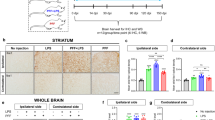
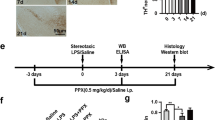
Abstract
Activation of microglia by classical inflammatory mediators can convert astrocytes into a neurotoxic A1 phenotype in a variety of neurological diseases1,2. Development of agents that could inhibit the formation of A1 reactive astrocytes could be used to treat these diseases for which there are no disease-modifying therapies. Glucagon-like peptide-1 receptor (GLP1R) agonists have been indicated as potential neuroprotective agents for neurologic disorders such as Alzheimer’s disease and Parkinson’s disease3,4,5,6,7,8,9,10,11,12,13. The mechanisms by which GLP1R agonists are neuroprotective are not known. Here we show that a potent, brain-penetrant long-acting GLP1R agonist, NLY01, protects against the loss of dopaminergic neurons and behavioral deficits in the α-synuclein preformed fibril (α-syn PFF) mouse model of sporadic Parkinson’s disease14,15. NLY01 also prolongs the life and reduces the behavioral deficits and neuropathological abnormalities in the human A53T α-synuclein (hA53T) transgenic mouse model of α-synucleinopathy-induced neurodegeneration16. We found that NLY01 is a potent GLP1R agonist with favorable properties that is neuroprotective through the direct prevention of microglial-mediated conversion of astrocytes to an A1 neurotoxic phenotype. In light of its favorable properties, NLY01 should be evaluated in the treatment of Parkinson’s disease and related neurologic disorders characterized by microglial activation.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Liddelow, S. A. & Barres, B. A. Reactive astrocytes: production, function, and therapeutic potential. Immunity 46, 957–967 (2017).
Liddelow, S. A. et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 541, 481–487 (2017).
Athauda, D. & Foltynie, T. The glucagon-like peptide 1 (GLP) receptor as a therapeutic target in Parkinson’s disease: mechanisms of action. Drug Discov. Today 21, 802–818 (2016).
Aviles-Olmos, I. et al. Exenatide and the treatment of patients with Parkinson’s disease. J. Clin. Invest. 123, 2730–2736 (2013).
Chen, S. et al. Glucagon-like peptide-1 protects hippocampal neurons against advanced glycation end product-induced tau hyperphosphorylation. Neuroscience 256, 137–146 (2014).
Harkavyi, A. & Whitton, P. S. Glucagon-like peptide 1 receptor stimulation as a means of neuroprotection. Br. J. Pharmacol. 159, 495–501 (2010).
Li, Y. et al. Liraglutide is neurotrophic and neuroprotective in neuronal cultures and mitigates mild traumatic brain injury in mice. J. Neurochem. 135, 1203–1217 (2015).
Li, Y. et al. GLP-1 receptor stimulation preserves primary cortical and dopaminergic neurons in cellular and rodent models of stroke and Parkinsonism. Proc. Natl Acad. Sci. USA 106, 1285–1290 (2009).
Meier, J. J. GLP-1 receptor agonists for individualized treatment of type 2 diabetes mellitus. Nat. Rev. Endocrinol. 8, 728–742 (2012).
Rachmany, L. et al. Exendin-4 induced glucagon-like peptide-1 receptor activation reverses behavioral impairments of mild traumatic brain injury in mice. Age (Dordr.) 35, 1621–1636 (2013).
Teramoto, S. et al. Exendin-4, a glucagon-like peptide-1 receptor agonist, provides neuroprotection in mice transient focal cerebral ischemia. J. Cereb. Blood Flow Metab. 31, 1696–1705 (2011).
Tweedie, D. et al. Blast traumatic brain injury-induced cognitive deficits are attenuated by preinjury or postinjury treatment with the glucagon-like peptide-1 receptor agonist, exendin-4. Alzheimers Dement. 12, 34–48 (2016).
Athauda, D. et al. Exenatide once weekly versus placebo in Parkinson’s disease: a randomised, double-blind, placebo-controlled trial. Lancet 390, 1664–1675 (2017).
Luk, K. C. et al. Pathological α-synuclein transmission initiates Parkinson-like neurodegeneration in nontransgenic mice. Science 338, 949–953 (2012).
Mao, X. et al. Pathological α-synuclein transmission initiated by binding lymphocyte-activation gene 3. Science 353, aah3374 (2016).
Lee, M. K. et al. Human α-synuclein-harboring familial Parkinson’s disease-linked Ala-53 → Thr mutation causes neurodegenerative disease with α-synuclein aggregation in transgenic mice. Proc. Natl Acad. Sci. USA 99, 8968–8973 (2002).
Cabezas, R. et al. Astrocytic modulation of blood brain barrier: perspectives on Parkinson’s disease. Front. Cell. Neurosci. 8, 211 (2014).
Gray, M. T. & Woulfe, J. M. Striatal blood–brain barrier permeability in Parkinson’s disease. J. Cereb. Blood Flow Metab. 35, 747–750 (2015).
Polinski, N. K. et al. Best practices for generating and using alpha-synuclein pre-formed fibrils to model parkinson’s disease in rodents. J. Parkinsons Dis. https://doi.org/10.3233/JPD-171248 (2018).
Brahmachari, S. et al. Activation of tyrosine kinase c-Abl contributes to α-synuclein-induced neurodegeneration. J. Clin. Invest. 126, 2970–2988 (2016).
Zhang, Y. et al. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J. Neurosci. 34, 11929–11947 (2014).
Kim, C., Lee, H. J., Masliah, E. & Lee, S. J. Non-cell-autonomous neurotoxicity of α-synuclein through microglial Toll-like receptor 2. Exp. Neurobiol. 25, 113–119 (2016).
Daher, J. P. et al. Neurodegenerative phenotypes in an A53T α-synuclein transgenic mouse model are independent of LRRK2. Hum. Mol. Genet. 21, 2420–2431 (2012).
Harms, A. S. et al. Peripheral monocyte entry is required for alpha-synuclein induced inflammation and neurodegeneration in a model of Parkinson disease. Exp. Neurol. 300, 179–187 (2018).
Martinez, F. O. & Gordon, S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep. 6, 13 (2014).
Allen Reish, H. E. & Standaert, D. G. Role of α-synuclein in inducing innate and adaptive immunity in Parkinson disease. J. Parkinsons Dis. 5, 1–19 (2015).
Goncalves, A. et al. Protective effect of a GLP-1 analog on ischemia–reperfusion induced blood–retinal barrier breakdown and inflammation. Invest. Ophthalmol. Vis. Sci. 57, 2584–2592 (2016).
Gullo, F. et al. Plant polyphenols and exendin-4 prevent hyperactivity and TNF-α release in LPS-treated in vitro neuron/astrocyte/microglial networks. Front. Neurosci. 11, 500 (2017).
Kim, S., Moon, M. & Park, S. Exendin-4 protects dopaminergic neurons by inhibition of microglial activation and matrix metalloproteinase-3 expression in an animal model of Parkinson’s disease. J. Endocrinol. 202, 431–439 (2009).
Lee, C.-H. et al. Activation of glucagon-like peptide-1 receptor promotes neuroprotection in experimental autoimmune encephalomyelitis by reducing neuroinflammatory responses. Mol. Neurobiol. 55, 3007–3020 (2018).
Li, Y. et al. Exendin-4 ameliorates motor neuron degeneration in cellular and animal models of amyotrophic lateral sclerosis. PLoS ONE 7, e32008 (2012).
Wu, H.-Y., Tang, X.-Q., Liu, H., Mao, X.-F. & Wang, Y.-X. Both classic Gs-cAMP/PKA/CREB and alternative Gs-cAMP/PKA/p38β/CREB signal pathways mediate exenatide-stimulated expression of M2 microglial markers. J. Neuroimmunol. 316, 17–22 (2018).
Ventorp, F. et al. Exendin-4 treatment improves LPS-induced depressive-like behavior without affecting pro-inflammatorycytokines. J. Parkinsons Dis. 7, 263–273 (2017).
Ji, C. et al. A novel dual GLP-1 and GIP receptor agonist is neuroprotective in the MPTP mouse model of Parkinson’s disease by increasing expression of BNDF. Brain Res. 1634, 1–11 (2016).
Salcedo, I., Tweedie, D., Li, Y. & Greig, N. H. Neuroprotective and neurotrophic actions of glucagon-like peptide-1: an emerging opportunity to treat neurodegenerative and cerebrovascular disorders. Br. J. Pharmacol. 166, 1586–1599 (2012).
Volpicelli-Daley, L. A., Luk, K. C. & Lee, V. M. Addition of exogenous α-synuclein preformed fibrils to primary neuronal cultures to seed recruitment of endogenous α-synuclein to Lewy body and Lewy neurite-like aggregates. Nat. Protoc. 9, 2135–2146 (2014).
Kim, T. H. et al. Mono-PEGylated dimeric exendin-4 as high receptor binding and long-acting conjugates for type 2 anti-diabetes therapeutics. Bioconjug. Chem. 22, 625–632 (2011).
Kim, T. H. et al. Site-specific PEGylated exendin-4 modified with a high molecular weight trimeric PEG reduces steric hindrance and increases type 2 antidiabetic therapeutic effects. Bioconjug. Chem. 23, 2214–2220 (2012).
Karuppagounder, S. S. et al. The c-Abl inhibitor, nilotinib, protects dopaminergic neurons in a preclinical animal model of Parkinson’s disease. Sci. Rep. 4, 4874 (2014).
Lee, Y. et al. Parthanatos mediates AIMP2-activated age-dependent dopaminergic neuronal loss. Nat. Neurosci. 16, 1392–1400 (2013).
Kim, S. et al. GBA1 deficiency negatively affects physiological α-synuclein tetramers and related multimers. Proc. Natl Acad. Sci. USA 115, 798–803 (2018).
Panicker, N. et al. Fyn kinase regulates microglial neuroinflammatory responses in cell culture and animal models of Parkinson’s disease. J. Neurosci. 35, 10058–10077 (2015).
Andrabi, S. A. et al. Iduna protects the brain from glutamate excitotoxicity and stroke by interfering with poly(ADP-ribose) polymer-induced cell death. Nat. Med. 17, 692–699 (2011).
Wang, Y. et al. A nuclease that mediates cell death induced by DNA damage and poly(ADP-ribose) polymerase-1. Science 354, aad6872 (2016).
Acknowledgements
All relevant ethical regulations were followed. This work was supported by grants from the NIH/NINDS NS38377 (H.S.K., V.L.D. and T.M.D.), NIH/NINDS NS082205 and NS098006 (H.S.K.), Maryland Stem Cell Research Foundation 2012-MSCRFE-0059 (H.S.K.), the JPB Foundation (T.M.D.), NIH/National Institute on Aging grant 1K01AG056841-01(X.M.), the American Parkinson Disease Association (APDA) Research Grant Awards (X.M.) and the National Research Foundation of Korea NRF-2016R1D1A1B03934847 (D.H.N.). We acknowledge the joint participation by the Adrienne Helis Malvin Medical Research Foundation and the Diana Helis Henry Medical Research Foundation through their direct engagement in the continuous active conduct of medical research in conjunction with The Johns Hopkins Hospital and the Johns Hopkins University School of Medicine and the Foundation’s Parkinson’s Disease Programs M-1 (T.M.D. and V.L.D.), M-2 (T.M.D. and V.L.D.), H-2014 (T.M.D.), M-2014 (H.S.K., T.M.D. and V.L.D.), H-1 (H.S.K.), H-2013 (H.S.K.). T.M.D. is the Leonard and Madlyn Abramson Professor in Neurodegenerative Diseases. We thank Neuraly Inc. for providing NLY01.
Author information
Authors and Affiliations
Contributions
S.P.Y. and T.-I.K. designed the majority of the experiments, performed the experiments, analyzed data and wrote the manuscript. N.P., S.M.K., Y.O., J.-S.P., S.-H.K., S.-U.K. and D.K. performed experiments and data interpretation. Y.J.P. injected NLY01 into mice. S.S.K. performed HPLC analysis. H.P., S.K., N.O., N.A.K., Sa.L., J.H.L., M.K. and D.A. performed sample preparation and helped with experiments. S.B. and X.M. provided and managed mice and PFF, and helped with data interpretation. K.C.L. and D.H.N. provided and made NLY01. V.V.R. performed mouse pharmacokinetic experiments and data analysis. Y.L., S.H.L., S.A.L. and B.A.B. preformed manuscript writing, review and editing. Z.M. provided human postmortem brain samples. V.L.D., Se.L., T.M.D. and H.S.K. supervised the project, formulated the hypothesis, designed experiments, analyzed data and wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
Z.M., V.L.D., Se.L., T.M.D. and H.S.K are co-founders of Neuraly Inc. and hold ownership equity in the company. This arrangement has been reviewed and approved by the Johns Hopkins University in accordance with its conflict of interest policies. V.V.R. is the CSO of Neuraly Inc.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–22 and Supplementary Tables 1–6
Rights and permissions
About this article
Cite this article
Yun, S.P., Kam, TI., Panicker, N. et al. Block of A1 astrocyte conversion by microglia is neuroprotective in models of Parkinson’s disease. Nat Med 24, 931–938 (2018). https://doi.org/10.1038/s41591-018-0051-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41591-018-0051-5
This article is cited by
-
α-Synuclein oligomers potentiate neuroinflammatory NF-κB activity and induce Cav3.2 calcium signaling in astrocytes
Translational Neurodegeneration (2024)
-
Hypoxia inducible factor-1α regulates microglial innate immune memory and the pathology of Parkinson’s disease
Journal of Neuroinflammation (2024)
-
Microglial ferroptotic stress causes non-cell autonomous neuronal death
Molecular Neurodegeneration (2024)
-
Targeting synapse function and loss for treatment of neurodegenerative diseases
Nature Reviews Drug Discovery (2024)
-
Microglia in neuroimmunopharmacology and drug addiction
Molecular Psychiatry (2024)