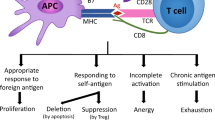
Abstract
Tumor-specific CD8+ T cells (TST) in patients with cancer are dysfunctional and unable to halt cancer progression. TST dysfunction, also known as exhaustion, is thought to be driven by chronic T cell antigen receptor (TCR) stimulation over days to weeks. However, we know little about the interplay between CD8+ T cell function, cell division and epigenetic remodeling within hours of activation. Here, we assessed early CD8+ T cell differentiation, cell division, chromatin accessibility and transcription in tumor-bearing mice and acutely infected mice. Surprisingly, despite robust activation and proliferation, TST had near complete effector function impairment even before undergoing cell division and had acquired hallmark chromatin accessibility features previously associated with later dysfunction/exhaustion. Moreover, continued tumor/antigen exposure drove progressive epigenetic remodeling, ‘imprinting’ the dysfunctional state. Our study reveals the rapid divergence of T cell fate choice before cell division in the context of tumors versus infection.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
RNA-seq and ATAC-seq data have been deposited in the Gene Expression Omnibus under super-series accession number GSE209712. KEGG, Hallmark and Reactome gene sets were retrieved from the Broad Institute’s MSigDB collections using msigbr v7.5.177 (https://www.gsea-msigdb.org/gsea/msigdb/index.jsp). Source data are provided with this paper. All other data that support the findings of this study are present in the article or are available from the corresponding author upon request.
Code availability
Code has been deposited in GitHub: https://github.com/abcwcm/Rudloff2022.
References
Williams, M. A. & Bevan, M. J. Effector and memory CTL differentiation. Annu. Rev. Immunol. 25, 171–192 (2007).
McLane, L. M., Abdel-Hakeem, M. S. & Wherry, E. J. CD8 T cell exhaustion during chronic viral infection and cancer. Annu. Rev. Immunol. 37, 457–495 (2019).
Waugh, K. A. et al. Molecular profile of tumor-specific CD8+ T cell hypofunction in a transplantable murine cancer model. J. Immunol. 197, 1477–1488 (2016).
Mognol, G. P. et al. Exhaustion-associated regulatory regions in CD8+ tumor-infiltrating T cells. Proc. Natl Acad. Sci. USA 114, E2776–E2785 (2017).
Philip, M. et al. Chromatin states define tumour-specific T cell dysfunction and reprogramming. Nature 545, 452–456 (2017).
Zhu, L. & Skoultchi, A. I. Coordinating cell proliferation and differentiation. Curr. Opin. Genet. Dev. 11, 91–97 (2001).
Kreslavsky, T. et al. beta-selection-induced proliferation is required for αβ T cell differentiation. Immunity 37, 840–853 (2012).
Bird, J. J. et al. Helper T cell differentiation is controlled by the cell cycle. Immunity 9, 229–237 (1998).
Tangye, S. G. & Hodgkin, P. D. Divide and conquer: the importance of cell division in regulating B-cell responses. Immunology 112, 509–520 (2004).
Scharer, C. D., Barwick, B. G., Guo, M., Bally, A. P. R. & Boss, J. M. Plasma cell differentiation is controlled by multiple cell division-coupled epigenetic programs. Nat. Commun. 9, 1698 (2018).
Kaech, S. M. & Ahmed, R. Memory CD8+ T cell differentiation: initial antigen encounter triggers a developmental program in naive cells. Nat. Immunol. 2, 415–422 (2001).
van Stipdonk, M. J., Lemmens, E. E. & Schoenberger, S. P. Naive CTLs require a single brief period of antigenic stimulation for clonal expansion and differentiation. Nat. Immunol. 2, 423–429 (2001).
Williams, M. A. & Bevan, M. J. Shortening the infectious period does not alter expansion of CD8 T cells but diminishes their capacity to differentiate into memory cells. J. Immunol. 173, 6694–6702 (2004).
Bevan, M. J. & Fink, P. J. The CD8 response on autopilot. Nat. Immunol. 2, 381–382 (2001).
Schietinger, A. et al. Tumor-specific T cell dysfunction is a dynamic antigen-driven differentiation program initiated early during tumorigenesis. Immunity 45, 389–401 (2016).
Philip, M. & Schietinger, A. CD8+ T cell differentiation and dysfunction in cancer. Nat. Rev. Immunol. 22, 209–223 (2022).
Scott, A. C. et al. TOX is a critical regulator of tumour-specific T cell differentiation. Nature 571, 270–274 (2019).
Okazaki, T. et al. PD-1 and LAG-3 inhibitory co-receptors act synergistically to prevent autoimmunity in mice. J. Exp. Med. 208, 395–407 (2011).
Ahn, E. et al. Role of PD-1 during effector CD8 T cell differentiation. Proc. Natl Acad. Sci. USA 115, 4749–4754 (2018).
Wherry, E. J., Blattman, J. N., Murali-Krishna, K., van der Most, R. & Ahmed, R. Viral persistence alters CD8 T cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J. Virol. 77, 4911–4927 (2003).
Buenrostro, J. D., Giresi, P. G., Zaba, L. C., Chang, H. Y. & Greenleaf, W. J. Transposition of native chromatin for fast and sensitive epigenomic profiling of open chromatin, DNA-binding proteins and nucleosome position. Nat. Methods 10, 1213–1218 (2013).
Sen, D. R. et al. The epigenetic landscape of T cell exhaustion. Science 354, 1165–1169 (2016).
Pauken, K. E. et al. Epigenetic stability of exhausted T cells limits durability of reinvigoration by PD-1 blockade. Science 354, 1160–1165 (2016).
Scott-Browne, J. P. et al. Dynamic changes in chromatin accessibility occur in CD8+ T cells responding to viral infection. Immunity 45, 1327–1340 (2016).
Schep, A. N., Wu, B., Buenrostro, J. D. & Greenleaf, W. J. chromVAR: inferring transcription-factor-associated accessibility from single-cell epigenomic data. Nat. Methods 14, 975–978 (2017).
Deng, W. et al. Recombinant Listeria promotes tumor rejection by CD8+ T cell-dependent remodeling of the tumor microenvironment. Proc. Natl Acad. Sci. USA 115, 8179–8184 (2018).
Harty, J. T., Tvinnereim, A. R. & White, D. W. CD8+ T cell effector mechanisms in resistance to infection. Annu. Rev. Immunol. 18, 275–308 (2000).
Sadler, A. J. & Williams, B. R. Interferon-inducible antiviral effectors. Nat. Rev. Immunol. 8, 559–568 (2008).
Martinez, G. J. et al. The transcription factor NFAT promotes exhaustion of activated CD8+ T cells. Immunity 42, 265–278 (2015).
Chapman, N. M., Boothby, M. R. & Chi, H. Metabolic coordination of T cell quiescence and activation. Nat. Rev. Immunol. 20, 55–70 (2020).
Tsao, H. W. et al. Batf-mediated epigenetic control of effector CD8+ T cell differentiation. Sci. Immunol. 7, eabi4919 (2022).
Yang, C. Y. et al. The transcriptional regulators Id2 and Id3 control the formation of distinct memory CD8+ T cell subsets. Nat. Immunol. 12, 1221–1229 (2011).
Roychoudhuri, R. et al. BACH2 regulates CD8+ T cell differentiation by controlling access of AP-1 factors to enhancers. Nat. Immunol. 17, 851–860 (2016).
Machlab, D. et al. monaLisa: an R/Bioconductor package for identifying regulatory motifs. Bioinformatics 38, 2624–2625 (2022).
Intlekofer, A. M. et al. Anomalous type 17 response to viral infection by CD8+ T cells lacking T-bet and eomesodermin. Science 321, 408–411 (2008).
Intlekofer, A. M. et al. Effector and memory CD8+ T cell fate coupled by T-bet and eomesodermin. Nat. Immunol. 6, 1236–1244 (2005).
Sullivan, B. M., Juedes, A., Szabo, S. J., von Herrath, M. & Glimcher, L. H. Antigen-driven effector CD8 T cell function regulated by T-bet. Proc. Natl Acad. Sci. USA 100, 15818–15823 (2003).
Pearce, E. L. et al. Control of effector CD8+ T cell function by the transcription factor Eomesodermin. Science 302, 1041–1043 (2003).
He, R. et al. Follicular CXCR5- expressing CD8+ T cells curtail chronic viral infection. Nature 537, 412–428 (2016).
Im, S. J. et al. Defining CD8+ T cells that provide the proliferative burst after PD-1 therapy. Nature 537, 417–421 (2016).
Utzschneider, D. T. et al. T cell factor 1-expressing memory-like CD8+ T cells sustain the immune response to chronic viral infections. Immunity 45, 415–427 (2016).
Wu, T. et al. The TCF1–Bcl6 axis counteracts type I interferon to repress exhaustion and maintain T cell stemness. Sci. Immunol. 1, eaai8593 (2016).
Abdel-Hakeem, M. S. et al. Epigenetic scarring of exhausted T cells hinders memory differentiation upon eliminating chronic antigenic stimulation. Nat. Immunol. 22, 1008–1019 (2021).
Hensel, N. et al. Memory-like HCV-specific CD8+ T cells retain a molecular scar after cure of chronic HCV infection. Nat. Immunol. 22, 229–239 (2021).
Yates, K. B. et al. Epigenetic scars of CD8+ T cell exhaustion persist after cure of chronic infection in humans. Nat. Immunol. 22, 1020–1029 (2021).
Liu, J. et al. CTCF mediates CD8+ effector differentiation through dynamic redistribution and genomic reorganization. J. Exp. Med. 220, e20221288 (2023).
Amezquita, R. A. marge: an API for analysis of motifs using HOMER in R. Preprint at bioRxiv https://doi.org/10.1101/249268 (2019).
Cao, Z., Sun, X., Icli, B., Wara, A. K. & Feinberg, M. W. Role of Kruppel-like factors in leukocyte development, function, and disease. Blood 116, 4404–4414 (2010).
Yamada, T., Park, C. S., Mamonkin, M. & Lacorazza, H. D. Transcription factor ELF4 controls the proliferation and homing of CD8+ T cells via the Kruppel-like factors KLF4 and KLF2. Nat. Immunol. 10, 618–626 (2009).
Guan, T. et al. ZEB1, ZEB2, and the miR-200 family form a counterregulatory network to regulate CD8+ T cell fates. J. Exp. Med. 215, 1153–1168 (2018).
Omilusik, K. D. et al. Transcriptional repressor ZEB2 promotes terminal differentiation of CD8+ effector and memory T cell populations during infection. J. Exp. Med. 212, 2027–2039 (2015).
Scott, C. L. & Omilusik, K. D. ZEBs: novel players in immune cell development and function. Trends Immunol. 40, 431–446 (2019).
Shih, H. Y. et al. Developmental acquisition of regulomes underlies innate lymphoid cell functionality. Cell 165, 1120–1133 (2016).
Hernandez, J., Aung, S., Marquardt, K. & Sherman, L. A. Uncoupling of proliferative potential and gain of effector function by CD8+ T cells responding to self-antigens. J. Exp. Med. 196, 323–333 (2002).
Curtsinger, J. M., Lins, D. C. & Mescher, M. F. Signal 3 determines tolerance versus full activation of naive CD8 T cells: dissociating proliferation and development of effector function. J. Exp. Med. 197, 1141–1151 (2003).
Otten, G. R. & Germain, R. N. Split anergy in a CD8+ T cell: receptor-dependent cytolysis in the absence of interleukin-2 production. Science 251, 1228–1231 (1991).
Utzschneider, D. T. et al. Early precursor T cells establish and propagate T cell exhaustion in chronic infection. Nat. Immunol. 21, 1256–1266 (2020).
Wherry, E. J. et al. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity 27, 670–684 (2007).
Staveley-O’Carroll, K. et al. In vivo ligation of CD40 enhances priming against the endogenous tumor antigen and promotes CD8+ T cell effector function in SV40 T antigen transgenic mice. J. Immunol. 171, 697–707 (2003).
Stahl, S. et al. Tumor agonist peptides break tolerance and elicit effective CTL responses in an inducible mouse model of hepatocellular carcinoma. Immunol. Lett. 123, 31–37 (2009).
Brockstedt, D. G. et al. Listeria-based cancer vaccines that segregate immunogenicity from toxicity. Proc. Natl Acad. Sci. USA 101, 13832–13837 (2004).
Andrews, S. FastQC: a quality control tool for high throughput sequence data. http://www.bioinformatics.babraham.ac.uk/projects/fastqc (2010).
Hartley, S. W. & Mullikin, J. C. QoRTs: a comprehensive toolset for quality control and data processing of RNA-Seq experiments. BMC Bioinformatics 16, 224 (2015).
R Core Team. R: a language and environment for statistical computing. R Foundation for Statistical Computing. https://www.R-project.org/ (2017).
Wickham, H. et al. ggplot2: Elegant Graphics for Data Analysis (2016).
Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 17, 10–12 (2011).
Dobin, A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 (2013).
Liao, Y., Smyth, G. K. & Shi, W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 30, 923–930 (2014).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014).
Korotkevich, G. et al. Fast gene-set enrichment analysis. Preprint at bioRxiv https://doi.org/10.1101/060012 (2021).
Subramanian, A. et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl Acad. Sci. USA 102, 15545–15550 (2005).
Liberzon, A. et al. Molecular signatures database (MSigDB) 3.0. Bioinformatics 27, 1739–1740 (2011).
Dolgalev, I. msigdbr: MSigDB gene sets for multiple organisms in a tidy data format. R package version 7.5.1.9001 https://igordot.github.io/msigdbr/ (2022).
Li, H. & Durbin, R. Fast and accurate short read alignment with Burrows–Wheeler transform. Bioinformatics 25, 1754–1760 (2009).
Li, H. et al. The sequence alignment/map format and SAMtools. Bioinformatics 25, 2078–2079 (2009).
Liu, T. Use model-based analysis of ChIP-seq (MACS) to analyze short reads generated by sequencing protein–DNA interactions in embryonic stem cells. Methods Mol. Biol. 1150, 81–95 (2014).
Stark, R. & Brown, G. DiffBind: differential binding analysis of ChIP-seq peak data. http://bioconductor.org/packages/release/bioc/html/DiffBind.html (2011).
Yu, G., Wang, L. G. & He, Q. Y. ChIPseeker: an R/Bioconductor package for ChIP peak annotation, comparison and visualization. Bioinformatics 31, 2382–2383 (2015).
Leek, J. T. et al. sva: surrogate variable analysis. R package version 3.44.0 (2022).
Weirauch, M. T. et al. Determination and inference of eukaryotic transcription factor sequence specificity. Cell 158, 1431–1443 (2014).
Ramirez, F. et al. deepTools2: a next generation web server for deep-sequencing data analysis. Nucleic Acids Res. 44, W160–W165 (2016).
Carroll, T. & Barrows, D. profileplyr: visualization and annotation of read signal over genomic ranges with profileplyr. R package version 1.12.0. (2022).
Gu, Z., Eils, R. & Schlesner, M. Complex heatmaps reveal patterns and correlations in multidimensional genomic data. Bioinformatics 32, 2847–2849 (2016).
Acknowledgements
We thank A. Schietinger and members of the laboratory of M.P. for helpful discussions. We thank E. Chiu, A. Dave, G. Anzarova and T. Bryson for technical assistance. We thank the Vanderbilt Division of Animal Care, D. Flaherty and the VUMC Flow Cytometry Shared Resource Core, A. Jones and the Vanderbilt Technologies for Advanced Genomics (VANTAGE) Core, and A. Viale and the Sloan Kettering Integrated Genomics Operation Core (IGO). We thank P. Lauer and Aduro Biotech for providing attenuated Listeria strains. We thank A. Schietinger, J. C. Rathmell and J. M. Balko for critical review of the manuscript. This work was supported by the following funding sources: V Foundation Scholar Award (to M.P.), National Institutes of Health (NIH) R37CA263614 (to M.P.), Serodino Family Adventure Allee Fund (to M.P.), Vanderbilt-Ingram Cancer Center (VICC) SPORE Career Enhancement Program (to M.P.) NIH P50CA098131, Vanderbilt Digestive Disease Research Center (VDDRC) Young Investigator and Pilot Award (to M.P.) NIH P30DK058404, Medical Scientist Training Program (MSTP) NIH T32GM007347 (to M.W.R.), NIH T32GM008554 (to N.R.F.), NIH T32CA009592 (to C.R.D.R) and NIH T32AR059039 (to M.M.E.). The VUMC Flow Cytometry Shared Resource is supported by the VICC (NIH P30CA68485) and the VDDRC (NIH P30DK058404). VANTAGE is supported by the VICC (NIH P30CA68485), the Vanderbilt Vision Center (NIH P30EY08126) and the NIH G20RR030956. IGO is supported by NIH P30CA08748, Cycle for Survival and the Marie-Josée and Henry R. Kravis Center for Molecular Oncology.
Author information
Authors and Affiliations
Contributions
M.W.R. and M.P. conceived and designed the study and analyzed and interpreted data. M.W.R. carried out experiments, assisted by N.R.F., J.J.R., C.R.D.R., M.M.E., K.A.M. and S.T.J. P.Z., F.D. and D.B. designed and performed computational analyses of RNA-seq and ATAC-seq data. M.W.R. and M.P. wrote the manuscript, with all authors contributing to the writing and providing feedback.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Immunology thanks Mohamed Abdel-Hakeem, Ansuman Satpathy, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: L. A. Dempsey, in collaboration with the Nature Immunology team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 TST undergo robust activation and proliferation but do not gain effector function.
a, Live CD8+ Thy1.1+ TCRTAG CFSE dilution and expression of surface markers at each time point from LMTAG-infected mice (spleens; green) or from tumor-bearing mice (blue), from liver tumors, tumor draining lymph nodes (tdLN), and spleens, shown together with naive in vivo control (N; grey). Each time point shows data concatenated from 3-4 biologic replicates. Three independent experiments were carried out with 3-4 mice/group. b, Counts of TCRTAG per organ at 12h, 36h, 48h, and 60h as well as later time points 5 and 21+ days. Each symbol represents an individual mouse with n = 3 per group. ****P <0.0001 determined by two-way ANOVA with post hoc Šidák multiple comparison test comparing the cumulative number of TCRTAG from tumor-bearing mice and infected mice. c, TNF, IFNγ, and CD107a production following 4h ex vivo TAG peptide stimulation in TCRTAG from LMTAG-infected mice (green) or from tumor-bearing mice (blue) at pooled 48 and 60h time points. GZMB and PRF1 expression was assessed immediately ex vivo. Naive in vivo TCRTAG is shown for comparison (N; grey). Dot plots are concatenated from 3 mice/group. d, IFNγ and TNF production by TCRTAG from spleens and livers of LMTAG-infected mice at 48h time point following 4h ex vivo TAG peptide stimulation (top) and summary plot of percentage TNF+IFNγ+ TCRTAG (bottom) points where bars represent mean and error bars represent standard deviation. ns = not statistically significant, determined by unpaired two-tailed Student’s t-test. e, Dot plots of no peptide stimulation controls from infected and tumor-bearing mice from pooled 48 and 60h time points. Two independent experiments were carried out with 2–4 mice/group.
Extended Data Fig. 2 Tumor-induced TST effector function impairment begins prior to cell division.
Live CD8+ Thy1.1+ TCRTAG analyzed from spleens of infected mice (green) and livers from tumor-bearing hosts (blue) at 6h, 12h, and 18h. a, Representative histograms of CD44, LAG3, and PD1 expression profiles. Two independent experiments were carried out with 3-4 mice/group. b, Ex vivo GZMB expression. Summary plots are shown to the right with each point representing an individual mouse and n = 3-4 per group. c, Independent experimental replicate of Fig. 2e showing summary plots of percentage TNF+IFNγ+ TCRTAG after peptide stimulation. Each symbol represents an individual mouse with n = 3-4 per group. ***P = 0.0003, ****P <0.0001 determined by two-way ANOVA with post hoc Tukey test.
Extended Data Fig. 3 Committed effector CD8 T cells rapidly lose function in tumor-bearing hosts.
a, Dot plots of TCRTAG TNF and IFNγ production from spleens and livers of LMTAG-infected animals following 4h TAG peptide stimulation. Gates set based on no peptide stimulation controls. b, Summary plots of percentage TNF+IFNγ+ TCRTAG following 4h TAG peptide stimulation, where open circles represent TCRTAG isolated from livers and closed circles from spleens of infected mice with n = 3 (12h, 7d) or 4 (36h) per group. Each point represents an individual animal, statistics determined by two-way ANOVA with post-hoc Tukey test. c, TCRTAG CFSE dilution and TNF and IFNγ production at 12h and 36h time points from tumor livers following 4h TAG peptide stimulation (left). Data is concatenated from 3-4 biological replicates/timepoint. Summary plots of percentage TNF+IFNγ+ TCRTAG (right) where each symbol represents an individual mouse with n = 3 (12h) or 4 (36h) per group and black bars represent mean. d, Summary plots of percentage TNF+IFNγ+ TCRTAG by cell division 36h post-transfer points where bars represent mean and error bars represent standard deviation. Each symbol represents an individual mouse with n = 3-4 per group.
Extended Data Fig. 4 Naive and effector TST robustly proliferate in mice with metastatic melanoma but lack effector function.
Live CD8+ Ly5.1+ TCROTI analyzed from spleens of LMOVA infected B6 (green) and B6 with pulmonary B16-OVA metastases (pink) at 16h. a, Representative histograms of CFSE, CD69, and CD44 expression profiles. b, Representative histograms of LAG3 and PD1 expression. c, Ex vivo GZMB expression. Summary plots are shown to the right with each point representing an individual mouse. d, CFSE dilution of E5d TCROTI transferred into time-matched LMOVA infected B6 (green) or B6 with pulmonary B16-OVA metastases (pink) at 24 and 48 hours following transfer. Naive TCROTI (N; grey) shown for comparison. *P = 0.0307 and ***P = 0.0008 determined using unpaired two-tailed Student’s t-test.
Extended Data Fig. 5 Gating strategy for sorting; dysfunction-associated epigenetic and transcriptional programming begins prior to cell division.
a, Gating strategy to sort TCRTAG from infected spleens or tumor livers for sequencing studies. b, Principal component analysis (PCA) of RNA-SEQ data comparing top 500 most variable genes between naive (N; grey) and TCRTAG differentiating during acute infection (green) and in tumors (blue) at 6, 12, 24h post-transfer. Each symbol represents a single biological replicate. c, Chromatin accessibility profile across the Pdcd1 locus with the exhaustion-associated −23kb peak boxed (left). Summary plot (right) shows the DESeq2-determined log2FC and FDR (two-sided Wald test with Benjamini-Hochberg correction) at the Pdcd1 −23kb peak for early E and T time points as compared to naive (N).
Extended Data Fig. 6 STAT1 motif enrichment is accompanied by increased gene expression in CD8 T cells activated during acute infection; tumor- and infection-activated CD8 T cells show similar enrichment of T cell activation-associated genes.
a, Heatmap showing expression of differentially-expressed genes with peaks containing STAT1 motifs (by ChromVar; Fig. 5e) in effector (E, left) or tumor (T, right) across time points. Heatmaps are z-score normalized across rows. b, Gene set enrichment analysis (GSEA) of E versus N or T versus N at 6h, 12h, and 24h post transfer for activation associated gene sets and KEGG, HALLMARK, and REACTOME gene sets. Color represents normalized enrichment score (NES) and circle size represents the negative log10 (FDR).
Extended Data Fig. 7 Pre-division tumor-induced TST chromatin remodeling is reinforced with time and tumor antigen exposure.
a, Chromatin accessibility heatmap showing naive (N) and TCRTAG from 6h to 60+d in liver tumors of ASTxCre (left) and in LMTAG-infected B6 (right). Each row represents one of 29,884 (left) or 31,756 (right) peaks (differentially accessible between at least one sequential time point comparison; FDR <0.05, |log2FC| >1.5) displayed over 2kb window centered on the peak summit. Scale units are RPGC normalized to 1x sequencing depth for 20 bp bins with blue indicating closed chromatin and red open chromatin. Peaks are clustered by k-means (k = 6). b, PCA comparing chromatin accessibility in TCRTAG from 6h to M during infection. Each symbol represents a single biological replicate. c, Upper panel shows peak changes for early (E24h) → intermediate (E5d) transition (log2FC E5d/E24h) versus int (E7d) → late (M) transition (log2FC M/E7d) (upper). Lower panel shows peak changes for early (T24h) → int (T5d) transition (log2FC T5d/T24h) versus int (T7d) → late (T14d) transition (log2FC T14d/T7d). Each point represents an individual peak colored according to the scheme in Fig. 5c. To the right are shown corresponding bar plots showing the number of peaks in e.
Extended Data Fig. 8 Early TST uniformly express TCF1.
a, Experimental scheme: CFSE-labeled naive TCRTAG (Thy1.1) were adoptively transferred into B6 (Thy1.2) or ASTxAlb-Cre mice (Thy1.2). TCRTAG were re-isolated at 36h (T36h), 5d (T5d), and 60d (T60d) from spleens and livers for flow cytometric analysis (Naive in vivo (N; grey); tumor (T; blue)). b, Dot plots of TST TCF1 and PD1 expression by CFSE dilution across time points. c, Dot plots comparing CD38 and CD101 (top) or TOX and TCF1 (bottom) expression in TST isolated from tumor-bearing livers across time points. Gates set based on N with inset numbers indicating percentage of cells in each gate. Plots are concatenated from 3-4 samples/time point.
Extended Data Fig. 9 Duration of tumor antigen exposure determines dysfunction stability and imprinting.
Chromatin accessibility profiles across selected gene loci for TCRTAG activated in tumor for 24h (T24h), 5d (T5d), 10d (T10d) pre-parking (blue) and post-parking (P24h, P5d, P10d) (purple) with naive (grey) and memory (green). a, Pdcd1 gene locus with −23 kb enhancer peak boxed in red. Summary plot (right) shows the DESeq2-determined log2FC and FDR (two-sided Wald test with Benjamini-Hochberg correction) at the Pdcd1 −23kb peak for pre- (blue) and post-park (purple) relative to naive. b, Tox locus with blue box representing region without epigenetic scarring and red box showing region with epigenetic scarring. Star denotes representative +100 kb peak from scar region used for quantification on right. Late dysfunctional TCRTAG activated in tumor (D35) added for comparison. Summary plot (right) shows the DESeq2-determined log2FC and FDR (two-sided Wald test with Benjamini-Hochberg correction) at the Tox +220 kb and +100 kb peaks for pre- (blue) and post-park (purple) relative to naive. c, Ifng locus with black box denoting +14kb peak open in memory but not in pre- or post-parking samples, and red box denoting +19kb peak open in both pre- and post-parking samples. Summary plot (right) shows the DESeq2-determined log2FC and FDR (two-sided Wald test with Benjamini-Hochberg correction) for the Ifng +14kb and +19 kb peak area pre- (blue) and post-park (purple) relative to memory.
Supplementary information
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Statistical source data.
Source Data Fig. 7
Statistical source data.
Source Data Extended Data Fig. 1
Source Data Extended Data Fig. 1.
Source Data Extended Data Fig. 2
Source Data Extended Data Fig. 2.
Source Data Extended Data Fig. 3
Source Data Extended Data Fig. 3.
Source Data Extended Data Fig. 4
Source Data Extended Data Fig. 4.
Source Data Extended Data Fig. 5
Source Data Extended Data Fig. 5.
Source Data Extended Data Fig. 7
Source Data Extended Data Fig. 7.
Source Data Extended Data Fig. 9
Source Data Extended Data Fig. 9.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rudloff, M.W., Zumbo, P., Favret, N.R. et al. Hallmarks of CD8+ T cell dysfunction are established within hours of tumor antigen encounter before cell division. Nat Immunol 24, 1527–1539 (2023). https://doi.org/10.1038/s41590-023-01578-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41590-023-01578-y
This article is cited by
-
Construction and validation of a metabolism-associated gene signature for predicting the prognosis, immune landscape, and drug sensitivity in bladder cancer
BMC Medical Genomics (2023)
-
The road not taken en route to T cell exhaustion
Nature Immunology (2023)
-
Trogocytosis of CAR molecule regulates CAR-T cell dysfunction and tumor antigen escape
Signal Transduction and Targeted Therapy (2023)