Abstract
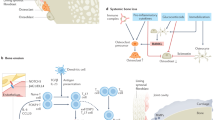
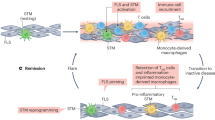
The immunopathogenesis of rheumatoid arthritis (RA) spans decades, beginning with the production of autoantibodies against post-translationally modified proteins (checkpoint 1). After years of asymptomatic autoimmunity and progressive immune system remodeling, tissue tolerance erodes and joint inflammation ensues as tissue-invasive effector T cells emerge and protective joint-resident macrophages fail (checkpoint 2). The transition of synovial stromal cells into autoaggressive effector cells converts synovitis from acute to chronic destructive (checkpoint 3). The loss of T cell tolerance derives from defective DNA repair, causing abnormal cell cycle dynamics, telomere fragility and instability of mitochondrial DNA. Mitochondrial and lysosomal anomalies culminate in the generation of short-lived tissue-invasive effector T cells. This differentiation defect builds on a metabolic platform that shunts glucose away from energy generation toward the cell building and motility programs. The next frontier in RA is the development of curative interventions, for example, reprogramming T cell defects during the period of asymptomatic autoimmunity.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Myasoedova, E., Davis, J., Matteson, E. L. & Crowson, C. S. Is the epidemiology of rheumatoid arthritis changing? Results from a population-based incidence study, 1985–2014. Ann. Rheum. Dis. 79, 440–444 (2020).
Mahler, M., Martinez-Prat, L., Sparks, J. A. & Deane, K. D. Precision medicine in the care of rheumatoid arthritis: focus on prediction and prevention of future clinically-apparent disease. Autoimmun. Rev. 19, 102506 (2020).
Eyre, S. et al. High-density genetic mapping identifies new susceptibility loci for rheumatoid arthritis. Nat. Genet. 44, 1336–1340 (2012).
Alarcón, G. S. Epidemiology of rheumatoid arthritis. Rheum. Dis. Clin. North Am. 21, 589–604 (1995).
Viatte, S. et al. Association of HLA-DRB1 haplotypes with rheumatoid arthritis severity, mortality, and treatment response. JAMA 313, 1645–1656 (2015).
Okada, Y. et al. Risk for ACPA-positive rheumatoid arthritis is driven by shared HLA amino acid polymorphisms in Asian and European populations. Hum. Mol. Genet. 23, 6916–6926 (2014).
Amariuta, T., Luo, Y., Knevel, R., Okada, Y. & Raychaudhuri, S. Advances in genetics toward identifying pathogenic cell states of rheumatoid arthritis. Immunol. Rev. 294, 188–204 (2020).
Hu, X. et al. Regulation of gene expression in autoimmune disease loci and the genetic basis of proliferation in CD4+ effector memory T cells. PLoS Genet. 10, e1004404 (2014).
Goronzy, J. J. & Weyand, C. M. Mechanisms underlying T cell ageing. Nat. Rev. Immunol. 19, 573–583 (2019).
Goronzy, J. J. & Weyand, C. M. Successful and maladaptive T cell aging. Immunity 46, 364–378 (2017).
Arbuckle, M. R. et al. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N. Engl. J. Med. 349, 1526–1533 (2003).
McClain, M. T. et al. The prevalence, onset, and clinical significance of antiphospholipid antibodies prior to diagnosis of systemic lupus erythematosus. Arthritis Rheum. 50, 1226–1232 (2004).
Aho, K., Heliövaara, M., Maatela, J., Tuomi, T. & Palosuo, T. Rheumatoid factors antedating clinical rheumatoid arthritis. J. Rheumatol. 18, 1282–1284 (1991).
Deane, K. D., Norris, J. M. & Holers, V. M. Preclinical rheumatoid arthritis: identification, evaluation, and future directions for investigation. Rheum. Dis. Clin. North Am. 36, 213–241 (2010).
van Delft, M. A. M. & Huizinga, T. W. J. An overview of autoantibodies in rheumatoid arthritis. J. Autoimmun. 110, 102392 (2020).
Ligier, S., Fortin, P. R. & Newkirk, M. M. A new antibody in rheumatoid arthritis targeting glycated IgG: IgM anti-IgG-AGE. Br. J. Rheumatol. 37, 1307–1314 (1998).
Raposo, B. et al. T cells specific for post-translational modifications escape intrathymic tolerance induction. Nat. Commun. 9, 353 (2018).
Berglin, E. et al. A combination of autoantibodies to cyclic citrullinated peptide (CCP) and HLA-DRB1 locus antigens is strongly associated with future onset of rheumatoid arthritis. Arthritis Res. Ther. 6, R303–R308 (2004).
Johansson, M., Arlestig, L., Hallmans, G. & Rantapää-Dahlqvist, S. PTPN22 polymorphism and anti-cyclic citrullinated peptide antibodies in combination strongly predicts future onset of rheumatoid arthritis and has a specificity of 100% for the disease. Arthritis Res. Ther. 8, R19 (2006).
Ge, C. & Holmdahl, R. The structure, specificity and function of anti-citrullinated protein antibodies. Nat. Rev. Rheumatol. 15, 503–508 (2019).
Kissel, T. et al. Antibodies and B cells recognising citrullinated proteins display a broad cross-reactivity towards other post-translational modifications. Ann. Rheum. Dis. 79, 472–480 (2020).
Makrygiannakis, D. et al. Smoking increases peptidylarginine deiminase 2 enzyme expression in human lungs and increases citrullination in BAL cells. Ann. Rheum. Dis. 67, 1488–1492 (2008).
Holers, V. M. et al. Rheumatoid arthritis and the mucosal origins hypothesis: protection turns to destruction. Nat. Rev. Rheumatol. 14, 542–557 (2018).
Khandpur, R. et al. NETs are a source of citrullinated autoantigens and stimulate inflammatory responses in rheumatoid arthritis. Sci. Transl. Med. 5, 178ra140 (2013).
Carmona-Rivera, C. et al. Synovial fibroblast-neutrophil interactions promote pathogenic adaptive immunity in rheumatoid arthritis. Sci. Immunol. 2, eaag3358 (2017).
Schönland, S. O. et al. Premature telomeric loss in rheumatoid arthritis is genetically determined and involves both myeloid and lymphoid cell lineages. Proc. Natl Acad. Sci. USA 100, 13471–13476 (2003).
Waase, I., Kayser, C., Carlson, P. J., Goronzy, J. J. & Weyand, C. M. Oligoclonal T cell proliferation in patients with rheumatoid arthritis and their unaffected siblings. Arthritis Rheum. 39, 904–913 (1996).
Gerlag, D. M. et al. Effects of B-cell directed therapy on the preclinical stage of rheumatoid arthritis: the PRAIRI study. Ann. Rheum. Dis. 78, 179–185 (2019).
Kissel, T. et al. On the presence of HLA-SE alleles and ACPA-IgG variable domain glycosylation in the phase preceding the development of rheumatoid arthritis. Ann. Rheum. Dis. 78, 1616–1620 (2019).
Scher, J. U. et al. Expansion of intestinal Prevotella copri correlates with enhanced susceptibility to arthritis. Elife 2, e01202 (2013).
Humby, F. et al. Synovial cellular and molecular signatures stratify clinical response to csDMARD therapy and predict radiographic progression in early rheumatoid arthritis patients. Ann. Rheum. Dis. 78, 761–772 (2019).
Lewis, M. J. et al. Molecular portraits of early rheumatoid arthritis identify clinical and treatment response phenotypes. Cell Rep. 28, 2455–2470.e5 (2019).
Ponchel, F. et al. T-cell subset abnormalities predict progression along the Inflammatory Arthritis disease continuum: implications for management. Sci. Rep. 10, 3669 (2020).
Bader, L. et al. Candidate markers for stratification and classification in rheumatoid arthritis. Front. Immunol. 10, 1488 (2019).
Pitaksalee, R. et al. Differential CpG DNA methylation in peripheral naive CD4+ T-cells in early rheumatoid arthritis patients. Clin. Epigenetics 12, 54 (2020).
Tuncel, J. et al. Self-reactive T cells induce and perpetuate chronic relapsing arthritis. Arthritis Res. Ther. 22, 95 (2020).
Weyand, C. M. & Goronzy, J. J. Immunometabolism in early and late stages of rheumatoid arthritis. Nat. Rev. Rheumatol. 13, 291–301 (2017).
Weyand, C. M., Shen, Y. & Goronzy, J. J. Redox-sensitive signaling in inflammatory T cells and in autoimmune disease. Free Radic. Biol. Med. 125, 36–43 (2018).
Wu, B., Goronzy, J. J. & Weyand, C. M. Metabolic fitness of T cells in autoimmune disease. Immunometabolism 2, e200017 (2020).
Li, Y., Goronzy, J. J. & Weyand, C. M. DNA damage, metabolism and aging in pro-inflammatory T cells: rheumatoid arthritis as a model system. Exp. Gerontol. 105, 118–127 (2018).
Wagner, U. G., Koetz, K., Weyand, C. M. & Goronzy, J. J. Perturbation of the T cell repertoire in rheumatoid arthritis. Proc. Natl Acad. Sci. USA 95, 14447–14452 (1998).
Koetz, K. et al. T cell homeostasis in patients with rheumatoid arthritis. Proc. Natl Acad. Sci. USA 97, 9203–9208 (2000).
Burgoyne, C. H. et al. Abnormal T cell differentiation persists in patients with rheumatoid arthritis in clinical remission and predicts relapse. Ann. Rheum. Dis. 67, 750–757 (2008).
Ponchel, F. et al. Dysregulated lymphocyte proliferation and differentiation in patients with rheumatoid arthritis. Blood 100, 4550–4556 (2002).
Weyand, C. M., Fujii, H., Shao, L. & Goronzy, J. J. Rejuvenating the immune system in rheumatoid arthritis. Nat. Rev. Rheumatol. 5, 583–588 (2009).
Weyand, C. M., Shao, L. & Goronzy, J. J. Immune aging and rheumatoid arthritis. Rheum. Dis. Clin. North Am. 36, 297–310 (2010).
Weyand, C. M., Yang, Z. & Goronzy, J. J. T cell aging in rheumatoid arthritis. Curr. Opin. Rheumatol. 26, 93–100 (2014).
Goronzy, J. J., Li, G., Yang, Z. & Weyand, C. M. The Janus head of T cell aging—autoimmunity and immunodeficiency. Front. Immunol. 4, 131 (2013).
Li, Y. et al. Deficient activity of the nuclease MRE11A induces T cell aging and promotes arthritogenic effector functions in patients with rheumatoid arthritis. Immunity 45, 903–916 (2016).
Shao, L. et al. Deficiency of the DNA repair enzyme ATM in rheumatoid arthritis. J. Exp. Med. 206, 1435–1449 (2009).
Burger, K., Ketley, R. F. & Gullerova, M. Beyond the trinity of ATM, ATR, and DNA-PK: multiple kinases shape the DNA damage response in concert with RNA metabolism. Front. Mol. Biosci. 6, 61 (2019).
Yang, Z. et al. Restoring oxidant signaling suppresses proarthritogenic T cell effector functions in rheumatoid arthritis. Sci. Transl. Med. 8, 331ra338 (2016).
Amsen, D., Backer, R. A. & Helbig, C. Decisions on the road to memory. Adv. Exp. Med. Biol. 785, 107–120 (2013).
Shao, L., Goronzy, J. J. & Weyand, C. M. DNA-dependent protein kinase catalytic subunit mediates T-cell loss in rheumatoid arthritis. EMBO Mol. Med. 2, 415–427 (2010).
Li, Y. et al. The DNA repair nuclease MRE11A functions as a mitochondrial protector and prevents T cell pyroptosis and tissue inflammation. Cell Metab. 30, 477–492 e6 (2019).
Syed, A. & Tainer, J. A. The MRE11–RAD50–NBS1 complex conducts the orchestration of damage signaling and outcomes to stress in DNA replication and repair. Annu. Rev. Biochem. 87, 263–294 (2018).
Mathur, A., Hayward, J. A. & Man, S. M. Molecular mechanisms of inflammasome signaling. J. Leukoc. Biol. 103, 233–257 (2018).
Mehta, P. & Manson, J. J. What is the clinical relevance of TNF inhibitor immunogenicity in the management of patients with rheumatoid arthritis? Front. Immunol. 11, 589 (2020).
Balsa, A. et al. Drug immunogenicity in patients with inflammatory arthritis and secondary failure to tumour necrosis factor inhibitor therapies: the REASON study. Rheumatology (Oxford) 57, 688–693 (2018).
Ponchel, F. et al. Interleukin-7 deficiency in rheumatoid arthritis: consequences for therapy-induced lymphopenia. Arthritis Res. Ther. 7, R80–R92 (2005).
Schmidt, D., Goronzy, J. J. & Weyand, C. M. CD4+ CD7– CD28– T cells are expanded in rheumatoid arthritis and are characterized by autoreactivity. J. Clin. Invest. 97, 2027–2037 (1996).
Weyand, C. M., Brandes, J. C., Schmidt, D., Fulbright, J. W. & Goronzy, J. J. Functional properties of CD4+ CD28– T cells in the aging immune system. Mech. Ageing Dev. 102, 131–147 (1998).
Yamada, H. et al. Interleukin-15 selectively expands CD57+ CD28– CD4+ T cells, which are increased in active rheumatoid arthritis. Clin. Immunol. 124, 328–335 (2007).
Weyand, C. M., Fulbright, J. W. & Goronzy, J. J. Immunosenescence, autoimmunity, and rheumatoid arthritis. Exp. Gerontol. 38, 833–841 (2003).
Liuzzo, G. et al. Perturbation of the T-cell repertoire in patients with unstable angina. Circulation 100, 2135–2139 (1999).
Liuzzo, G. et al. Monoclonal T-cell proliferation and plaque instability in acute coronary syndromes. Circulation 101, 2883–2888 (2000).
Gerli, R. et al. CD4+CD28– T lymphocytes contribute to early atherosclerotic damage in rheumatoid arthritis patients. Circulation 109, 2744–2748 (2004).
Ormseth, M. J. et al. Telomere length and coronary atherosclerosis in rheumatoid arthritis. J. Rheumatol. 43, 1469–1474 (2016).
Liuzzo, G. et al. Molecular fingerprint of interferon-γ signaling in unstable angina. Circulation 103, 1509–1514 (2001).
Fasth, A. E. R., Björkström, N. K., Anthoni, M., Malmberg, K.-J. & Malmström, V. Activating NK-cell receptors co-stimulate CD4+CD28– T cells in patients with rheumatoid arthritis. Eur. J. Immunol. 40, 378–387 (2010).
Warrington, K. J., Takemura, S., Goronzy, J. J. & Weyand, C. M. CD4+,CD28– T cells in rheumatoid arthritis patients combine features of the innate and adaptive immune systems. Arthritis Rheum. 44, 13–20 (2001).
Snyder, M. R., Nakajima, T., Leibson, P. J., Weyand, C. M. & Goronzy, J. J. Stimulatory killer Ig-like receptors modulate T cell activation through DAP12-dependent and DAP12-independent mechanisms. J. Immunol. 173, 3725–3731 (2004).
Snyder, M. R., Lucas, M., Vivier, E., Weyand, C. M. & Goronzy, J. J. Selective activation of the c-Jun NH2-terminal protein kinase signaling pathway by stimulatory KIR in the absence of KARAP/DAP12 in CD4+ T cells. J. Exp. Med. 197, 437–449 (2003).
Pereira, B. I. et al. Sestrins induce natural killer function in senescent-like CD8+ T cells. Nat. Immunol. 21, 684–694 (2020).
Zhang, F. et al. Defining inflammatory cell states in rheumatoid arthritis joint synovial tissues by integrating single-cell transcriptomics and mass cytometry. Nat. Immunol. 20, 928–942 (2019).
Fonseka, C. Y. et al. Mixed-effects association of single cells identifies an expanded effector CD4+ T cell subset in rheumatoid arthritis. Sci. Transl. Med. 10, eaaq0305 (2018).
Colmegna, I. et al. Defective proliferative capacity and accelerated telomeric loss of hematopoietic progenitor cells in rheumatoid arthritis. Arthritis Rheum. 58, 990–1000 (2008).
Makowski, L., Chaib, M. & Rathmell, J. C. Immunometabolism: from basic mechanisms to translation. Immunol. Rev. 295, 5–14 (2020).
Shen, Y. et al. Metabolic control of the scaffold protein TKS5 in tissue-invasive, proinflammatory T cells. Nat. Immunol. 18, 1025–1034 (2017).
Yang, Z., Fujii, H., Mohan, S. V., Goronzy, J. J. & Weyand, C. M. Phosphofructokinase deficiency impairs ATP generation, autophagy, and redox balance in rheumatoid arthritis T cells. J. Exp. Med. 210, 2119–2134 (2013).
Yang, Z., Goronzy, J. J. & Weyand, C. M. The glycolytic enzyme PFKFB3/phosphofructokinase regulates autophagy. Autophagy 10, 382–383 (2014).
Yang, Z., Matteson, E. L., Goronzy, J. J. & Weyand, C. M. T-cell metabolism in autoimmune disease. Arthritis Res. Ther. 17, 29 (2015).
Weyand, C. M., Wu, B. & Goronzy, J. J. The metabolic signature of T cells in rheumatoid arthritis. Curr. Opin. Rheumatol. 32, 159–167 (2020).
Ling, G. S. et al. C1q restrains autoimmunity and viral infection by regulating CD8+ T cell metabolism. Science 360, 558–563 (2018).
Wen, Z. et al. N-myristoyltransferase deficiency impairs activation of kinase AMPK and promotes synovial tissue inflammation. Nat. Immunol. 20, 313–325 (2019).
Krishnan, S. et al. Alterations in lipid raft composition and dynamics contribute to abnormal T cell responses in systemic lupus erythematosus. J. Immunol. 172, 7821–7831 (2004).
Fernandez, D. & Perl, A. Metabolic control of T cell activation and death in SLE. Autoimmun. Rev. 8, 184–189 (2009).
Fernandez, D. R. et al. Activation of mammalian target of rapamycin controls the loss of TCRζ in lupus T cells through HRES-1/Rab4-regulated lysosomal degradation. J. Immunol. 182, 2063–2073 (2009).
Choi, S.-C. et al. Inhibition of glucose metabolism selectively targets autoreactive follicular helper T cells. Nat. Commun. 9, 4369 (2018).
Kono, M. et al. Pyruvate dehydrogenase phosphatase catalytic subunit 2 limits Th17 differentiation. Proc. Natl Acad. Sci. USA 115, 9288–9293 (2018).
Kono, M. et al. Pyruvate kinase M2 is requisite for Th1 and Th17 differentiation. JCI Insight 4, e127395 (2019).
Taylor, P. C. & Law, S. T. When the first visit to the rheumatologist is established rheumatoid arthritis. Best Pract. Res. Clin. Rheumatol. 33, 101479 (2020).
Verburg, R. J. et al. Outcome of intensive immunosuppression and autologous stem cell transplantation in patients with severe rheumatoid arthritis is associated with the composition of synovial T cell infiltration. Ann. Rheum. Dis. 64, 1397–1405 (2005).
Law, S. T. & Taylor, P. C. Role of biological agents in treatment of rheumatoid arthritis. Pharmacol. Res. 150, 104497 (2019).
Nygaard, G. & Firestein, G. S. Restoring synovial homeostasis in rheumatoid arthritis by targeting fibroblast-like synoviocytes. Nat. Rev. Rheumatol. 16, 316–333 (2020).
Takemura, S. et al. Lymphoid neogenesis in rheumatoid synovitis. J. Immunol. 167, 1072–1080 (2001).
Grumbach, I. M. & Nguyen, E. K. Metabolic stress: mitochondrial function in neointimal formation. Arterioscler. Thromb. Vasc. Biol. 39, 991–997 (2019).
Wadey, K., Lopes, J., Bendeck, M. & George, S. Role of smooth muscle cells in coronary artery bypass grafting failure. Cardiovasc. Res. 114, 601–610 (2018).
Tinajero, M. G. & Gotlieb, A. I. Recent developments in vascular adventitial pathobiology: the dynamic adventitia as a complex regulator of vascular disease. Am. J. Pathol. 190, 520–534 (2020).
Croft, A. P. et al. Rheumatoid synovial fibroblasts differentiate into distinct subsets in the presence of cytokines and cartilage. Arthritis Res. Ther. 18, 270 (2016).
Mizoguchi, F. et al. Functionally distinct disease-associated fibroblast subsets in rheumatoid arthritis. Nat. Commun. 9, 789 (2018).
Croft, A. P. et al. Distinct fibroblast subsets drive inflammation and damage in arthritis. Nature 570, 246–251 (2019).
Finch, R. et al. Results of a phase 2 study of RG6125, an anti-cadherin-11 monoclonal antibody, in rheumatoid arthritis patients with an inadequate response to anti-TNFalpha therapy. Ann. Rheum. Dis. 78, abstr. OP0224 (2019).
Orr, C. et al. Synovial tissue research: a state-of-the-art review. Nat. Rev. Rheumatol. 13, 463–475 (2017).
Davies, L. C., Jenkins, S. J., Allen, J. E. & Taylor, P. R. Tissue-resident macrophages. Nat. Immunol. 14, 986–995 (2013).
Chakarov, S. et al. Two distinct interstitial macrophage populations coexist across tissues in specific subtissular niches. Science 363, eaau0964 (2019).
Molawi, K. & Sieweke, M. H. Monocytes compensate Kupffer cell loss during bacterial infection. Immunity 42, 10–12 (2015).
Murray, P. J. Immune regulation by monocytes. Semin. Immunol. 35, 12–18 (2018).
Murray, P. J. Macrophage polarization. Annu. Rev. Physiol. 79, 541–566 (2017).
Udalova, I. A., Mantovani, A. & Feldmann, M. Macrophage heterogeneity in the context of rheumatoid arthritis. Nat. Rev. Rheumatol. 12, 472–485 (2016).
Herenius, M. M. et al. Monocyte migration to the synovium in rheumatoid arthritis patients treated with adalimumab. Ann. Rheum. Dis. 70, 1160–1162 (2011).
Culemann, S. et al. Locally renewing resident synovial macrophages provide a protective barrier for the joint. Nature 572, 670–675 (2019).
Kuo, D. et al. HBEGF+ macrophages in rheumatoid arthritis induce fibroblast invasiveness. Sci. Transl. Med. 11, eaau8587 (2019).
Kang, Y. M. et al. CD8 T cells are required for the formation of ectopic germinal centers in rheumatoid synovitis. J. Exp. Med. 195, 1325–1336 (2002).
Crotty, S. Follicular helper CD4 T cells (TFH). Annu. Rev. Immunol. 29, 621–663 (2011).
Bocharnikov, A. V. et al. PD-1hiCXCR5– T peripheral helper cells promote B cell responses in lupus via MAF and IL-21. JCI Insight 4, e130062 (2019).
Rao, D. A. et al. Pathologically expanded peripheral T helper cell subset drives B cells in rheumatoid arthritis. Nature 542, 110–114 (2017).
Wang, S. et al. IL-21 drives expansion and plasma cell differentiation of autoreactive CD11chiT-bet+ B cells in SLE. Nat. Commun. 9, 1758 (2018).
Wu, B. et al. Succinyl-CoA ligase deficiency in pro-inflammatory and tissue-invasive T cells. Cell Metab. (in the press).
Acknowledgements
This work was supported by the National Institutes of Health (R01 AR042527, R01 HL117913, R01 AI108906, R01 HL142068 and P01 HL129941 to C.M.W.; and R01 AI108891, R01 AG045779, U19 AI057266, R01 AI129191 and IO1 BX001669 to J.J.G.) and the Encrantz Family Discovery Fund.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Editor recognition statement L. A. Dempsey was the primary editor on this article and managed its editorial process and peer review in collaboration with the rest of the editorial team.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Weyand, C.M., Goronzy, J.J. The immunology of rheumatoid arthritis. Nat Immunol 22, 10–18 (2021). https://doi.org/10.1038/s41590-020-00816-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41590-020-00816-x
This article is cited by
-
Impaired immune tolerance mediated by reduced Tfr cells in rheumatoid arthritis linked to gut microbiota dysbiosis and altered metabolites
Arthritis Research & Therapy (2024)
-
Cayratia albifolia C.L.Li exerts anti-rheumatoid arthritis effect by inhibiting macrophage activation and neutrophil extracellular traps (NETs)
Chinese Medicine (2024)
-
Targeted therapies of inflammatory diseases with intracellularly gelated macrophages in mice and rats
Nature Communications (2024)
-
Production and use of antigen tetramers to study antigen-specific B cells
Nature Protocols (2024)
-
Identifying the causal relationship between immune factors and osteonecrosis: a two-sample Mendelian randomization study
Scientific Reports (2024)