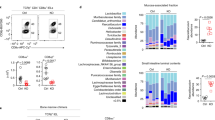
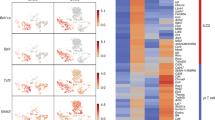
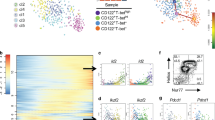
Abstract
Mesenteric lymph node (mLN) T cells undergo tissue adaptation upon migrating to intestinal lamina propria and epithelium, ensuring appropriate balance between tolerance and resistance. By combining mouse genetics with single-cell and chromatin analyses, we uncovered the molecular imprinting of gut epithelium on T cells. Transcriptionally, conventional and regulatory (Treg) CD4+ T cells from mLN, lamina propria and intestinal epithelium segregate based on the gut layer they occupy; trajectory analysis suggests a stepwise loss of CD4 programming and acquisition of an intraepithelial profile. Treg cell fate mapping coupled with RNA sequencing and assay for transposase-accessible chromatin followed by sequencing revealed that the Treg cell program shuts down before an intraepithelial program becomes fully accessible at the epithelium. Ablation of CD4-lineage-defining transcription factor ThPOK results in premature acquisition of an intraepithelial lymphocyte profile by mLN Treg cells, partially recapitulating epithelium imprinting. Thus, coordinated replacement of the circulating lymphocyte program with site-specific transcriptional and chromatin changes is necessary for tissue imprinting.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All generated raw and processed sequencing data for this study (all main figures) can be accessed at GEO: GSE146292 and BioProject: PRJNA610060. Publicly available data on ThPOK motif were obtained24 and can be accessed at GEO: GSE116506 and BioProject: PRJNA478869. All other data that support the findings of this study are available from the corresponding author upon request.
References
Mucida, D. et al. Oral tolerance in the absence of naturally occurring Tregs. J. Clin. Invest. 115, 1923–1933 (2005).
Bilate, A. M. & Lafaille, J. J. Induced CD4+Foxp3+ regulatory T cells in immune tolerance. Annu. Rev. Immunol. 30, 733–758 (2012).
Furusawa, Y. et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 504, 446–450 (2013).
Hadis, U. et al. Intestinal tolerance requires gut homing and expansion of FoxP3+ regulatory T cells in the lamina propria. Immunity 34, 237–246 (2011).
Sujino, T. et al. Tissue adaptation of regulatory and intraepithelial CD4+ T cells controls gut inflammation. Science 352, 1581–1586 (2016).
Atarashi, K. et al. Induction of colonic regulatory T cells by indigenous Clostridium species. Science 331, 337–341 (2011).
Atarashi, K. et al. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature 500, 232–236 (2013).
Olivares-Villagómez, D. & Van Kaer, L. Intestinal intraepithelial lymphocytes: sentinels of the mucosal barrier. Trends Immunol. 39, 264–275 (2018).
McDonald, B. D., Jabri, B. & Bendelac, A. Diverse developmental pathways of intestinal intraepithelial lymphocytes. Nat. Rev. Immunol. 18, 514–525 (2018).
Vantourout, P. & Hayday, A. Six-of-the-best: unique contributions of γδ T cells to immunology. Nat. Rev. Immunol. 13, 88–100 (2013).
Reis, B. S., Rogoz, A., Costa-Pinto, F. A., Taniuchi, I. & Mucida, D. Mutual expression of the transcription factors Runx3 and ThPOK regulates intestinal CD4+ T cell immunity. Nat. Immunol. 14, 271–280 (2013).
Reis, B. S., Hoytema van Konijnenburg, D. P., Grivennikov, S. I. & Mucida, D. Transcription factor T-bet regulates intraepithelial lymphocyte functional maturation. Immunity 41, 244–256 (2014).
Konkel, J. E. et al. Control of the development of CD8αα+ intestinal intraepithelial lymphocytes by TGF-β.Nat. Immunol. 12, 312–319 (2011).
Bilate, A. M. et al. Tissue-specific emergence of regulatory and intraepithelial T cells from a clonal T cell precursor. Sci. Immunol. 1, eaaf7471 (2016).
Mucida, D. et al. Transcriptional reprogramming of mature CD4+ helper T cells generates distinct MHC class II–restricted cytotoxic T lymphocytes. Nat. Immunol. 14, 281–289 (2013).
Rubtsov, Y. P. et al. Stability of the regulatory T cell lineage in vivo. Science 329, 1667–1671 (2010).
Setoguchi, R. et al. Repression of the transcription factor Th-POK by Runx complexes in cytotoxic T cell development. Science 319, 822–825 (2008).
Becht, E. et al. Dimensionality reduction for visualizing single-cell data using UMAP. Nat. Biotechnol. 37, 38–44 (2019).
Zemmour, D. et al. Single-cell gene expression reveals a landscape of regulatory T cell phenotypes shaped by the TCR. Nat. Immunol. 19, 291–301 (2018).
Miragaia, R. J. et al. Single-cell transcriptomics of regulatory T cells reveals trajectories of tissue adaptation. Immunity 50, 493–504.e7 (2019).
Bilate, A. M. et al. T cell receptor is required for differentiation, but not maintenance, of intestinal CD4+ intraepithelial lymphocytes. Immunity 53, 1001–1014 e1020 (2020).
Buenrostro, J. D., Giresi, P. G., Zaba, L. C., Chang, H. Y. & Greenleaf, W. J. Transposition of native chromatin for fast and sensitive epigenomic profiling of open chromatin, DNA-binding proteins and nucleosome position. Nat. Methods 10, 1213–1218 (2013).
Badis, G. et al. Diversity and complexity in DNA recognition by transcription factors. Science 324, 1720–1723 (2009).
Ciucci, T. et al. The emergence and functional fitness of memory CD4+ T cells require the transcription factor Thpok. Immunity 50, 91–105.e4 (2019).
Machanick, P. & Bailey, T. L. MEME-ChIP: motif analysis of large DNA datasets. Bioinformatics 27, 1696–1697 (2011).
Egawa, T. & Littman, D. R. ThPOK acts late in specification of the helper T cell lineage and suppresses Runx-mediated commitment to the cytotoxic T cell lineage. Nat. Immunol. 9, 1131–1139 (2008).
Naoe, Y. et al. Repression of interleukin-4 in T helper type 1 cells by Runx/Cbfβ binding to the Il4 silencer. J. Exp. Med. 204, 1749–1755 (2007).
Powrie, F., Leach, M. W., Mauze, S., Caddle, L. B. & Coffman, R. L. Phenotypically distinct subsets of CD4+ T cells induce or protect from chronic intestinal inflammation in C. B-17 scid mice. Int. Immunol. 5, 1461–1471 (1993).
DiSpirito, J. R. et al. Molecular diversification of regulatory T cells in nonlymphoid tissues. Sci. Immunol. 3, eaat5861 (2018).
Delacher, M. et al. Precursors for nonlymphoid-tissue Treg cells reside in secondary lymphoid organs and are programmed by the transcription factor BATF. Immunity 52, 295–312 (2020).
Samstein, R. M. et al. Foxp3 exploits a pre-existent enhancer landscape for regulatory T cell lineage specification. Cell 151, 153–166 (2012).
Bruno, L. et al. Runx proteins regulate Foxp3 expression. J. Exp. Med. 206, 2329–2337 (2009).
Kitoh, A. et al. Indispensable role of the Runx1-Cbfβ transcription complex for in vivo-suppressive function of FoxP3+ regulatory T cells. Immunity 31, 609–620 (2009).
Klunker, S. et al. Transcription factors RUNX1 and RUNX3 in the induction and suppressive function of Foxp3+ inducible regulatory T cells. J. Exp. Med. 206, 2701–2715 (2009).
Carpenter, A. C. et al. Control of regulatory T cell differentiation by the transcription factors Thpok and LRF. J. Immunol. 199, 1716–1728 (2017).
Kanamori, M., Nakatsukasa, H., Okada, M., Lu, Q. & Yoshimura, A. Induced regulatory T cells: their development, stability, and applications. Trends Immunol. 37, 803–811 (2016).
Masopust, D. & Soerens, A. G. Tissue-resident T cells and other resident leukocytes. Annu. Rev. Immunol. 37, 521–546 (2019).
Fonseca, R. et al. Developmental plasticity allows outside-in immune responses by resident memory T cells. Nat. Immunol. 21, 412–421 (2020).
Gebhardt, T. et al. Different patterns of peripheral migration by memory CD4+ and CD8+ T cells. Nature 477, 216–219 (2011).
Ricardo-Gonzalez, R. R. et al. Tissue signals imprint ILC2 identity with anticipatory function. Nat. Immunol. 19, 1093–1099 (2018).
Cohen, M. et al. Lung single-cell signaling interaction map reveals basophil role in macrophage imprinting. Cell 175, 1031–1044.e18 (2018).
Chassaing, B. et al. Fecal lipocalin 2, a sensitive and broadly dynamic non-invasive biomarker for intestinal inflammation. PloS ONE 7, e44328 (2012).
Boyman, O., Kovar, M., Rubinstein, M. P., Surh, C. D. & Sprent, J. Selective stimulation of T cell subsets with antibody-cytokine immune complexes. Science 311, 1924–1927 (2006).
Webster, K. E. et al. In vivo expansion of T reg cells with IL-2-mAb complexes: induction of resistance to EAE and long-term acceptance of islet allografts without immunosuppression. J. Exp. Med. 206, 751–760 (2009).
Trombetta, J. J. et al. Preparation of single-cell RNA-seq libraries for next generation sequencing. Curr. Protoc. Mol. Biol. 107, 4.22.1–4.22.17 (2014).
Stuart, T. et al. Comprehensive integration of single-cell data. Cell 177, 1888–1902.e21 (2019).
Gu, Z., Eils, R. & Schlesner, M. Complex heatmaps reveal patterns and correlations in multidimensional genomic data. Bioinformatics 32, 2847–2849 (2016).
Cao, J. et al. The single-cell transcriptional landscape of mammalian organogenesis. Nature 566, 496–502 (2019).
Street, K. et al. Slingshot: cell lineage and pseudotime inference for single-cell transcriptomics. BMC Genomics 19, 477 (2018).
Bray, N. L., Pimentel, H., Melsted, P. & Pachter, L. Near-optimal probabilistic RNA-seq quantification. Nat. Biotechnol. 34, 525–527 (2016).
Frankish, A. et al. GENCODE reference annotation for the human and mouse genomes. Nucleic Acids Res. 47, D766–D773 (2019).
Pimentel, H., Bray, N. L., Puente, S., Melsted, P. & Pachter, L. Differential analysis of RNA-seq incorporating quantification uncertainty. Nat. Methods 14, 687–690 (2017).
R Core Team. R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, 2020).
Ritchie, M. E. et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 43, e47 (2015).
Sergushichev, S. A. An algorithm for fast preranked gene set enrichment analysis using cumulative statistic calculation. Preprint at bioRxiv https://doi.org/10.1101/060012 (2016).
Langmead, B. & Salzberg, S. L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 9, 357–359 (2012).
Fornes, O. et al. JASPAR 2020: update of the open-access database of transcription factor binding profiles. Nucleic Acids Res. 48, D87–D92 (2020).
Heinz, S. et al. Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol. Cell 38, 576–589 (2010).
Bailey, T. L. & Machanick, P. Inferring direct DNA binding from ChIP–seq. Nucleic Acids Res. 40, e128 (2012).
Jolma, A. et al. DNA-binding specificities of human transcription factors. Cell 152, 327–339 (2013).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014).
Li, H. et al. The Sequence Alignment/Map format and SAMtools. Bioinformatics 25, 2078–2079 (2009).
Acknowledgements
We are grateful to A. Rogoz and S. Gonzalez for exceptional animal care, mouse colony management and genotyping and the Rockefeller University employees for continuous assistance. We thank K. Gordon and K. Chhosphel for assistance with cell sorting. We thank C. Zhao and the entire Genomics Core of Rockefeller University for library preparation for 10X Genomics and assistance with all sequencing platforms used in this paper. We are grateful to U. Schaefer (The Rockefeller University) for help with ChIP protocols and guidance and S. Larsen for help with the ATAC–seq. We thank T. Egawa (Washington University in St. Louis) for providing anti-ThPOK ChIP antibody. We thank B. Reis and G. Victora for suggestions and critical reading of the manuscript and all the members of the Mucida laboratory for fruitful discussions. This work was supported by the Leona M. and Harry B. Helmsley Charitable Trust, the Black Family Metastasis Center, the Kavli Foundation, the Burroughs Wellcome Fund PATH Award and National Institute of Health grants no. AI144827, no. DK113375 and no. DK093674 (D.M.).
Author information
Authors and Affiliations
Contributions
D.M. conceived the study. M.L., A.M.B. and D.M. designed experiments and wrote the manuscript. M.L. and A.M.B. performed experiments. T.B.R.C. performed all bioinformatics analyses. T.B.R.C., M.L. and A.M.B. analyzed experiments. T.S. helped with discussion and setting up mouse strains. M.L., A.M.B., T.B.R.C. and D.M. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Immunology thanks Laura Mackay and Harinder Singh for their contribution to the peer review of this work. Peer reviewer reports are available. L. A. Dempsey was the primary editor on this article and managed its editorial process and peer review in collaboration with the rest of the editorial team.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Related to Fig. 1.
a–k, iFoxp3TomThPOKGFP mice were treated with tamoxifen for 10 weeks, and Tomato– and Tomato+ CD4+ T cells from mesenteric lymph nodes (mLN), lamina propria (LP) and intestinal epithelium (IE) were sorted for scRNA-Seq using 10X Genomics platform. Sorted Tomato– (blue gates) and Tomato+ (red gates) cells were pooled in a 2:1 ratio per tissue, resulting in 3 separate libraries. a, b, CD4+ T cells from mLN, LP and IE before (a) and after sorting (b). c, Number of sequenced genes (left) and RNA molecules (middle) per cluster and percent of mitochondrial DNA (right) per library. d, Top expressed genes per UMAP cluster. Circle size represents proportion of cells per cluster expressing the indicated gene and color represents expression level. e, Expression levels of the motility signature among all sequenced cells. f, Expression levels of Sell (top), Klf2 (middle), and Ccr7 (bottom) of cells from indicated tissues. g, tdTomato gene expression in 6,668 sequenced cells. h,i, Expression levels of Th1 signature (h) and Cd8a (i) among all sequenced cells. j, Expression levels of indicated genes among IE clusters. k, Expression heatmap of selected genes in pre-IEL clusters.
Extended Data Fig. 2 Related to Fig. 2.
a–d, Foxp3eGFP-Cre-ERT2xRosa26lsl-tdTomatoxZbtb7bGFP(iFoxp3TomThPOKGFP) mice were treated with tamoxifen for 10 weeks, Tomato– and Tomato+ CD4+ T cells from mesenteric lymph nodes (mLN), lamina propria (LP) and intestinal epithelium (IE) were sorted for scRNA-Seq using 10X Genomics platform. Sorted Tomato– and Tomato+ cells were pooled in a 2:1 ratio per tissue, resulting in 3 separate libraries. a–c, mLN-derived cells were re-clustered into 8 new clusters (0–7). a, Re-clustered mLN cells with number indicating the new clusters, consistent throughout the figure. b, Expression levels of top differentially expressed genes among re-clustered cells. c, Proportion of original UMAP clusters per re-clustered mLN clusters (X-axis), as indicated. Top 3 original UMAP cluster contained within re-clustered mLN indicated in a box. d, Scatter plot of gene expression ranked based on pseudotime principle component 1 (PC1).
Extended Data Fig. 3 Related to Fig. 3.
a–j, iFoxp3TomThPOKGFP mice were treated with tamoxifen for 10 weeks and induced Treg cells (iTreg; CD4+Tomato+GFPHineuropilin-1–CD8α–), pre-IELs (CD4+Tomato+ GFPLoCD8α–), exTreg-IELs (CD4+Tomato+GFPLoCD8α+), and CD4-IELs (CD4+ Tomato–GFPLoCD8α+) were sorted in bulk from the IE. Assay for transposase-accessible chromatin (ATAC) or RNA libraries were prepared followed by sequencing of indicated populations. iTreg cells were also sorted from the mLN for RNA-seq. a, Gating strategy used for sorting of CD4+ T cells in sequential order. Note that this gating strategy was used in all FACS data shown in this study. b, Sorting strategy of the indicated populations. CD8α and Tomato expression among CD4+ CD8βLo T cells (left) from IE (top) and mLN (bottom). ThPOK and neuropilin-1 expression among Tomato+CD8α– (red), Tomato+CD8α+ (purple) and Tomato–CD8α+ (green) cells from IE (top) and mLN (bottom). c, Levels of ThPOK expression in each sorted population in the IE. d, Mapped accessible chromatin regions relative to promoters of all genes in ATAC-Seq data (top) and their relative read counts per genomic regions (bottom). e, ATAC-Seq peak annotations as indicated per cell type. f, Percent of total differentially accessible chromatin regions as follows: 5′UTR and promoters (Promoter; red), 3′UTR with exons and introns (Gene Body; blue), transcriptional termination site (TTS; gray) and intergenic (black). g, Euclidean distance correlation of chromatin accessibility profiles of all samples. h, Volcano representation of differentially expressed genes between IE iTreg (higher expression in blue) and mLN iTreg cells (higher expression in red), performed by Wald pairwise comparison test, padj < 0.05 values were considered significant. i, Treg cell signature from clusters 1- 3 and IEL signature from cluster 5 of the bulk RNA-seq heatmap (Fig. 3d) overlaid onto the scRNA-Seq UMAP from Fig. 1. j, Curated list of gene set enrichment analysis determined using the fgsea package with padj < 0.05 of Hallmark pathways (black), chemical genetic perturbations pathways (white), and immune pathways (Retinoic acid (RA) pathways; green, Interferon pathways; blue, CD8-program; gray, and Treg cell program; silver). Significant differentially accessible chromatin regions padj < 0.01(d–g) and significant differentially expressed genes padj < 0.05 in RNA-Seq (h-j). Each sample for ATAC-Seq was from n = 9 (1st) or n = 6 (2nd) pooled mice over 2 independent experiments. 15,000 or 19,000 iTreg cells, 30,000 or 17,000 pre-IELs, 12,000 or 11,000 exTreg-IELs and 40,000 CD4-IELs were used (d–g). Each sample for RNA-Seq (d-f) consisted of 650 or 800 cells per mouse, n = 2 or 3 mice over 2 independent experiments (h–j).
Extended Data Fig. 4 Related to Fig. 4.
a–c, iFoxp3TomThPOKGFP mice were treated with tamoxifen for 10 weeks and induced Treg cells (iTreg; CD4+Tomato+GFPHineuropilin-1–CD8α–), pre-IELs (CD4+Tomato+ GFPLoCD8α–), exTreg-IELs (CD4+Tomato+GFPLoCD8α+), and CD4-IELs (CD4+ Tomato–GFPLoCD8α+) were sorted in bulk from the IE. Assay for transposase-accessible chromatin (ATAC) or RNA libraries were prepared followed by sequencing of indicated populations. a, Pearson’s correlation (r) of log2 fold changes of RNA-Seq vs ATAC-Seq of total ATAC peaks averaged (top) or at promoters only (bottom) of indicated cell types. P value (p) for r coefficient as indicated. b, ATAC-Seq peaks of indicated populations and ThPOK chromatin immunoprecipitation followed by sequencing (ChIP-Seq) of in vivo differentiated Foxp3+ splenic Treg cells displayed on the integrative genomics viewer (IGV) in select regions as indicated. Overlap regions between ChIP- and ATAC-Seq indicated on bottom. c, Numbers of differentially expressed genes (DEGs, n = 2 or 3 per cell type) (silver for increase, gray for decrease) in between cell types in sequential progression as performed by Wald pairwise test as indicated, with differentially accessible chromatin regions (DACR, n = 2 per cell type) within those genes (red for increase, blue for decrease in accessibility). Significant DACR padj < 0.01 and significant DEG padj < 0.05 in RNA-Seq. Each sample for ATAC-Seq was from n = 9 (1st) or n = 6 (2nd) pooled mice over 2 independent experiments. 15,000 or 19,000 iTreg cells, 30,000 or 17,000 pre-IELs, 12,000 or 11,000 exTreg-IELs and 40,000 CD4-IELs were used. Each sample for RNA-Seq consisted of 650 or 800 cells per mouse, n = 2–4 mice over 2 independent experiments.
Extended Data Fig. 5 Related to Fig. 5.
a, b, Flow cytometry analysis of CD45+TCRβ+CD4+CD8βLo Tomato+ cells in the IE and mLN of Zbtb7bfl/+xRunx3fl/+xRosa26|s|tdTomatoxFoxp3CreER (iFoxp3), iFoxp3xRunx3fl/+ (iFoxp3(ΔThPOK)), iFoxp3xZbtb7bfl/+xRunx3fl/fl (iFoxp3(ΔRunx3)), iFoxp3xZbtb7bfl/flxRunx3fl/fl (iFoxp3(ΔThPOK ΔRunx3)) mice after 10 weeks of tamoxifen treatment. a, Contour plot (left) and frequency (right) of total Foxp3+ cells among Tomato+ CD4+ T cells. b, Contour plot (left) and frequency (right) of total CD8α+ cells among Tomato+ CD4+ T cells (**p = 0.0036). c, Neuropilin-1 expression among Foxp3+ Treg cells in the intestinal epithelium (IE) and mesenteric lymph nodes (mLN) of WT mice. Histogram (left) and frequency (right) (****p < 0.0001). d-g, nTreg (neuropilin-1–) or iTreg cells (neuropilin-1+) were sorted from spleens and mLNs of CD45.2 Zbtb7bfl/+xRunx3fl/+xRosa26|s|tdTomatoxFoxp3CreER (iFoxp3), iFoxp3xRunx3fl/+ (iFoxp3(ΔThPOK)), iFoxp3xZbtb7bfl/+xRunx3fl/fl (iFoxp3(ΔRunx3)), iFoxp3xZbtb7bfl/flxRunx3fl/fl (iFoxp3(ΔThPOK ΔRunx3)) mice after tamoxifen administration and co-transferred with CD45.1 naïve CD4+ T cells to Rag1–/– hosts. CD45.2+TCRβ+CD4+CD8β-Tomato+ lymphocytes from the IE and mLN were analyzed 10 weeks after transfer. d, Experimental layout. e, Frequencies of total Foxp3 cells after nTreg (left) or iTreg (right) cell transfer. f–g, Body weight of Rag1-/- recipients after nTreg (left) or iTreg (right) cell transfers (f) and levels of fecal lipocalin-2 before and after per cell type transferred (g). Dashed lines represent colitis control transfer of naïve CD45.1+CD4+ T cells only. a-b, WT n = 6, ΔThPOK n = 9, ΔRunx3 n = 7, ΔThPOKΔRunx3 n = 11. c, IE n = 7, mLN n = 8. e-g, nTreg (WT n = 6, ΔThPOK n = 7, ΔRunx3 n = 6, ΔThPOKΔRunx3 n = 5) and iTreg (WT n = 5, ΔThPOK n = 6, ΔRunx3 n = 6, ΔThPOKΔRunx3 n = 6.) Data are expressed as mean ± SEM of individual mice (3 separate experiments). *p < 0.05, **p < 0.01, ***p < 0.001 [one-way ANOVA and Bonferonni test (a,b,e–g) and unpaired parametric two-tailed student’s t test (c)].
Extended Data Fig. 6 Related to Fig. 5.
a, b, Induced Treg cells (iTreg; Tomato+CD8α–neuropilin-1–) and Treg-derived CD4-IELs (exTreg-IELs; Tomato+CD8α+) were sorted from Zbtb7bfl/flxRunx3fl/+xRosa26|s|tdTomato xFoxp3CreER (iFoxp3(ΔThPOK)) mice after 10 weeks of tamoxifen administration followed by RNA-Sequencing from IE or mLN. Numbers of differentially expressed genes (DEGs) (silver for increase, gray for decrease) with total putative ThPOK targets or ThPOK targets at promoters (blue and purple, respectively; left) or putative Runx3 targets (red, right) at indicated comparisons between wild-type (WT) cells only (a) and WT and ΔThPOK cells (b). Each sample for RNA-Seq consisted of 650 or 800 cells per mouse, n = 2–4 mice over 3 independent experiments.
Supplementary information
Rights and permissions
About this article
Cite this article
London, M., Bilate, A.M., Castro, T.B.R. et al. Stepwise chromatin and transcriptional acquisition of an intraepithelial lymphocyte program. Nat Immunol 22, 449–459 (2021). https://doi.org/10.1038/s41590-021-00883-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41590-021-00883-8
This article is cited by
-
Universal recording of immune cell interactions in vivo
Nature (2024)
-
Downregulation of chemokine receptor 9 facilitates CD4+CD8αα+ intraepithelial lymphocyte development
Nature Communications (2023)
-
Regulatory T cells in the face of the intestinal microbiota
Nature Reviews Immunology (2023)
-
Localization and movement of Tregs in gastrointestinal tract: a systematic review
Inflammation and Regeneration (2022)