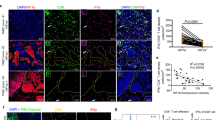
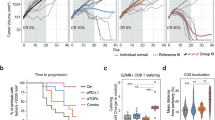
Abstract
CD8+ T cells are critical for elimination of cancer cells. Factors within the tumor microenvironment (TME) can drive these cells to a hypofunctional state known as exhaustion. The most terminally exhausted T (tTex) cells are resistant to checkpoint blockade immunotherapy and might instead limit immunotherapeutic efficacy. Here we show that intratumoral CD8+ tTex cells possess transcriptional features of CD4+Foxp3+ regulatory T cells and are similarly capable of directly suppressing T cell proliferation ex vivo. tTex cell suppression requires CD39, which generates immunosuppressive adenosine. Restricted deletion of CD39 in endogenous CD8+ T cells resulted in slowed tumor progression, improved immunotherapy responsiveness and enhanced infiltration of transferred tumor-specific T cells. CD39 is induced on tTex cells by tumor hypoxia, thus mitigation of hypoxia limits tTex suppression. Together, these data suggest tTex cells are an important regulatory population in cancer and strategies to limit their generation, reprogram their immunosuppressive state or remove them from the TME might potentiate immunotherapy.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
Publicly available fastq files were downloaded from the Gene Expression Omnibus under accession code GSE123235. All other data are present in the article and supplementary files or are available from the corresponding author upon reasonable request. Source data are provided with this paper.
References
Blank, C. U. et al. Defining ‘T cell exhaustion’. Nat. Rev. Immunol. 19, 665–674 (2019).
Im, S. J. et al. Defining CD8+ T cells that provide the proliferative burst after PD-1 therapy. Nature 537, 417–421 (2016).
Miller, B. C. et al. Subsets of exhausted CD8+ T cells differentially mediate tumor control and respond to checkpoint blockade. Nat. Immunol. 20, 326–336 (2019).
Bruni, D., Angell, H. K. & Galon, J. The immune contexture and Immunoscore in cancer prognosis and therapeutic efficacy. Nat. Rev. Cancer 20, 662–680 (2020).
Sade-Feldman, M. et al. Defining T cell states associated with response to checkpoint immunotherapy in melanoma. Cell 175, 998–1013 (2018).
DePeaux, K. & Delgoffe, G. M. Metabolic barriers to cancer immunotherapy. Nat. Rev. Immunol. 21, 785–797 (2021).
Jayaprakash, P., Vignali, P. D. A., Delgoffe, G. M. & Curran, M. A. Hypoxia reduction sensitizes refractory cancers to immunotherapy. Annu. Rev. Med. 73, 251–265 (2022).
Ford, B. R. et al. Tumor microenvironmental signals reshape chromatin landscapes to limit the functional potential of exhausted T cells. Sci. Immunol. 7, eabj9123 (2022).
Wherry, E. J. & Kurachi, M. Molecular and cellular insights into T cell exhaustion. Nat. Rev. Immunol. 15, 486–499 (2015).
Collier, J. L., Weiss, S. A., Pauken, K. E., Sen, D. R. & Sharpe, A. H. Not-so-opposite ends of the spectrum: CD8+ T cell dysfunction across chronic infection, cancer and autoimmunity. Nat. Immunol. 22, 809–819 (2021).
Hardardottir, L. et al. The new old CD8+ T cells in the immune paradox of pregnancy. Front. Immunol. 12, 765730 (2021).
Pagès, F. et al. International validation of the consensus Immunoscore for the classification of colon cancer: a prognostic and accuracy study. Lancet 391, 2128–2139 (2018).
Scharping, N. E. et al. Mitochondrial stress induced by continuous stimulation under hypoxia rapidly drives T cell exhaustion. Nat. Immunol. 22, 205–215 (2021).
Zandberg, D. P. et al. Tumor hypoxia is associated with resistance to PD-1 blockade in squamous cell carcinoma of the head and neck. J. Immunother. Cancer 9, e002088 (2021).
Najjar, Y. G. et al. Tumor cell oxidative metabolism as a barrier to PD-1 blockade immunotherapy in melanoma. JCI Insight 4, e124989 (2019).
Canale, F. P. et al. CD39 expression defines cell exhaustion in tumor-infiltrating CD8+ T cells. Cancer Res. 78, 115–128 (2018).
Magnuson, A. M. et al. Identification and validation of a tumor-infiltrating Treg transcriptional signature conserved across species and tumor types. Proc. Natl Acad. Sci. USA 115, E10672–E10681 (2018).
Watson, M. J. et al. Metabolic support of tumour-infiltrating regulatory T cells by lactic acid. Nature 591, 645–651 (2021).
Zhao, Y. et al. IL-4 induces a suppressive IL-10-producing CD8+ T cell population via a Cdkn2a-dependent mechanism. J. Leukoc. Biol. 94, 1103–1112 (2013).
Jin, H.-T. et al. Cooperation of Tim-3 and PD-1 in CD8 T-cell exhaustion during chronic viral infection. Proc. Natl Acad. Sci. USA 107, 14733–14738 (2010).
Guo, Y. et al. Metabolic reprogramming of terminally exhausted CD8+ T cells by IL-10 enhances anti-tumor immunity. Nat. Immunol. 22, 746–756 (2021).
Seo, N., Hayakawa, S., Takigawa, M. & Tokura, Y. Interleukin-10 expressed at early tumour sites induces subsequent generation of CD4(+) T-regulatory cells and systemic collapse of antitumour immunity. Immunology 103, 449–457 (2001).
Hanna, B. S. et al. Interleukin-10 receptor signaling promotes the maintenance of a PD-1int TCF-1+ CD8+ T cell population that sustains anti-tumor immunity. Immunity 54, 2825–2841 (2021).
Li, J. et al. KIR+CD8+ T cells suppress pathogenic T cells and are active in autoimmune diseases and COVID-19. Science 376, eabi9591 (2022).
Cao, X. et al. Granzyme B and perforin are important for regulatory T cell-mediated suppression of tumor clearance. Immunity 27, 635–646 (2007).
Maj, T. et al. Oxidative stress controls regulatory T cell apoptosis and suppressor activity and PD-L1-blockade resistance in tumor. Nat. Immunol. 18, 1332–1341 (2017).
Deaglio, S. et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J. Exp. Med. 204, 1257–1265 (2007).
Gupta, P. K. et al. CD39 expression identifies terminally exhausted CD8+ T cells. PLoS Pathog. 11, e1005177 (2015).
Schuler, P. J. et al. Human CD4+ CD39+ regulatory T cells produce adenosine upon co-expression of surface CD73 or contact with CD73+ exosomes or CD73+ cells. Clin. Exp. Immunol. 177, 531–543 (2014).
Rothweiler, S. et al. Selective deletion of ENTPD1/CD39 in macrophages exacerbates biliary fibrosis in a mouse model of sclerosing cholangitis. Purinergic Signal. 15, 375–385 (2019).
Menk, A. V. et al. Early TCR signaling induces rapid aerobic glycolysis enabling distinct acute T cell effector functions. Cell Rep. 22, 1509–1521 (2018).
Doedens, A. L. et al. Hypoxia-inducible factors enhance the effector responses of CD8(+) T cells to persistent antigen. Nat. Immunol. 14, 1173–1182 (2013).
McKeown, S. R. Defining normoxia, physoxia and hypoxia in tumours-implications for treatment response. Br. J. Radiol. 87, 20130676 (2014).
Scharping, N. E., Menk, A. V., Whetstone, R. D., Zeng, X. & Delgoffe, G. M. Efficacy of PD-1 blockade is potentiated by metformin-induced reduction of tumor hypoxia. Cancer Immunol. Res. 5, 9–16 (2017).
Tirosh, I. et al. Dissecting the multicellular ecosystem of metastatic melanoma by single-cell RNA-seq. Science 352, 189–196 (2016).
Wherry, E. J. et al. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity 27, 670–684 (2007).
Linsley, P. S. & Long, S. A. Enforcing the checkpoints: harnessing T-cell exhaustion for therapy of T1D. Curr. Opin. Endocrinol. Diabetes Obes. 26, 213–218 (2019).
Perrot, I. et al. Blocking antibodies targeting the CD39/CD73 immunosuppressive pathway unleash immune responses in combination cancer therapies. Cell Rep. 27, 2411–2425 (2019).
Allard, D., Allard, B. & Stagg, J. On the mechanism of anti-CD39 immune checkpoint therapy. J. Immunother. Cancer 8, e000186 (2020).
Lee, Y. G. et al. Modulation of BCL-2 in both T cells and tumor cells to enhance chimeric antigen receptor T cell Immunotherapy against cancer. Cancer Discov. 12, 2372–2391 (2022).
Horton, B. L., Williams, J. B., Cabanov, A., Spranger, S. & Gajewski, T. F. Intratumoral CD8+ T-cell apoptosis is a major component of T-cell dysfunction and impedes antitumor immunity. Cancer Immunol. Res 6, 14–24 (2018).
Boison, D. & Yegutkin, G. G. Adenosine metabolism: emerging concepts for cancer therapy. Cancer Cell 36, 582–596 (2019).
Guo, S., Han, F. & Zhu, W. CD39 - A bright target for cancer immunotherapy. Biomed. Pharmacother. 151, 113066 (2022).
Zhang, H. et al. The role of NK cells and CD39 in the immunological control of tumor metastases. Oncoimmunology 8, e1593809 (2019).
Tøndell, A. et al. Ectonucleotidase CD39 and checkpoint signalling receptor programmed death 1 are highly elevated in intratumoral immune cells in non-small-cell lung cancer. Transl. Oncol. 13, 17–24 (2020).
Duhen, T. et al. Co-expression of CD39 and CD103 identifies tumor-reactive CD8 T cells in human solid tumors. Nat. Commun. 9, 2724 (2018).
Gallerano, D. et al. Genetically driven CD39 expression shapes human tumor-infiltrating CD8+ T-cell functions. Int. J. Cancer 147, 2597–2610 (2020).
Rivas, J. R. et al. Interleukin-10 suppression enhances T-cell antitumor immunity and responses to checkpoint blockade in chronic lymphocytic leukemia. Leukemia 35, 3188–3200 (2021).
Sawant, D. V. et al. Adaptive plasticity of IL-10+ and IL-35+ Treg cells cooperatively promotes tumor T cell exhaustion. Nat. Immunol. 20, 724–735 (2019).
Oft, M. IL-10: master switch from tumor-promoting inflammation to antitumor immunity. Cancer Immunol. Res. 2, 194–199 (2014).
Pfannenstiel, L. W. et al. Immune-checkpoint blockade opposes CD8+ T-cell suppression in human and murine cancer. Cancer Immunol. Res. 7, 510–525 (2019).
Naing, A. et al. PEGylated IL-10 (pegilodecakin) induces systemic immune activation, CD8+ T cell invigoration and polyclonal T cell expansion in cancer patients. Cancer Cell 34, 775–791 (2018).
Acknowledgements
The authors thank all members of the Delgoffe laboratory for helpful and invigorating discussions. We also thank friends and collaborators at the University of Pittsburgh, especially A. Burr and the Hand laboratory. This work was supported by a National Institutes of Health (NIH) Director’s New Innovator Award (DP2AI136598); the National Institute of Allergy and Infectious Disease (R01AI171483, R01AI166598); the Hillman Fellows for Innovative Cancer Research Program; a Stand Up to Cancer–American Association for Cancer Research Innovative Research Grant (SU2C-AACR-IRG-04-16); the Alliance for Cancer Gene Therapy; the UPMC Hillman Cancer Center Skin Cancer and Head and Neck Cancer SPOREs (P50CA121973 and P50CA097190; NIH); the Mark Foundation for Cancer Research’s Emerging Leader Award; and a Cancer Research Institute-Lloyd J. Old STAR Award; and the Sy Holzer Endowed Immunotherapy Fund (all to G.M.D.). Trainees on this manuscript were supported by grants T32CA082084 (NIH) (to P.D.A.V., M.J.W., K.D. and B.R.F.), F30CA247034 and T32GM008208 (NIH) (to P.D.A.V.), F31AI149971 (NIH) (to M.J.W.), F31CA247129 (NIH) (to K.D.). This work used the UPMC Hillman Cancer Center Flow Cytometry and Animal Facilities, supported in part by grant P30CA047904 (NIH). This work was supported by the Health Sciences Sequencing Core at UPMC Children’s Research Hospital of Pittsburgh and the University of Pittsburgh Center for Research Computing. Some images were derived from Biorender.com. We also acknowledge the authors of important papers that we could not cite due to space constraints.
Author information
Authors and Affiliations
Contributions
P.D.A.V. conceived and performed the majority of the experiments, compiled and analyzed data and wrote the manuscript. K.D. performed TIL analysis and suppression assays in the B16-ND4– and axitinib/metformin experiments and contributed to the editing of the text. M.J.W. carried out initial experiments and performed several assays characterizing CD39 overexpression in effector T cells. C.Y. performed critical experiments involving cell death in tTex cells. B.R.F. performed bioinformatic analysis of publicly available data sets comparing Foxp3+ Treg cells to CD8+ TILs and contributed to the editing of the text. K.L. performed detailed statistical analysis on many of the figures. N.K.M. helped characterize CD39 overexpression in Teff cells. N.E.S. supported generation of data characterizing tTex cells. A.V.M. supported generation of metabolic assay data in CD39 overexpression experiments. S.C.R. generously donated the Entpd1f/f mice. A.C.P. supported the bioinformatic analysis. D.B.R. performed several experiments and helped direct the research. G.M.D. conceived of the study, directed the research, obtained funding and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
A.V.M. is currently an employee of Novasenta. G.M.D. declares competing financial interests and has submitted patents targeting exhausted T cells that are licensed or pending and is entitled to a share in net income generated from licensing of these patent rights for commercial development. G.M.D. consults for and/or is on the scientific advisory board of BlueSphere Bio, Century Therapeutics, Nanna Therapeutics, Novasenta, Pieris Pharmaceuticals and Western Oncolytics/Kalivir; has grants from bluebird bio, Novasenta, Pfizer, Pieris Pharmaceuticals, TCR2 and Western Oncolytics/Kalivir. G.M.D. owns stock in Novasenta, BlueSphere Bio and RemplirBio. The other authors declare no competing interests.
Peer review
Peer review information
Nature Immunology thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editor: N. Bernard, in collaboration with the Nature Immunology team. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 PD-1hiTim-3+ terminally exhausted CD8+ T cells are a numerically dominant in tumor and express numerous Treg cell-associated effector molecules.
(a) Gating strategy for isolating CD8+ T cells in B16-F10 tumor or tumor-draining lymph nodes. (b) Quantified distribution of inhibitory receptor expression on CD8+ TIL from Fig. 1a. (c) Total cell numbers per size-matched B16-F10 tumor or draining lymph node. (d) Quantification from Fig. 1c. Mean fluorescence intensity (MFI) of CD4+Foxp3+ Treg cell-associated genes among tumor-infiltrating T cell populations. Statistics are one-way ANOVA (C,D) with ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001 and ∗∗∗∗p < 0.0001.
Extended Data Fig. 2 Tumor infiltrating CD8+ T cells upregulate Treg cell signature upon terminal differentiation.
(a) Gene set enrichment analysis (GSEA) of tumor-infiltrating Treg cell signature on bulk SLAMF6+ progenitor and Tim-3+ terminally exhausted CD8+ T cell transcripts from Miller et al. (b, c) Heatmap displaying DESeq2 of log2 normalized transcript expression of genes from the tumor-infiltrating Treg cell signature gene set in tetramer+ (b) or bulk (c) progenitor and terminally exhausted CD8 + T cells. Values are transformed log2 (TPM) scaled to row. (d) Heatmap of log2 normalized DESeq2-defined differentially expressed genes (DEG) between progenitor and terminally exhausted CD8+ T cells.
Extended Data Fig. 3 Quantification of tumor sizes and TIL populations from preclinical models with diverse sensitivity to immunotherapy.
(a) Tumor sizes from suppression assay experiments in Figs. 1 and 2(b) (f) Average calculated percent suppression at 1:4 suppressor to responder ratio from all replicate experiments in Fig. 1 and supplementary Fig. 3r. (G) Percent of CD8+PD-1+Tim-3+ tTexh cells in various murine tumor models. Bivariant plots and histograms are representative of ≥3 experiments. Statistics are one-way ANOVA (A-C) with ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001 and ∗∗∗∗p < 0.0001.
Extended Data Fig. 4 tTex cells do not suppress via IL-10 secretion or direct cytotoxicity.
(a, b) Day 14 B16-F10 tumor sizes from Il10–/– experiments and matched CD8+ T cell infiltrate. (c) Suppression assay of B16-F10 infiltrating tTex cells co-cultured with isotype IgG or neutralizing IL-10 antibody at 2.5 or 5.0 𝜇g/mL. (d) Gating strategy for calculation of cell viability in suppression assay populations. (e) Calculated averages of ‘responder’ or ‘APC’ population cell viability per dilution series of ‘suppressor’ T cells from Fig. 1g. (f) Cell viability of responder T cell populations from Fig. 3b. (g) Suppression assay of B16-F10 infiltrating tTex cells co-cultured with CTV-labeled responding T cells with either live or Mytomycin C (MC)-killed T cell-depleted splenocytes, or anti-CD3/anti-CD28 bound microbeads. (h) Cell viability of ‘suppressor’ populations from Fig. 1g. (i) Cell viability of ‘suppressor’ populations from Fig. 3d, e. Statistics are Mann-Whitney (A,B,F,H), linear regression (C) and one-way ANOVA (G,I) with ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001 and ∗∗∗∗p < 0.0001.
Extended Data Fig. 5 tTex cells suppress through CD39-mediated extracellular ATP depletion and adenosine production.
(a, b) Human CD8+ T cell populations from five melanoma biopsies from treatment-naïve patients and corresponding CD39 expression. (c) Sorting strategy for Fig. 4b. (d) Percent CD8+ T cells expressing CD39 in various tissues in a B16-F10 tumor-bearing C57/BL6 mice. (e, f) Suppression assays of B16-F10 infiltrating CD4+ Treg cells, co-cultured (E) with activated CTV-labeled C57/BL or Nt5e– responding T cells or (F) with DMSO vehicle or A2AR/A2BR small-molecule inhibitor AB928 at 3 𝜇g/mL. Statistics are one-way ANOVA (B), and linear regression (E,F) with ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001 and ∗∗∗∗p < 0.0001.
Extended Data Fig. 6 Cd4-driven Cre recombinase expression efficiently deletes CD39 on CD8+ TIL.
(a, b) TIL analysis of CD39 and inhibitory expression in CD8+ T cells from Cd4CreEntpd1f/f mice. (c) Cell counts per milligram of tumor mass from experiments in Extended Data Fig. 6a, b. (d) Tumor areas at day 14 from experiments in Fig. 2f. (e) Suppression assay of Cd4CreEntpd1f/f Thy1.1+CD44+ OT-I Teff cells isolated from day 8 of acute VacciniaOVA infection. (f) CD39 expression on TIL tTex cells or VacciniaOVA OT-I T cells in suppression assay co-cultures. (g) Suppression assay of B16-F10-derived CD8+ tTex cells co-cultured with OT-I TCR transgenic CD8+ T cells. Culture were stimulated with either anti-CD3 antibodies as before, or OT-I specific peptide, SIIFEKL. (h–j) Flow cytometric analysis of TIL following suppression assay from Extended Data Fig. 6g. Statistics are Mann-Whitney (B–D,H-J) and linear regression (E,G) with ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001 and ∗∗∗∗p < 0.0001.
Extended Data Fig. 7 Enforced CD39 expression on CD8+ T cells limits metabolic reprogramming via disruption of TCR signaling.
(a) Extracellular acidification rate (ECAR) in in-Seahorse activation of rested CD8 + T cells stability transduced with pMSCV or pMSCV-Entpd1. (b) Delta-maximal ECAR quantified by basal ECAR – maximal ECAR; minutes till maximal ECAR per sample well. (c) Oxygen consumption rate (OCR) of cells from Extended Data Fig. 7c with (d) quantified basal OCR. (e) Intracellular calcium flux in day 7 transduced T cells restimulated in a calcium-buffered solution with anti-CD3/anti-CD28-bound microbeads in the presence of calcium indicators, Fluo-4 and Fura Red. (f) Cytokine production of transduced T cells following 24-hour anti-CD3/anti-CD28-bound microbeads stimulation. (g) ELISA of supernatants from repeat experiments of Extended Data Fig. 7f. Statistics are Mann-Whitney (B,D,G) and one-way ANOVA (E,F) with ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001 and ∗∗∗∗p < 0.0001.
Extended Data Fig. 8 E8i-driven Cre recombinase expression efficiently deletes CD39 on CD8+ TIL.
(a) CD39 expression in day 14 B16-F10 tumor-infiltrating CD8+ T cells from Entpd1f/f or E8iCre-ERT2Entpd1f/f mice. (b) Cytokine production following PMA/Ionomycin re-simulation of day 14 CD8+ TIL. (c, d) Infiltration of adoptively transferred pmel-I T cells into tumor-draining lymph nodes (dLN; C) and (D) quantification of pmel-I percentages in a representative experiment. (e) Ex vivo cytokine production of pmel-I T cells following gp100 re-stimulation. (f) TIL analysis of CD39 expression in CD8+ T cells from E8iCre-ERT2Entpd1f/f mice treated for three consecutive days with tamoxifen. (g, h) Representative flow cytometry plots from Fig. 7e, f. Statistics are one-way ANOVA (B,D,E) and Mann-Whitney (F) with ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001 and ∗∗∗∗p < 0.0001.
Extended Data Fig. 9 Tumor hypoxia enforces CD39 expression on tTex cells.
(a) Histogram overlay displaying hypoxia exposure in CD8+ dLN and TIL from B16-F10 tumors. (b) CD39 staining in exhausted T cells from B16-F10 or MC38, and (c) Pimonidazole staining of bulk TIL. (d) Continuous Activation under Hypoxia (CS + H) assay. In brief, naïve T cells are activated for 24 hours, then split into treatment groups of removal (acute stimulation) or continued presence (continuous stimulation) of anti-CD3/anti-CD28-bound microbeads and cultured under atmospheric oxygen tensions (~20% O2) or tumor hypoxic conditions (1.5% O2) for 5 days. (e) Inhibitory receptor staining in murine day 6 CS + H cells. (f–h) Validation of humanized CS + H assay with healthy donor PBMC-derived CD8+ T cells via staining of (F) inhibitory receptors and (g) enzymes of adenosine metabolism. (h) 24-hour PMA/Ionomycin re-stimulation of human CS + H cells. Statistics are Mann-Whitney (B,C), one-way ANOVA (H) with ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001 and ∗∗∗∗p < 0.0001.
Extended Data Fig. 10 Tumor hypoxia mitigation as a therapeutic target to lessen tTex cell-mediated suppression.
(a) Extracellular flux analysis showing validation of mitochondrial respiration knockdown (via OCR measurement) in B16ND4– tumor cells versus parental B16-F10. (b) PD-1 and Tim-3 staining in TIL from day 14 wild-type B16-F10 or B16ND4– tumors. (c) Schematic of treatment plan for therapeutic alleviation of tumor hypoxia. (d) Tumor sizes at treatment initiation and sacrifice in Axitinib/metformin experiments. (e) PD-1 and Tim-3 staining in TIL from Axitinib/metformin experiments in Extended Data Fig. 9d. (f) Suppression assays of CD4+Foxp3+ Treg cells from Fig. 9c. (g) Suppression assays of CD4+Foxp3+ Treg cells from Fig. 9f. Statistics are Mann-Whitney (B), one-way ANOVA (E) and linear regression (F,G) with ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001 and ∗∗∗∗p < 0.0001.
Supplementary information
Source data
Source Data Fig. 1
Raw data for all Prism plots.
Source Data Fig. 2
Raw data for all Prism plots.
Source Data Fig. 3
Raw data for all Prism plots.
Source Data Fig. 4
Raw data for all Prism plots.
Source Data Fig. 5
Raw data for all Prism plots.
Source Data Fig. 5
Unmodified immunoblots, scanned in.
Source Data Fig. 6
Raw data for all Prism plots.
Source Data Fig. 7
Raw data for all Prism plots.
Source Data Fig. 8
Raw data for all Prism plots.
Source Data Extended Data Fig. 1
Raw data for all Prism plots.
Source Data Extended Data Fig. 3
Raw data for all Prism plots.
Source Data Extended Data Fig. 4
Raw data for all Prism plots.
Source Data Extended Data Fig. 5
Raw data for all Prism plots.
Source Data Extended Data Fig. 6
Raw data for all Prism plots.
Source Data Extended Data Fig. 7
Raw data for all Prism plots.
Source Data Extended Data Fig. 8
Raw data for all Prism plots.
Source Data Extended Data Fig. 9
Raw data for all Prism plots.
Source Data Extended Data Fig. 10
Raw data for all Prism plots.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Vignali, P.D.A., DePeaux, K., Watson, M.J. et al. Hypoxia drives CD39-dependent suppressor function in exhausted T cells to limit antitumor immunity. Nat Immunol 24, 267–279 (2023). https://doi.org/10.1038/s41590-022-01379-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41590-022-01379-9
This article is cited by
-
Spatial features of specific CD103+CD8+ tissue-resident memory T cell subsets define the prognosis in patients with non-small cell lung cancer
Journal of Translational Medicine (2024)
-
Cancer cell metabolism and antitumour immunity
Nature Reviews Immunology (2024)
-
Associations between HIFs and tumor immune checkpoints: mechanism and therapy
Discover Oncology (2024)
-
Targeting the epigenome to reinvigorate T cells for cancer immunotherapy
Military Medical Research (2023)
-
CD39 identifies a specific CD8 + T cell population in lung adenocarcinoma-related metastatic pleural effusion
BMC Immunology (2023)