turing Institute. The study was sponsored by the University of Birmingham, UK. The study funders and sponsors had no role in the design and conduct of the study; collection, management, analysis and interpretation of the data; preparation, review or approval of the manuscript; or decision to submit the manuscript for publication. M.J.C. is a National Institute for Health Research (NIHR) Senior Investigator and receives funding from the National Institute for Health Research (NIHR) Birmingham Biomedical Research Centre; the NIHR Surgical Reconstruction and Microbiology Research Centre and NIHR ARC West Midlands at the University of Birmingham and University Hospitals Birmingham NHS Foundation Trust; Health Data Research UK; Innovate UK (part of UK Research and Innovation); the Health Foundation; Macmillan Cancer Support; and UCB Pharma. A.D. and J.D. are also NIHR Senior Investigators. The views expressed in this article are those of the author(s) and not necessarily those of the NIHR, or the Department of Health and Social Care. S.J.V. receives funding from the Engineering and Physical Sciences Research Council, UK Research and Innovation (UKRI), Accenture, Warwick Impact Fund, Health Data Research UK and European Regional Development Fund. S.R. is an employee of the Medical Research Council (UKRI). D.M. is supported by a University of Ottawa Research Chair. M.K.E. is supported by the U.S. Food and Drug Administration (FDA), and D.P. is supported in part by the Office of the Director at the National Library of Medicine (NLM), US National Institutes of Health (NIH). A.B. is supported by a National Institutes of Health (NIH) award 7K01HL141771-02. This article may not be consistent with NIH and/or FDA’s views or policies. It reflects only the views and opinions of the authors.
Author information
Authors and Affiliations
Consortia
Contributions
Concept and design, and acquisition, analysis and interpretation of data, all authors; drafting of the manuscript, X.L., S.C.R., D.M., M.J.C. and A.K.D.; obtaining of funding, M.J.C., C.Y., C.H. and A.K.D. The SPIRIT-AI and CONSORT-AI Working Group consists of two groups that have been key in the development of the guidelines: the SPIRIT-AI and CONSORT-AI Steering Group, which was responsible for overseeing the consensus process and guidelines development methodology (Alastair K. Denniston, An-Wen Chan, Ara Darzi, Christopher Holmes, Christopher Yau, David Moher, Hutan Ashrafian, Jonathan J. Deeks, Lavinia Ferrante di Ruffano, Livia Faes, Melanie J. Calvert, Pearse A. Keane, Samantha Cruz Rivera, Sebastian J. Vollmer and Xiaoxuan Liu); and the SPIRIT-AI and CONSORT-AI Consensus Group, which was responsible for reaching consensus on the content and wording of the items within the checklists (Aaron Y. Lee, Adrian Jonas, Andre Esteva, Andrew L. Beam, An-Wen Chan, Maria Beatrice Panico, Cecilia S. Lee, Charlotte Haug, Christopher J. Kelly, Christopher Yau, Cynthia Mulrow, Cyrus Espinoza, David Moher, Dina Paltoo, Elaine Manna, Gary Price, Gary S Collins, Hugh Harvey, James Matcham, Joao Monteiro, John Fletcher, M. Khair ElZarrad, Lavinia Ferrante Di Ruffano, Luke Oakden-Rayner, Melanie J. Calvert, Melissa McCradden, Pearse A. Keane, Richard Savage, Robert Golub, Rupa Sarkar and Samuel Rowley).
Corresponding author
Ethics declarations
Competing interests
M.J.C. has received personal fees from Astellas, Takeda, Merck, Daiichi Sankyo, Glaukos, GlaxoSmithKline and the Patient-Centered Outcomes Research Institute (PCORI) outside the submitted work. P.A.K. is a consultant for DeepMind Technologies, Roche, Novartis and Apellis, and has received speaker fees or travel support from Bayer, Allergan, Topcon and Heidelberg Engineering. C.J.K. is an employee of Google and owns Alphabet stock. A.E. is an employee of Salesforce CRM. R.S. is an employee of Pinpoint Science. J. Matcham was an employee of AstraZeneca at the time of this study. J. Monteiro is Chief Editor of the journal Nature Medicine; he has recused himself from any aspect of decision-making on this manuscript and played no part in the assignment of this manuscript to in-house editors or peer reviewers, and was also separated and blinded from the editorial process from submission inception to decision.
Additional information
Peer review information Javier Carmona was the primary editor on this article, and managed its editorial process and peer review in collaboration with the rest of the editorial team.
Supplementary information
Supplementary Information
Supplementary Figures 1–2, Supplementary Tables 1–2, Supplementary Note.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Liu, X., Cruz Rivera, S., Moher, D. et al. Reporting guidelines for clinical trial reports for interventions involving artificial intelligence: the CONSORT-AI extension. Nat Med 26, 1364–1374 (2020). https://doi.org/10.1038/s41591-020-1034-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41591-020-1034-x
This article is cited by
-
Artificial intelligence in the risk prediction models of cardiovascular disease and development of an independent validation screening tool: a systematic review
BMC Medicine (2024)
-
Artificial intelligence and urology: ethical considerations for urologists and patients
Nature Reviews Urology (2024)
-
Predicting non-muscle invasive bladder cancer outcomes using artificial intelligence: a systematic review using APPRAISE-AI
npj Digital Medicine (2024)
-
To warrant clinical adoption AI models require a multi-faceted implementation evaluation
npj Digital Medicine (2024)
-
Knowledge-based, computerized, patient clinical decision support system for perioperative pain, nausea and constipation management: a clinical feasibility study
Journal of Clinical Monitoring and Computing (2024)