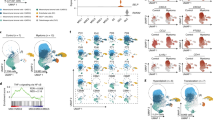
Abstract
Human bone marrow permanently harbors high numbers of neutrophils, and a tumor-supportive bias of these cells could significantly impact bone marrow-confined malignancies. In individuals with multiple myeloma, the bone marrow is characterized by inflammatory stromal cells with the potential to influence neutrophils. We investigated myeloma-associated alterations in human marrow neutrophils and the impact of stromal inflammation on neutrophil function. Mature neutrophils in myeloma marrow are activated and tumor supportive and transcribe increased levels of IL1B and myeloma cell survival factor TNFSF13B (BAFF). Interactions with inflammatory stromal cells induce neutrophil activation, including BAFF secretion, in a STAT3-dependent manner, and once activated, neutrophils gain the ability to reciprocally induce stromal activation. After first-line myeloid-depleting antimyeloma treatment, human bone marrow retains residual stromal inflammation, and newly formed neutrophils are reactivated. Combined, we identify a neutrophil–stromal cell feed-forward loop driving tumor-supportive inflammation that persists after treatment and warrants novel strategies to target both stromal and immune microenvironments in multiple myeloma.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
The single-cell RNA-sequencing datasets described in this study are available on ArrayExpress under accession number E-MTAB-12760. CellRanger output files are available through Mendeley Data (https://data.mendeley.com/datasets/sm7fvt8hsg) (ref. 77). Datasets can also be interactively explored at www.bmbrowser.org. Bulk RNA-sequencing data of neutrophils and ADSCs acquired after coculture are available on EGA under accession number EGAD00001010080. Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact. Source data are provided with this paper.
Code availability
The code generated during this study to analyze the single-cell datasets is available through GitHub at https://github.com/MyelomaRotterdam. Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact.
References
Diakos, C. I., Charles, K. A., McMillan, D. C. & Clarke, S. J. Cancer-related inflammation and treatment effectiveness. Lancet Oncol. 15, e493–e503 (2014).
Kumar, S. K. et al. Multiple myeloma. Nat. Rev. Dis. Prim. 3, 17046 (2017).
Kawano, Y. et al. Targeting the bone marrow microenvironment in multiple myeloma. Immunol. Rev. 263, 160–172 (2015).
de Jong, M. M. E. et al. The multiple myeloma microenvironment is defined by an inflammatory stromal cell landscape. Nat. Immunol. 22, 769–780 (2021).
Liu, Q. et al. The CXCL8–CXCR1/2 pathways in cancer. Cytokine Growth Factor Rev. 31, 61–71 (2016).
Wright, H. L., Cross, A. L., Edwards, S. W. & Moots, R. J. Effects of IL-6 and IL-6 blockade on neutrophil function in vitro and in vivo. Rheumatology 53, 1321–1331 (2014).
Zheng, Z. et al. Lung mesenchymal stromal cells influenced by TH2 cytokines mobilize neutrophils and facilitate metastasis by producing complement C3. Nat. Commun. 12, 6202 (2021).
Shaul, M. E. & Fridlender, Z. G. Tumour-associated neutrophils in patients with cancer. Nat. Rev. Clin. Oncol. 16, 601–620 (2019).
Hedrick, C. C. & Malanchi, I. Neutrophils in cancer: heterogeneous and multifaceted. Nat. Rev. Immunol. 22, 173–187 (2022).
Shen, M. et al. Tumor-associated neutrophils as a new prognostic factor in cancer: a systematic review and meta-analysis. PLoS ONE 9, e98259 (2014).
Templeton, A. J. et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J. Natl Cancer Inst. 106, dju124 (2014).
Coffelt, S. B. et al. IL-17-producing γδ T cells and neutrophils conspire to promote breast cancer metastasis. Nature 522, 345–348 (2015).
Sagiv, J. Y. et al. Phenotypic diversity and plasticity in circulating neutrophil subpopulations in cancer. Cell Rep. 10, 562–573 (2015).
Furze, R. C. & Rankin, S. M. Neutrophil mobilization and clearance in the bone marrow. Immunology 125, 281–288 (2008).
Sender, R. et al. The total mass, number, and distribution of immune cells in the human body. Proc. Natl Acad. Sci. USA 120, e2308511120 (2023).
Botta, C., Gullà, A., Correale, P., Tagliaferri, P. & Tassone, P. Myeloid-derived suppressor cells in multiple myeloma: pre-clinical research and translational opportunities. Front. Oncol. 4, 348 (2014).
Perez, C. et al. Immunogenomic identification and characterization of granulocytic myeloid-derived suppressor cells in multiple myeloma. Blood 136, 199–209 (2020).
Romano, A. et al. High-density neutrophils in MGUS and multiple myeloma are dysfunctional and immune-suppressive due to increased STAT3 downstream signaling. Sci. Rep. 10, 1983 (2020).
Szudy-Szczyrek, A. et al. Prognostic value of pretreatment neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios in multiple myeloma patients treated with thalidomide-based regimen. Ann. Hematol. 99, 2881–2891 (2020).
Shi, L. et al. Elevated neutrophil-to-lymphocyte ratio and monocyte-to-lymphocyte ratio and decreased platelet-to-lymphocyte ratio are associated with poor prognosis in multiple myeloma. Oncotarget 8, 18792–18801 (2017).
Giallongo, C. et al. TLR4 signaling drives mesenchymal stromal cells commitment to promote tumor microenvironment transformation in multiple myeloma. Cell Death Dis. 10, 704 (2019).
Dimitrov, D. et al. Comparison of methods and resources for cell–cell communication inference from single-cell RNA-seq data. Nat. Commun. 13, 3224 (2022).
Jia, S. H. et al. Pre-B cell colony-enhancing factor inhibits neutrophil apoptosis in experimental inflammation and clinical sepsis. J. Clin. Invest. 113, 1318–1327 (2004).
Venkateshaiah, S. U. et al. NAMPT/PBEF1 enzymatic activity is indispensable for myeloma cell growth and osteoclast activity. Exp. Hematol. 41, 547–557 (2013).
Managò, A. et al. Extracellular nicotinate phosphoribosyltransferase binds Toll like receptor 4 and mediates inflammation. Nat. Commun. 10, 4116 (2019).
Hengeveld, P. J. & Kersten, M. J. B-cell activating factor in the pathophysiology of multiple myeloma: a target for therapy? Blood Cancer J. 5, e282 (2015).
Bossen, C. & Schneider, P. BAFF, APRIL and their receptors: structure, function and signaling. Semin. Immunol. 18, 263–275 (2006).
Shah, N., Chari, A., Scott, E., Mezzi, K. & Usmani, S. Z. B-cell maturation antigen (BCMA) in multiple myeloma: rationale for targeting and current therapeutic approaches. Leukemia 34, 985–1005 (2020).
Hamarsheh, S. A. & Zeiser, R. NLRP3 inflammasome activation in cancer: a double-edged sword. Front. Immunol. 11, 1444 (2020).
Yang, Y., Wang, H., Kouadir, M., Song, H. & Shi, F. Recent advances in the mechanisms of NLRP3 inflammasome activation and its inhibitors. Cell Death Dis. 10, 128 (2019).
Lust, J. A. et al. Induction of a chronic disease state in patients with smoldering or indolent multiple myeloma by targeting interleukin 1β-induced interleukin 6 production and the myeloma proliferative component. Mayo Clin. Proc. 84, 114–122 (2009).
Hofbauer, D. et al. β2-Microglobulin triggers NLRP3 inflammasome activation in tumor-associated macrophages to promote multiple myeloma progression. Immunity 54, 1772–1787 (2021).
Tong, D. et al. The roles of the COX2/PGE2/EP axis in therapeutic resistance. Cancer Metastasis Rev. 37, 355–368 (2018).
Munder, M. et al. Suppression of T-cell functions by human granulocyte arginase. Blood 108, 1627–1634 (2006).
Fragioudaki, M. et al. Serum BAFF levels are related to angiogenesis and prognosis in patients with multiple myeloma. Leuk. Res. 36, 1004–1008 (2012).
Moreaux, J. R. M. et al. BAFF and APRIL protect myeloma cells from apoptosis induced by interleukin 6 deprivation and dexamethasone. Blood 103, 3148–3157 (2004).
Busch, K. et al. Fundamental properties of unperturbed haematopoiesis from stem cells in vivo. Nature 518, 542–546 (2015).
Schäffler, A. & Büchler, C. Adipose tissue-derived stromal cells—basic and clinical implications for novel cell-based therapies. Stem Cells 25, 818–827 (2007).
Schust, J., Sperl, B., Hollis, A., Mayer, T. U. & Berg, T. Stattic: a small-molecule inhibitor of STAT3 activation and dimerization. Chem. Biol. 13, 1235–1242 (2006).
Friščić, J. et al. The complement system drives local inflammatory tissue priming by metabolic reprogramming of synovial fibroblasts. Immunity 54, 1002–1021 (2021).
Molejon, M. I. et al. Deciphering the cellular source of tumor relapse identifies CD44 as a major therapeutic target in pancreatic adenocarcinoma. Oncotarget 6, 7408–7423 (2015).
Yang, C. et al. Inducible formation of leader cells driven by CD44 switching gives rise to collective invasion and metastases in luminal breast carcinomas. Oncogene 38, 7113–7132 (2019).
Owattanapanich, W., Suphadirekkul, K., Kunacheewa, C., Ungprasert, P. & Prayongratana, K. Risk of febrile neutropenia among patients with multiple myeloma or lymphoma who undergo inpatient versus outpatient autologous stem cell transplantation: a systematic review and meta-analysis. BMC Cancer 18, 1126 (2018).
Lemancewicz, D. et al. Evaluation of TNF superfamily molecules in multiple myeloma patients: correlation with biological and clinical features. Leuk. Res. 37, 1089–1093 (2013).
Yu, P. F. et al. TNFα-activated mesenchymal stromal cells promote breast cancer metastasis by recruiting CXCR2+ neutrophils. Oncogene 36, 482–490 (2017).
Grégoire, M. et al. Neutrophils trigger a NF-κB dependent polarization of tumor-supportive stromal cells in germinal center B-cell lymphomas. Oncotarget 6, 16471–16487 (2015).
Friedrich, M. et al. IL-1-driven stromal–neutrophil interactions define a subset of patients with inflammatory bowel disease that does not respond to therapies. Nat. Med. 27, 1970–1981 (2021).
Zou, S. et al. Targeting STAT3 in cancer immunotherapy. Mol. Cancer 19, 145 (2020).
Chong, P. S. Y., Chng, W. J. & de Mel, S. STAT3: a promising therapeutic target in multiple myeloma. Cancers 11, 731 (2019).
Heo, S.-K. et al. Radotinib inhibits multiple myeloma cell proliferation via suppression of STAT3 signaling. PLoS ONE 17, e0265958 (2022).
Wang, Y. et al. YL064 directly inhibits STAT3 activity to induce apoptosis of multiple myeloma cells. Cell Death Discov. 4, 44 (2018).
Tai, Y.-T. et al. Role of B-cell-activating factor in adhesion and growth of human multiple myeloma cells in the bone marrow microenvironment. Cancer Res. 66, 6675–6682 (2006).
Neri, P. et al. Neutralizing B-cell-activating factor antibody improves survival and inhibits osteoclastogenesis in a severe combined immunodeficient human multiple myeloma model. Clin. Cancer Res. 13, 5903–5909 (2007).
Pilcher, W. et al. Cross center single-cell RNA sequencing study of the immune microenvironment in rapid progressing multiple myeloma. NPJ Genom. Med. 8, 3 (2023).
Moreaux, J. R. M. et al. The level of TACI gene expression in myeloma cells is associated with a signature of microenvironment dependence versus a plasmablastic signature. Blood 106, 1021–1030 (2005).
Xu, G. et al. BLyS expression and JNK activation may form a feedback loop to promote survival and proliferation of multiple myeloma cells. Cytokine 60, 505–513 (2012).
Hope, C. et al. TPL2 kinase regulates the inflammatory milieu of the myeloma niche. Blood 123, 3305–3315 (2014).
Costes, V. et al. Interleukin-1 in multiple myeloma: producer cells and their role in the control of IL-6 production. Br. J. Haematol. 103, 1152–1160 (1998).
Vangsted, A. J. et al. A functional polymorphism in the promoter region of the IL1B gene is associated with risk of multiple myeloma. Br. J. Haematol. 158, 515–518 (2012).
Hao, Y. et al. Integrated analysis of multimodal single-cell data. Cell 184, 3573–3587 (2021).
Stuart, T. et al. Comprehensive integration of single-cell data. Cell 177, 1888–1902 (2019).
Waltman, L. & van Eck, N. J. A smart local moving algorithm for large-scale modularity-based community detection. Eur. Phys. J. B 86, 471 (2013).
McInnes, L., Healy, J., Saul, N. & Großberger, L. UMAP: uniform manifold approximation and projection. J. Open Source Softw. 3, 861 (2018).
Cao, J. et al. The single-cell transcriptional landscape of mammalian organogenesis. Nature 566, 496–502 (2019).
Cheng, W. C., Saleh, F., Abuaisha Karim, B., Hughes, F. J. & Taams, L. S. Comparative analysis of immune cell subsets in peripheral blood from patients with periodontal disease and healthy controls. Clin. Exp. Immunol. 194, 380–390 (2018).
Kolde, R. Pheatmap: pretty heatmaps. R package version 1 (2012).
Mootha, V. K. et al. PGC-1α-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat. Genet. 34, 267–273 (2003).
Subramanian, A. et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl Acad. Sci. USA 102, 15545–15550 (2005).
Bunis, D. G., Andrews, J., Fragiadakis, G. K., Burt, T. D. & Sirota, M. dittoSeq: universal user-friendly single-cell and bulk RNA sequencing visualization toolkit. Bioinformatics 36, 5535–5536 (2020).
Türei, D., Korcsmáros, T. & Saez-Rodriguez, J. OmniPath: guidelines and gateway for literature-curated signaling pathway resources. Nat. Methods 13, 966–967 (2016).
Patro, R., Duggal, G., Love, M. I., Irizarry, R. A. & Kingsford, C. Salmon provides fast and bias-aware quantification of transcript expression. Nat. Methods 14, 417–419 (2017).
Soneson, C., Love, M. I. & Robinson, M. D. Differential analyses for RNA-seq: transcript-level estimates improve gene-level inferences. F1000Res 4, 1521 (2015).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014).
Liberzon, A. et al. The Molecular Signatures Database (MSigDB) hallmark gene set collection. Cell Syst. 1, 417–425 (2015).
Blighe, K., Rana, S. & Lewis, M. EnhancedVolcano: publication-ready volcano plots with enhanced colouring and labeling. R package version 1 (2019).
Wickham, H. ggplot2: Elegant Graphics for Data Analysis, 2nd edn (Springer-Verlag, 2016).
de Jong, M. M. E. & Cupedo, T. A stromal cell-neutrophil axis promotes tumor-supportive bone marrow inflammation in newly diagnosed and treated myeloma. Mendeley Data https://doi.org/10.17632/sm7fvt8hsg.2 (2023).
Durie, B. G. M. et al. International uniform response criteria for multiple myeloma. Leukemia 20, 1467–1473 (2006).
Acknowledgements
We thank members of Myeloma Research Rotterdam and the Department of Hematology for critical discussions and reading of the paper and the participants, families and nurses for their contributions to this study. We thank T. Langerak (Department of Immunology, Erasmus MC, Rotterdam) for providing peripheral blood samples of healthy individuals. H.B. was supported by the Wilhelm–Sander Foundation. This work was supported by an International Myeloma Society and Paula and Rodger Riney Foundation translational research award (T.C.).
Author information
Authors and Affiliations
Contributions
Conceptualization: M.M.E.d.J. and T.C. Methodology: M.M.E.d.J. and T.C. Investigation: M.M.E.d.J., C.F., N.P., M.V., T.v.H., S.T., Á.C., M.K.A. and M.B.-H. Formal analysis: M.M.E.d.J., C.F., N.P., M.V., T.v.H., R.M.H., G.v.B., M.A.S., Á.C., M.K.A., M.B.-H., H.B. and T.C. Resources: P.C.v.d.W., P.M., F.G., M.v.D., A.B. and P.S. Data curation: M.M.E.d.J., R.M.H. and G.v.B. Visualization: M.M.E.d.J. Writing, original draft: M.M.E.d.J. and T.C. Writing, review and editing: all authors. Funding acquisition: T.C. and P.S. Supervision: T.C. and P.S. Project administration: T.C. and P.S.
Corresponding authors
Ethics declarations
Competing interests
M.B.-H. has received speaker’s fees from Sanofi and Pfizer and workshop sponsoring from Novartis. F.G. has received honoraria from Amgen, Celgene, Janssen, Takeda, BMS, AbbVie and GlaxoSmithKline and is on advisory boards for Roche, Adaptive Biotechnologies, Oncopeptides, bluebird bio and Pfizer. P.M. is on advisory boards for and has received honoraria from Janssen. A.B. is on advisory boards for and has received honoraria from Janssen, Sanofi, Amgen and BMS. P.S. has received research funding from Karyopharm, Janssen, Amgen, Celgene and BMS and is on the advisory board for Pfizer. T.C. has received research support from Janssen. The other authors declare no competing interests.
Peer review
Peer review information
Nature Immunology thanks Ivan Ballesteros and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: L. A. Dempsey, in collaboration with the Nature Immunology team. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Related to Fig. 1.
A) Gating strategy for neutrophils and monocytes/macrophages of unfractionated bone marrow from aspirates for single-cell RNA sequencing experiments B) Gating strategy for quantification of mature neutrophils, monocytes and macrophages in unfractionated bone marrow aspirates. Fractions were calculated as percentages of ‘live cells’-gate (as depicted in (A)) C) UMAP of the combined dataset of 47,497 cells from NDMM patients and 34,531 cells from controls showing 16 clusters identified by marker genes in (D) and (E). Colors represent clusters D) Marker gene transcription to demarcate the 3 major myeloid populations: progenitors (MPO+), mononuclear phagocytes (CD14+) and neutrophils (MME+ marks mature neutrophils) E) Transcription of marker genes of the myeloid dataset F) Monocle 3 pseudotime analysis of the myeloid dataset. ‘S’ represents an arbitrarily selected starting point of trajectory calculations G) Cluster distribution of myeloid clusters per condition. Colors represent clusters H) Cluster distribution of myeloid clusters per patient. Colors represent clusters. ‘MM’, MM-patient. ‘CBM’, control I) Chord diagram visualizing frequency of interactions between MSC subsets from 4 and myeloid subsets of MM patients J) Predicted ligand–receptor interactions between MSC clusters from 4 and myeloid clusters from MM patients. Expression magnitudes and interaction specificity values were calculated using the statistical framework of liana22 K) Cluster distribution of neutrophil clusters per patient. Colors represent clusters. ‘MM’, MM-patient. ‘CBM’, control L) Percentages of neutrophils in the 6 remaining clusters per individual in each condition M) Transcription of interferon-response genes comparing non-cancer controls to NDMM patients FSC, forward scatter. SSC, sideward scatter. GMP, granulocyte-monocyte progenitor. Data are presented as mean ± SEM. Significance was calculated in L using the Mann–Whitney U test (two-tailed). NS P > 0.05.
Extended Data Fig. 2 Related to Figs. 1, 2 and 3.
A) Enrichment plots for selected Hallmark genesets comparing cluster MatNeu2 to cluster MatNeu1 in NDMM. FDR, false discovery rate; Nom. P, nominal p-value; NES, normalized enrichment score. B) Transcription of selected genes comparing MatNeu1 to MatNeu2 and ImmNeu in NDMM. C) TNFSF13B and IL1B transcription in neutrophil objects generated using Harmony (sample ID, sex and sequencing batch as co-variates). D) Gating strategy for CXCR1/CXCR2 expression on (im)mature neutrophils, including FITC/PE levels of relevant isotype controls and representative plot of CXCR1/CXCR2 expression on CD15+ neutrophils. E) Pre-gating for live CD10+ neutrophils to quantify CD62L and CD11b(act). F) CD11c+ and C3AR+ frequencies, or MFI of CD45, CXCR1 or CD66b on CD10+ blood neutrophils (n = 6 NDMM, and n = 5 controls). G) Absolute number of U266 or NCI-H929 cultured overnight in medium with or without BAFF. Lines depict paired samples H) UMAPs of 23,069 mononuclear phagocytes from NDMM patients and 15,489 mononuclear phagocytes from controls. Clusters identified by marker genes in (I). I) Transcription of marker genes in the mononuclear phagocyte dataset. J) IL1B transcription in mononuclear phagocytes. K) Quantification of IL1B-transcribing cells in pro-inflammatory macrophage cluster (n = 6 NDMM, and n = 4 controls). L) Percentage of pro-inflammatory macrophages (n = 6 NDMM, and n = 4 controls). M) IL1B-transcribing cells in the dendritic cell cluster (n = 6 NDMM, and n = 4 controls). N) UMAPs of 7,457 progenitors from NDMM and 10,102 progenitors from controls identified by marker genes in (O). O) Transcription of marker genes in the progenitor dataset. P) IL1B-transcribing cells in the GMP cluster (n = 6 NDMM, and n = 4 controls) Q) Transcription of TNFSF13B in mononuclear phagocytes R) TNFSF13B-transcribing cells in classical monocytes, pro-inflammatory macrophages and anti-inflammatory macrophages (n = 6 NDMM, and n = 4 controls). GMP, granulocyte-monocyte progenitors. Data are presented as mean ± SEM. Significance calculated in A using pre-ranked gene set enrichment analysis (GSEA), in F, K-M, P, and R using Mann–Whitney U test (two-tailed), and in G using Willcoxon Rank Sum test (two-tailed); *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, NS P > 0.05.
Extended Data Fig. 3 Related to Fig. 4.
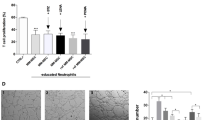
A) iMSC-related gene transcription in primary BM MSC with or without recombinant IL-1β. B) Gating for CD10+ neutrophils after coculture with (i)MSC, including representative plots after culture. C) C3AR, CD11b(act), and CD11c frequencies, or MFI of CD66b and CD45 on CD10+ neutrophils cultured alone (‘neutro only’), with rhIL-1β, or on ADSC-derived iMSC (n = 4; 3 experiments). D) BAFF protein in supernatant of neutrophils cultured alone, with IL-1β, or on ADSC-derived iMSC (3 donors; 2 experiments) E) BAFF or F) IL-1β protein in supernatant of (i)MSC cultured alone, neutrophils cultured alone, or neutrophils cultured on non-inflammatory ADSC-derived MSC or iMSC (4 donors; 2 experiments) G) BAFF H) or IL-1β protein in supernatant of neutrophils cultured alone, with different quantities of myeloma cells, or on ADSC-derived iMSC (4 donors; 2 experiments) I) Selected transcripts in unstimulated or IL-1β-stimulated ADSC. J) iMSC-signature enrichment plots (from DEGs of scRNA-seq in4), and selected Hallmark genesets for neutrophils cultured on iMSC or cultured alone. FDR, false discovery rate; Nom. P, nominal p-value; NES, normalized enrichment score K) C3AR, CD11b(act), and CD11c frequencies, or MFI of CD66b and CD45 on CD10+ neutrophils cultured alone, or on ADSC-derived MSC or iMSC (6 donors; 3 experiments). L) BAFF or M) IL-1β protein in supernatant of neutrophils cultured alone, or on ADSC-derived MSC, or iMSC (6 donors; 3 experiments) N) Volcano plot depicting DEGs of neutrophils cultured on ADSC-derived iMSC or cultured alone. Log2FoldChange cutoff, 1; adjusted p-value cutoff 10-4. Genes in red are related to activation O) Enrichment plots for MatNeu2 signature generated from data in Fig. 1 comparing neutrophils cultured on iMSC to cultured alone, or cultured on non-inflammatory MSC to neutrophils cultured alone. Data presented as mean ± SEM. Significance calculated in A and D using Mann-Whitney U test (two-tailed), in C, E, F, G, H, K, L and M using Wilcoxon Rank Sum test (two-tailed), in J and O using pre-ranked gene set enrichment analysis, and in I and N using Wald test (two-tailed) followed by Benjamini–Hochberg correction. *P ≤ 0.05, **P ≤ 0.01, ****P ≤ 0.0001, NS P > 0.05. Lines depict paired samples.
Extended Data Fig. 4 Related to Figs. 5 and 6.
A) SOCS3 in neutrophils cultured alone, with G-CSF (positive control), DMSO or Stattic (5 donors; 3 experiments). B) DAPI-negative neutrophils cultured alone, on ADSC-derived MSC or iMSC with DMSO, or on iMSC with Stattic (5 donors; 5 experiments). C) MFI of CD10 on neutrophils cultured with ADSC-derived MSC, iMSC + DMSO, or iMSC + Stattic. (6 donors; 5 experiments) D) Histograms of pSTAT3, pSTAT1, and pSTAT5 in HL60 cells treated with G-CSF (pSTAT3); GM-CSF (pSTAT5); or no cytokines (pSTAT1 was constitutively present) in the presence of Static or DMSO (n = 3 [pSTAT3, pSTAT1]; n = 5 [pSTAT5]) E) MFI of CD45 or CD66b on neutrophils cultured on ADSC-derived MSC or iMSC in the presence of Stattic/DMSO (6 donors; 5 experiments) F) Anti-human IL-6 or isotype control with 100 ng/mL recombinant IL-6 on luc2P-reporter cells. RLU, relative light units G) MFI of CD66b, CXCR1 and CD45 on CD10+ neutrophils cultured alone, on ADSC-derived MSC or iMSC in the presence of anti-IL6 or isotype (5 donors; 3 experiments). H) Recombinant IL-6 on luc2P-reporter cells. I) CD11b(act), CD11c, and C3AR frequencies, or MFI of CD66b, CXCR1 and CD45 on CD10+ neutrophils cultured alone, with IL-6, or on ADSC-derived iMSC (n = 5; 3 experiments). J) BAFF protein in supernatant of neutrophils cultured alone, with IL-6, or on ADSC-derived iMSC (n = 6; 3 experiments). K) IL-1β protein in supernatant of neutrophils cultured as in (J). L) CXCL8 and IL6 in MSC with or without IL-1β, or with ADSC-derived (i)MSC-conditioned neutrophils, with or without anti-IL-1β or isotype (3 donors; 3 experiments). M) CXCL8, IL6 and LIF in primary BM-derived MSC with or without IL-1β, with primary BM-derived (i)MSC-conditioned neutrophils, with or without anti-IL-1β or isotype (3 donors; 3 experiments). N) Neutrophil transcripts in indicated cultures after neutrophil removal (n = 5; 3 experiments). Data as mean ± SEM. Significance calculated in A, B, and C using Mann-Whitney U test (two-tailed), in D, E, G, I-K, M using Wilcoxon Rank Sum test (two-tailed) and in B and C using Wald test (two-tailed) followed by Benjamini–Hochberg correction. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, ****P ≤ 0.0001, NS P > 0.05. Lines depict paired samples.
Extended Data Fig. 5 Related to Figs. 6, 7 and 8.
A) Transcription of iMSC-related genes in unstimulated MSC (blue), and MSC cultured in the supernatant of iMSC (green), with MSC-conditioned neutrophils (grey) or cultured with iMSC-conditioned neutrophils (orange). (i)MSC were generated from ADSC. Dotted lined depict paired samples (n = 5, collected over 3 experiments) B) Enrichment plots for the iMSC-signature (generated from DEGs of single cell RNA sequencing results in4), and iMSC-like cells signature (generated from data in Extended Data Fig. 3I) comparing MSC cultured with iMSC-conditioned neutrophils with MSC cultured in supernatant of iMSC C) Transcription of iMSC-related genes in unstimulated or IL-1β stimulated ADSC, or co-cultured with myeloma cell lines (n = 3, collected over 3 experiments) D) Transcription of SELP and E) PTGS2 in the non-hematopoietic compartment of controls and patients post-consolidation. F) Cluster distribution per patient in non-hematopoietic object. Colors represent clusters. ‘TMM’, treated MM-patient (post-consolidation). ‘CBM’, control G) Percentages of MSCs within the discrete MSC clusters per individual (n = 7 treated patients, and n = 6 controls) H) Percentage of MSC with a positive ‘iMSC-like cell’ score split by treatment-type and MRD-status after consolidation therapy (VTD, bortezomib, thalidomide, dexamethasone; D-VTD, daratumumab, bortezomib, thalidomide, dexamethasone; MRD, minimal residual disease)(n = 7 treated patients, and n = 6 controls) I) Transcription of IL6 and CXCL8 comparing non-cancer controls to patients after consolidation therapy in the non-hematopoietic object generated using Harmony (with sample ID, sex and sequencing batch as co-variates) J) Marker gene transcription in the 9 neutrophil clusters, split by condition (light green, controls; dark green, treated MM patients) K) Transcription of selected genes comparing clusters MatNeu2a and MatNeu2b to clusters MatNeu1 and ImmNeu in MM patients post consolidation L) Cluster distribution per patient in post-treatment neutrophil object. Colors represent clusters. ‘TMM’, treated MM-patient (post-consolidation). ‘CBM’, control. Data are presented as mean ± SEM. Significance was calculated in A using the Wald test (two-tailed) followed by a Benjamini–Hochberg correction, in B using pre-ranked geneset enrichment analysis (GSEA), and in H and G using the Mann–Whitney U test (two-tailed), ***P ≤ 0.001, ****P ≤ 0.0001, NS P > 0.05.
Extended Data Fig. 6 Related to Fig. 8.
A) Predicted ligand–receptor interactions between MSC cluster from Fig. 7 and neutrophil clusters of MM patients after treatment. Expression magnitudes and interaction specificity values were calculated using the statistical framework of liana22 B) Predicted ligand–receptor interactions between MSC cluster from Fig. 7 and neutrophil clusters of MM patients at diagnosis. Expression magnitudes and interaction specificity values were calculated using the statistical framework of liana22 C) Transcription of IL1B and TNFSF13B comparing non-cancer controls to patients after consolidation therapy in the neutrophil object generated using Harmony (with sample ID, sex and sequencing batch as co-variates) D) Transcription of CCL2, CCL3, CXCL2 and IL1B in macrophages comparing those of treated MM patients with those of NDMM patients and controls E) BAFF protein in BM plasma of N = 18 controls, N = 24 NDMM patients and N = 18 patients after induction treatment F) BAFF protein in BM plasma of N = 52 patients after consolidation therapy, split by MRD status post-consolidation G) BAFF protein in BM plasma of N = 52 patients after consolidation therapy, split by IMWG Uniform Response criteria therapy response78 H) IL-1β protein in BM plasma of N = 52 patients after consolidation therapy, split by MRD status post-consolidation I) IL-1β protein in BM plasma of N = 52 patients after consolidation therapy, split by IMWG Uniform Response criteria therapy response78. PR, partial response. VGPR, very good partial response. CR, complete response. sCR, stringent complete response. Data are presented as mean ± SEM. Significance was calculated in B-F, using the Mann–Whitney U test (two-tailed), *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, ****P ≤ 0.0001.
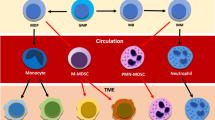
Extended Data Fig. 7 Model.
Interaction with myeloma-specific inflammatory mesenchymal stromal cells (iMSC) leads to an activated phenotype and transcriptome in mature bone marrow neutrophils. Once activated, neutrophils display features of inflammasome priming and secrete IL-1β, which in turn can induce de novo stromal inflammation. Moreover, via iMSC-induced STAT3-signaling, activated mature neutrophils secrete BAFF, a ligand for myeloma-expressed BCMA that induces myeloma cell survival and proliferation. Both iMSC and activated, BAFF-producing neutrophils remain present in the bone marrow of patients after treatment. At diagnosis, when tumor burden is high, BAFF protein is consumed, and detected at low levels in bone marrow plasma. However, when patients are in remission, and tumor cells are mostly gone, continued BAFF production by neutrophils leads to high levels of BAFF protein in the bone marrow, potentially providing residual, therapy-resistant clones with a survival benefit. Therefore, by generating pro-tumor bone marrow inflammation that persists after treatment, neutrophil – stromal cell interactions may impact progression and recurrence of disease.
Supplementary information
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Statistical source data.
Source Data Fig. 7
Statistical source data.
Source Data Fig. 8
Statistical source data.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 6
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
de Jong, M.M.E., Fokkema, C., Papazian, N. et al. An IL-1β-driven neutrophil–stromal cell axis fosters a BAFF-rich protumor microenvironment in individuals with multiple myeloma. Nat Immunol (2024). https://doi.org/10.1038/s41590-024-01808-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41590-024-01808-x
This article is cited by
-
The enduring neutrophilâstroma dance of multiple myeloma
Nature Immunology (2024)