Abstract
Few cancers can be targeted efficiently by engineered T cell strategies. Here, we show that γδ T cell antigen receptor (γδ TCR)-mediated cancer metabolome targeting can be combined with targeting of cancer-associated stress antigens (such as NKG2D ligands or CD277) through the addition of chimeric co-receptors. This strategy overcomes suboptimal γ9δ2 TCR engagement of αβ T cells engineered to express a defined γδ TCR (TEGs) and improves serial killing, proliferation and persistence of TEGs. In vivo, the NKG2D-CD28WT chimera enabled control only of liquid tumors, whereas the NKG2D-4-1BBCD28TM chimera prolonged persistence of TEGs and improved control of liquid and solid tumors. The CD277-targeting chimera (103-4-1BB) was the most optimal co-stimulation format, eradicating both liquid and solid tumors. Single-cell transcriptomic analysis revealed that NKG2D-4-1BBCD28TM and 103-4-1BB chimeras reprogram TEGs through NF-κB. Owing to competition with naturally expressed NKG2D in CD8+ TEGs, the NKG2D-4-1BBCD28TM chimera mainly skewed CD4+ TEGs toward adhesion, proliferation, cytotoxicity and less exhausted signatures, whereas the 103-4-1BB chimera additionally shaped the CD8+ subset toward a proliferative state.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
Sequence data for this study have been deposited into Gene Expression Omnibus under the accession code GSE244053. Source data are provided with this paper.
Code availability
In this study, we used published and publicly available software packages to conduct all data analyses. Source data and scripts for the single-cell RNA-sequencing analyses and visualization are deposited in Zenodo (https://doi.org/10.5281/zenodo.8378941).
References
Sebestyen, Z. et al. RhoB mediates phosphoantigen recognition by Vγ9Vδ2 T cell receptor. Cell Rep. 15, 1973–1985 (2016).
Rigau, M. et al. Butyrophilin 2A1 is essential for phosphoantigen reactivity by γδ T cells. Science 367, eaay5516 (2020).
Karunakaran, M. M. et al. Butyrophilin-2A1 directly binds germline-encoded regions of the Vγ9Vδ2 TCR and is essential for phosphoantigen sensing. Immunity 52, 487–498 (2020).
Mamedov, M. R. et al. CRISPR screens decode cancer cell pathways that trigger γδ T cell detection. Nature 621, 188–195 (2023).
Harly, C. et al. Key implication of CD277/butyrophilin-3 (BTN3A) in cellular stress sensing by a major human γδ T-cell subset. Blood 120, 2269–2279 (2012).
Hsiao, C. C. et al. Ligand-induced interactions between butyrophilin 2A1 and 3A1 internal domains in the HMBPP receptor complex. Cell Chem. Biol. 29, 985–995 (2022).
Yuan, L. et al. Phosphoantigens glue butyrophilin 3A1 and 2A1 to activate Vγ9Vδ2 T cells. Nature 621, 840–848 (2023).
Vyborova, A. et al. γ9δ2T cell diversity and the receptor interface with tumor cells. J. Clin. Invest. 130, 4637–4651 (2020).
Gründer, C. et al. γ9 and δ2CDR3 domains regulate functional avidity of T cells harboring γ9δ2TCRs. Blood 120, 5153–5162 (2012).
Straetemans, T. et al. Untouched GMP-ready purified engineered immune cells to treat cancer. Clin. Cancer Res. 21, 3957–3968 (2015).
de Witte, M. et al. First in human clinical responses and persistence data on TEG001: a next generation of engineered αβ T cells targeting AML and MM with a high affinity γ9δ2TCR. Blood 140, 12737–12739 (2022).
Dekkers, J. F. et al. Uncovering the mode of action of engineered T cells in patient cancer organoids. Nat. Biotechnol. 41, 60–69 (2022).
Fuertes, M. B., Domaica, C. I. & Zwirner, N. W. Leveraging NKG2D ligands in immuno-oncology. Front. Immunol. 12, 713158 (2021).
Marcu-Malina, V. et al. Redirecting αβ T cells against cancer cells by transfer of a broadly tumor-reactive γδ T-cell receptor. Blood 118, 50–59 (2011).
Benyamine, A. et al. BTN3A is a prognosis marker and a promising target for Vγ9Vδ2 T cells based-immunotherapy in pancreatic ductal adenocarcinoma (PDAC). Oncoimmunology 7, e1372080 (2017).
Palakodeti, A. et al. The molecular basis for modulation of human Vγ9Vδ2 T cell responses by CD277/butyrophilin-3 (BTN3A)-specific antibodies. J. Biol. Chem. 287, 32780–32790 (2012).
De Gassart, A. et al. Development of ICT01, a first-in-class, anti-BTN3A antibody for activating Vγ9Vδ2 T cell-mediated antitumor immune response. Sci. Transl. Med. 13, eabj0835 (2021).
Mansilla-Soto, J. et al. HLA-independent T cell receptors for targeting tumors with low antigen density. Nat. Med. 28, 345–352 (2022).
Oda, S. K. et al. A Fas-4-1BB fusion protein converts a death to a pro-survival signal and enhances T cell therapy. J. Exp. Med. 217, e20191166 (2020).
Liu, X. et al. A chimeric switch-receptor targeting PD1 augments the efficacy of second-generation CAR T cells in advanced solid tumors. Cancer Res. 76, 1578–1590 (2016).
Melenhorst, J. J. et al. Decade-long leukaemia remissions with persistence of CD4+ CAR T cells. Nature 602, 503–509 (2022).
Anderson, N. D. et al. Transcriptional signatures associated with persisting CD19 CAR-T cells in children with leukemia. Nat. Med. 29, 1700–1709 (2023).
Xia, A. et al. T cell dysfunction in cancer immunity and immunotherapy. Front. Immunol. 10, 1719 (2019).
Vandenberghe, P. et al. Ligation of the CD5 or CD28 molecules on resting human T cells induces expression of the early activation antigen CD69 by a calcium- and tyrosine kinase-dependent mechanism. Immunology 78, 210–217 (1993).
Kunzmann, V. et al. Stimulation of γδ T cells by aminobisphosphonates and induction of antiplasma cell activity in multiple myeloma. Blood 96, 384–392 (2000).
Weinkove, R. et al. Selecting costimulatory domains for chimeric antigen receptors: functional and clinical considerations. Clin. Transl. Immunol. 8, e1049 (2019).
Kuball, J. et al. Cooperation of human tumor-reactive CD4+ and CD8+ T cells after redirection of their specificity by a high-affinity p53A2.1-specific TCR. Immunity 22, 117–129 (2005).
Braham, M. V. J. et al. Cellular immunotherapy on primary multiple myeloma expanded in a 3D bone marrow niche model. Oncoimmunology 7, e1434465 (2018).
Eagle, R. A., Jafferji, I. & Barrow, A. D. Beyond stressed self: evidence for NKG2D ligand expression on healthy cells. Curr. Immunol. Rev. 5, 22–34 (2009).
Majzner, R. G. & Mackall, C. L. Clinical lessons learned from the first leg of the CAR T cell journey. Nat. Med. 25, 1341–1355 (2019).
Kawalekar, O. U. et al. Distinct signaling of coreceptors regulates specific metabolism pathways and impacts memory development in CAR T cells. Immunity 44, 380–390 (2016).
Philipson, B. I. et al. 4-1BB costimulation promotes CAR T cell survival through noncanonical NF-κB signaling. Sci. Signal. 13, eaay8248 (2020).
Payne, K. K. et al. BTN3A1 governs antitumor responses by coordinating αβ and γδ T cells. Science 369, 942–949 (2020).
Qi, C. et al. Claudin18.2-specific CAR T cells in gastrointestinal cancers: phase 1 trial interim results. Nat. Med. 28, 1189–1198 (2022).
Majzner, R. G. et al. GD2-CAR T cell therapy for H3K27M-mutated diffuse midline gliomas. Nature 603, 934–941 (2022).
Guerra, N. & Lanier, L. L. Emerging concepts on the NKG2D receptor–ligand axis in health and diseases. Front. Immunol. 11, 562 (2020).
Gumber, D. & Wang, L. D. Improving CAR-T immunotherapy: overcoming the challenges of T cell exhaustion. EBioMedicine 77, 103941 (2022).
Deng, Q. et al. Characteristics of anti-CD19 CAR T cell infusion products associated with efficacy and toxicity in patients with large B cell lymphomas. Nat. Med. 26, 1878–1887 (2020).
Fraietta, J. A. et al. Determinants of response and resistance to CD19 chimeric antigen receptor (CAR) T cell therapy of chronic lymphocytic leukemia. Nat. Med. 24, 563–571 (2018).
Scott, A. C. et al. TOX is a critical regulator of tumour-specific T cell differentiation. Nature 571, 270–274 (2019).
Philip, M. & Schietinger, A. CD8+ T cell differentiation and dysfunction in cancer. Nat. Rev. Immunol. 22, 209–223 (2022).
Lim, W. A. & June, C. H. The principles of engineering immune cells to treat cancer. Cell 168, 724–740 (2017).
Peng, W. et al. PD-1 blockade enhances T-cell migration to tumors by elevating IFN-γ inducible chemokines. Cancer Res. 72, 5209–5218 (2012).
Oh, D. Y. & Fong, L. Cytotoxic CD4+ T cells in cancer: expanding the immune effector toolbox. Immunity 54, 2701–2711 (2021).
Hashimoto, K. et al. Single-cell transcriptomics reveals expansion of cytotoxic CD4 T cells in supercentenarians. Proc. Natl Acad. Sci. USA 116, 24242–24251 (2019).
Li, G. et al. 4-1BB enhancement of CAR T function requires NF-κB and TRAFs. JCI Insight 3, e121322 (2018).
Hirabayashi, K. et al. Dual targeting CAR-T cells with optimal costimulation and metabolic fitness enhance antitumor activity and prevent escape in solid tumors. Nat. Cancer 2, 904–918 (2021).
Chen, S. et al. Prognostic and therapeutic significance of BTN3A proteins in tumors. J. Cancer 12, 4505–4512 (2021).
Liang, F. et al. Comprehensive analysis of BTN3A1 in cancers: mining of omics data and validation in patient samples and cellular models. FEBS Open Bio. 11, 2586–2599 (2021).
Abramson, J. S. et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet 396, 839–852 (2020).
Azangou-Khyavy, M. et al. CRISPR/Cas: from tumor gene editing to T cell-based immunotherapy of cancer. Front. Immunol. 11, 2062 (2020).
Textor, A. et al. CD28 co-stimulus achieves superior CAR T cell effector function against solid tumors than 4-1BB co-stimulus. Cancers 13, 1050 (2021).
Pichler, A. C. et al. TCR-independent CD137 (4-1BB) signaling promotes CD8+-exhausted T cell proliferation and terminal differentiation. Immunity 56, 1631–1648 (2023).
Driehuis, E., Kretzschmar, K. & Clevers, H. Establishment of patient-derived cancer organoids for drug-screening applications. Nat. Protoc. 15, 3380–3409 (2020).
Driehuis, E. et al. Oral mucosal organoids as a potential platform for personalized cancer therapy. Cancer Discov. 9, 852–871 (2019).
Huch, M. et al. Long-term culture of genome-stable bipotent stem cells from adult human liver. Cell 160, 299–312 (2015).
Saltsman, J. A. et al. A human organoid model of aggressive hepatoblastoma for disease modeling and drug testing. Cancers 12, 2668 (2020).
Miao, Y. et al. Next-generation surrogate Wnts support organoid growth and deconvolute Frizzled pleiotropy in vivo. Cell Stem Cell 27, 840–851 (2020).
Jutz, S. et al. Assessment of costimulation and coinhibition in a triple parameter T cell reporter line: simultaneous measurement of NF-κB, NFAT and AP-1. J. Immunol. Methods 430, 10–20 (2016).
Doench, J. G. et al. Optimized sgRNA design to maximize activity and minimize off-target effects of CRISPR–Cas9. Nat. Biotechnol. 34, 184–191 (2016).
Muraro, M. J. et al. A single-cell transcriptome atlas of the human pancreas. Cell Syst. 3, 385–394 (2016).
Wu, T. et al. clusterProfiler 4.0: a universal enrichment tool for interpreting omics data. Innovation 2, 100141 (2021).
Acknowledgements
We thank the staff of the Flow Core Facility at the University Medical Center Utrecht for their kind assistance. We thank W. Megchelenbrink at Princess Máxima Center for Pediatric Oncology, Utrecht, for suggestions related to statistical evaluations. Funding for this study was provided by KWF 6790 and 7601, 11393, 11979, 12586, 13043, 13493, Gadeta and Oncode Accelerator (https://www.oncodeaccelerator.nl) to J.K. S.M. is supported by the German Research Foundation through BIOSS EXC294 and CIBSS EXC 2189, SFB1479 (441891347, P15), FOR2799 (MI1942/3-1, 395236335) and MI1942/5-1 (501436442).
Author information
Authors and Affiliations
Contributions
Conceptualization: P.H.-L., E.v.D., D.X.B. and J.K. Methodology: P.H.-L., E.v.D., D.X.B. and J.K. Formal analysis: P.B. Investigation: P.H.-L., E.v.D., P.B., S.H., A.M., L.H.v.H., C.R., C.S., M.Z., I.J., M.J.T.N., A.C., T.A.K., R.M., J.Z., E.K., T.S. and S.M. Resources: H.C., R.d.B., H.G.S., J.R., W.C.P. and S.M. Writing, original draft: P.H.-L., E.v.D., P.B., Z.S., D.X.B. and J.K. Supervision: D.X.B. and J.K.
Corresponding author
Ethics declarations
Competing interests
J.K. was a shareholder of Gadeta. J.K., Z.S., E.v.D. and D.X.B. are inventors on patents with γδ TCR-related topics. J.K., Z.S., D.X.B., P.H.-L., A.M. and A.C. are inventors on patents with CD277-related topics. For the full disclosure of H.C., see https://www.uu.nl/staff/JCClevers/Additional%20functions. J.R. has advisory roles for Merck-Serono, Pierre Fabre, Servier, BMS, Roche, Bayer and GSK (all payments to the institution) and institutional scientific grants from Bristol Myers Squibb, Merck, Delphi, HUB4 Organoids, Cleara, Pierre Fabre, Servier, Xilis and GSK. J.R. is also a board member for the Foundation Hubrecht Organoid Biobank. All other authors have no competing interests.
Peer review
Peer review information
Nature Immunology thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editor: N. Bernard, in collaboration with the Nature Immunology team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Type I and Type II NKG2D chimeric co-receptors design, expression and activity upon CD3 and NKG2D stimulation.
(a) Schematic diagram of Type I and Type II chimeric NKG2D-chimeras. As the natural orientation of NKG2D (a Type II membrane protein) differs from the costimulatory proteins ICOS, CD28 and 4-1BB (all Type I membrane proteins), two different chimeras types were generated. A ‘Type I’ design with extracellular (EC) domain of NKG2D, and the hinge (H), transmembrane (TM) and cytoplasmic (CP) domains of the different costimulatory proteins, or a ‘Type II’ design where the cytoplasmic signaling domain of the costimulatory proteins was fused to the transmembrane and extracellular domain of NKG2D. Signal peptide is indicated as SP. N-terminal and C-terminal are represented by N and C respectively. NKG2D, ICOS, CD28 and 4-1BB domains are colored in purple, green, orange and blue respectively. (b) Expression of Type I and Type II chimeric NKG2D co-receptors in Jurkat-76 cells. For ‘Type I’ chimeras, differential, but high surface expression was observed, with the strongest surface expression for NKG2D-CD28wt. Of the ‘Type II’ designs, only NKG2D-CD28wt, was marginally expressed. (c) Surface expression of γδTCR-Cl5 and Type I NKG2D co-receptors or NKG2DWT on primary CD4+ T-cells after transduction and αβ depletion. (d) As NKG2D-CD28wt showed increased surface expression, we generated Type I NKG2D co-receptors containing CD28 transmembrane (TM) and hinge (H) domains. Surface expression of γδTCR-Cl5 and NKG2DWT, or new chimeras containing CD28 TM domain on CD4+ T-cells after transduction and αβ depletion was assessed. Using the same CD28 hinge and TM for all chimeras resulted in higher and more comparable surface expression. Chimera designs with CD28 hinge and TM (later referred as: NKG2D-ICOSCD28TM, NKG2D-CD28WT and NKG2D-4-1BBCD28TM) were selected for further testing (e) Expression of CD69 on CD4+ TEGs upon stimulation with CD3 (0.2 µg/ml and/or NKG2D (5 µg/ml) antibodies, or CD3/CD28 dynabeads. MFI relative to the unstimulated condition is shown. N = 2 independent experiments with two biological replicates each. Data represents mean ± SD. Significance was calculated using One way ANOVA with Tukey correction for multiple comparisons.
Extended Data Fig. 2 Introduction of NKG2D-4-1BB-chimera increases TEG001 IFNγ release in response to tumor cells, but does not impact short-term killing.
(a) Surface expression of NKG2D-ligands in TEG001 targeted (K562, SCC9, RPMI-8226 and Daudi) or no targeted (HL60) tumor cells. MFI was measured by flow cytometry using NKG2D-Fc and IgG-Fc fusion proteins. Fold change was calculated per type of cells as MFI, measured using NKG2D-Fc relative to IgG-Fc condition. (b) Transduced CD4+ T-cells were incubated with K562, Daudi or HL60 at several pamidronate concentrations. After 18 hours, supernatants were harvested and analyzed for IFNγ secretion by ELISA. N = 3 independent experiments. Data represent mean ± SD, significance was calculated using Two Way ANOVA with Dunnett correction. For K562: 3µM PAM (**P = 0.0075), 11 µM PAM (**P = 0.0075), 33 µM PAM (*P = 0.0112). (c) Transduced CD8+ (75%) were tested against K562, RPMI-8226, SCC9, Daudi and HL60 in a 51Cr-release assay (E:T, 10:1, 3:1, 1:1, 0,33:1, 0,11:1). 51Cr-release was measured in the supernatant after 5 hours. Specific lysis was calculated using the formula (experimental cpm - basal cpm)/(maximal cpm - basal cpm) x100 with maximal lysis determined in the presence of 5% triton, and basal lysis in the absence of effector cells. N = 2 independent experiments (for K562, SCC9 and HL60, N = 1 with biological replicates). Data represent mean.
Extended Data Fig. 3 In vitro assessment of TEGs.
(a) Surface expression of endogenous NKG2D in CD4+ and CD8+ αβT-cells. (b) CD8+ transduced T-cells were labeled with CTV and stimulated with CD3 and NKG2D antibodies for six days. On Day 6, MFI was assessed by flow cytometry. N = 2 independent experiments with two biological replicates each. Data represent Trace violet MFI mean ± SD, significance was calculated using One Way ANOVA with Holm-sidak. For NKG2D 1µg/ml: NKG2DWT (***P = 0.0002); For NKG2D 2.5 µg/ml: NKG2DWT (**P = 0.0012). For NKG2D 5 µg/ml: TEG-LM1 (**P = 0.0027). (c) CD4+ transduced cells were labeled using CTV and co-cultured with HL60 or RPMI-8226 tumor cells. On Day 6, proliferation was assessed by flow cytometry. Histogram data shows CTV intensity and percentage of proliferating cells, taking LM1 without targets as a control for gating.
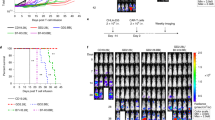
Extended Data Fig. 4 In vivo monitoring of tumor growth by BLI in multiple myeloma xenograft.
(a) RPMI-8226-luciferase tumor growth in NSG mice treated with different TEGs. Pictures show BLI signal of all the mice on Days 15, 27, 43, 49, 56. Data generated from one experiment with N = 10 mice per treatment. (b) Comparison of BLI signal, including mean and single curves, from mice treated with TEG-LM1 (mock) (grey), TEG001 (blue), TEG001-NKG2DWT (purple), TEG001-NKG2D-ICOSCD28TM (green), TEG001-NKG2D-CD28WT (red), TEG001-NKG2D-4-1BBCD28TM (orange).
Extended Data Fig. 5 Therapeutic effects of TEGs co-expressing NKG2D chimeric co-receptors in a head and neck xenograft model.
(a) Schematic diagram of experimental setup to evaluate efficacy of TEGs co-expressing NKG2D-chimeras against SCC9 luciferase tumor. Male and female NSG mice were irradiated and injected s.c. with 0,5 × 106 SCC9 tumor cells. On Days 1 and 7, 107 mice were treated with TEG-LM1 (mock) (N = 12), TEG001 (N = 11), TEG001-NKG2D-CD28WT (N = 11) or TEG-NKG2D-4-1BBCD28TM (N = 11). Weekly BLI and bleeding were performed, and IL2 and PAM was administered every three weeks. Data generated from one experiment. (b) Tumor size was measured weekly. Data represent mean ± SEM. Significance was calculated for tumor outgrowth over time using mixed-effect model test with repeated measures by comparing TEG-LM1 or TEG001 to the rest of the treatments. TEG-LM1 vs TEG001-NKG2D-4-1BBCD28TM (***P = 0.0002), TEG001 vs TEG001-NKG2D-4-1BBCD28TM (*P = 0.0148). (c) BLI were measured weekly to assess tumor outgrowth. Data represent single curves and mean for TEG-LM1 (mock) (grey), TEG001 (blue), TEG001-NKG2D-CD28WT (red) and TEG001-NKG2D-4-1BBCD28TM (orange). Significance was calculated for tumor outgrowth over time using mixed-effect model test with repeated measures by comparing TEG-LM1 or TEG001 to the rest of the treatments. TEG-LM1 vs TEG001 (P = 0.5907), TEG001 vs TEG001-NKG2D-CD28WT (P = 0.5570), TEG001 vs TEG001-NKG2D-4-1BBCD28TM (***P = 0.0003).
Extended Data Fig. 6 In vitro recognition of patient-derived tumor organoids by TEGs.
(a) Surface expression of NKG2D-ligands in patient-derived liver and head neck tumor organoids. MFI was measured by flow cytometry using NKG2D-Fc and IgG-Fc fusion proteins. Data represent the mean of NKG2D-Fc staining, relative to IgG-Fc condition. N = 2 independent experiments for all organoids but HCC pt1 (N = 1) (b) Transduced CD4+ and CD8+ T-cells were incubated with the different organoids with 30-60 µM PAM. After 18 hours, supernatants were harvested and analyzed for IFNγ secretion by ELISA. Data represent mean of fold change normalized to TEG001 ± SD. N = 4 (HN1, HN2, HN3, HCC pt1), N = 2 (HB pt10) or N = 1 (HB pt13) independent experiments. Significance was calculated using unpaired one-tailed T test. Data represent mean ± SD (c) Transduced CD4+ T-cells were labeled with CTV and co-cultured with patient-derived liver and head and neck tumor organoids in presence of 100 µM PAM. On Day 6 MFI was assessed by flow cytometry. Representative graph of two independent experiments is shown. Data represent Cell Trace violet MFI mean of biological replicates. (d) Histogram data shows CTV intensity and percentage of proliferating cells, taking LM1 without targets as control for gating.
Extended Data Fig. 7 Separated clustering of CD4 and CD8 subsets for the different TEGs.
a,b, CD4+ enriched cell from the TEG/CD28/41BB cell types (422, 404 and 351 cells, 1177 cells in total) split by chimera types. Cells are annotated by clusterID (a) or chimera types (b). (c) Percentage representation of the clusters (annotated by the main GO-terms) across the CD4(+) chimera types. (d) Density dot plot of the percentage of cells in a CD4(+) cluster that express a given gene ('percent expressed') and the scaled average expression of canonical marker. e,f, CD8+ enriched cell from the TEG/CD28/41BB cell types (417, 415, 346, and cells, 1178 cells in total) split by chimera types. Cells are annotated by clusterID (e) or chimera types (f). (g) Percentage representation of the clusters (annotated by the main GO-terms) across the CD8(+) chimera types. (h) Density dot plot of the percentage of cells in a CD8(+) cluster that express a given gene (‘percent expressed’) and the scaled average expression of canonical marker.
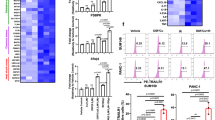
Extended Data Fig. 8 Decreased expression of PD-1 and enhanced expression of granzyme B and NF-κB in TEG001-NKG2D-4-1BBCD28TM.
(a) Expression of the exhaustion marker PD-1 (MFI) on CD4+ TEGs assessed by FACS after 4 rounds of stimulation with RPMI-8226, SCC9 or Fadu tumor cells in presence of 10 µM PAM. Opened symbols represent 1:1 E:T conditions. Closed symbols represent 3:1 E:T conditions. Data represent mean ± SD. Significance was calculated using One Way ANOVA with Tukey correction. N = 8 independent experiments for RPMI-8226, N = 6 for SCC9 and N = 4 for Fadu. (b) Percentage of granzyme B+ cells within CD4+TEG001 and TEG001-NKG2D-4-1BBCD28TM assessed by FACS for three different T-cell donors (ds136 in red; ds190 in orange; ds232 in blue) after 4 rounds of stimulation with RPMI-8226, SCC9 or Fadu in presence of 10 uM PAM at E:T 3:1 (closed symbols) or 1:1 (opened symbols). Donors 136/190: N = 6 independent experiments for RPMI-8226, N = 6 for SCC9, N = 4 for Fadu; Donor 232: N = 3 independent experiments for RPMI-8226, N = 4 for SCC9, N = 4 for Fadu. Opened symbols represent 1:1 E:T conditions. Closed symbols represent 3:1 E:T conditions. Dotted line represents 40%. Significance was calculated using a one-tailed unpaired t test. (c) Percentage of GFP-positive cells after co-culture of Jurkat NF-κB and Jurkat NFAT reporter cell lines with RPMI-8226 tumor cells. N = 3 independent experiments. Data represents mean ± SEM. Statistical significance was calculated using One Way ANOVA followed by Fisher’s LSD test comparison. For NF-κB: NF-κB parental (*P = 0.0247), TEG001-NKG2D-CD28WT (P = 0.2842), TEG001-NKG2D-4-1BBCD28TM (*P = 0.0405). For NFAT: NFAT parental (*P = 0.0377), TEG001-NKG2D-CD28WT (P = 0.9387), TEG001-NKG2D-4-1BBCD28TM (P = 0.9624).
Extended Data Fig. 9 Knock-out (KO) of endogenous NKG2D improves killing and proliferation activity of in CD8+ TEG001-NKG2D-4-1BB.
(a) Schematic overview showing annealing of crRNA guides (guide A and guide B) and NKG2D. (b, c) Percentage of NKG2D+ cells within CD8+TEG cells after electroporation with a negative control crRNA guide (grey) or NKG2D crRNA guides (blue). For TEG001, the percentage of NKG2D+ cells was reduced from 77% in the negative control to 21% after KO. Due to the high expression of the NKG2D chimera, the KO percentage on TEG001-NKG2D-4-1BBCD28TM could not be assessed by FACS. As the percentage of KO cells in TEG001 was similar amongst biological replicates, the same efficiency was assumed for TEG001-NKG2D-4-1BBCD28TM. Significance was calculated using a two-tailed unpaired t test. Data represents mean ± SD. N = 2 independent experiments. (d) RPMI-8226 tumor cell line expressing luciferase and stroma cells were cultured in Matrigel constituting 3D bone marrow niche. After four days, CD8+ negative control and NKG2D-KO TEGs were added, together with PAM (10 μM PAM). Six days later, living tumor cells were quantified by FACS. Tumor cell numbers were normalized to mock treatment (TEG-LM1). N = 1 with biological replicates. Data represent mean ± SD. (e) Negative control (gray) and NKG2D KO (blue) CD8+TEGs were stained with CTV and co-cultured with RPMI-8226 or SCC9 (1:1, E:T) in presence of 100 uM pam. After 6 days, proliferation was assessed by FACS.
Extended Data Fig. 10 Addition of 103-4-1BB-chimera to TEG001 impacts transcriptomics and proliferation capacity of CD4+ and CD8+ TEGs.
a) Surface expression of γδTCR-Cl5 and 103-4-BB chimeric receptor on T-cells after transduction and αβ depletion, assessed by flow cytometry. (b) Transduced CD4+ and CD8+ T-cells were labeled with CTV and co-cultured with RPMI-8226 and SCC9 tumor cells in presence of 100 µM PAM. On Day 6, MFI was assessed by flow cytometry. Representative histograms of two independent experiments are shown. (c) Density dot plot of the percentage of cells in a CD4+ cluster that express a given gene (ʻpercent expressed’), and the scaled average expression of canonical marker. (d) Density dot plot of the percentage of cells in a CD8+ cluster that express a given gene (ʻpercent expressed’) and the scaled average expression of canonical marker.
Supplementary information
Supplementary Information
Supplementary Figs. 1–4.
Supplementary Table 1
Cytokine and chemokine production after coculturing of TEGs and RPMI-8226 cells in a 3D model system. Significance was calculated using One Way ANOVA with Dunnett correction.
Supplementary Table 2
P values of the Fisher′s exact tests comparing cluster abundances to the same clusters in TEG001; related to Fig. 4.
Supplementary Table 3
Top 50 differentially expressed genes per clusters; related to Fig. 4a,e.
Supplementary Table 4
P values of the Fisher′s exact test comparing the per-cluster relative abundance values of CD4+ and CD8+ T cells; related to Fig. 4c.
Supplementary Table 5
P values of the Fisher′s exact test comparing the per-cluster relative abundance values of CD4+ and CD8+ T cells; related to Extended Data Fig. 7a–h.
Supplementary Table 6
Top 50 differentially expressed genes per chimera type; related to Fig. 4b,f.
Supplementary Table 7
P values of the Fisher′s exact tests comparing cluster abundances to the same clusters in TEG001 (of the matching CD4/CD8 group); related to Fig. 5d,g.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 8
Statistical source data.
Source Data Extended Data Fig. 9
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hernández-López, P., van Diest, E., Brazda, P. et al. Dual targeting of cancer metabolome and stress antigens affects transcriptomic heterogeneity and efficacy of engineered T cells. Nat Immunol 25, 88–101 (2024). https://doi.org/10.1038/s41590-023-01665-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41590-023-01665-0