Abstract
Understanding resistance to antibody to programmed cell death protein 1 (PD-1; anti-PD-1) is crucial for the development of reversal strategies. In anti-PD-1-resistant models, simultaneous anti-PD-1 and vaccine therapy reversed resistance, while PD-1 blockade before antigen priming abolished therapeutic outcomes. This was due to induction of dysfunctional PD-1+CD38hi CD8+ cells by PD-1 blockade in suboptimally primed CD8 cell conditions induced by tumors. This results in erroneous T cell receptor signaling and unresponsiveness to antigenic restimulation. On the other hand, PD-1 blockade of optimally primed CD8 cells prevented the induction of dysfunctional CD8 cells, reversing resistance. Depleting PD-1+CD38hi CD8+ cells enhanced therapeutic outcomes. Furthermore, non-responding patients showed more PD-1+CD38+CD8+ cells in tumor and blood than responders. In conclusion, the status of CD8+ T cell priming is a major contributor to anti-PD-1 therapeutic resistance. PD-1 blockade in unprimed or suboptimally primed CD8 cells induces resistance through the induction of PD-1+CD38hi CD8+ cells that is reversed by optimal priming. PD-1+CD38hi CD8+ cells serve as a predictive and therapeutic biomarker for anti-PD-1 treatment. Sequencing of anti-PD-1 and vaccine is crucial for successful therapy.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
For the clinical data, the cohorts were not collected specifically for this study and are already published. The references describing the participants of the human research and clinical data have been provided in this published article. In vitro, in vivo, flow cytometry and clinical data are included in this published article and its Supplementary Information. All other relevant data are available from the corresponding author upon reasonable request.
Change history
24 September 2019
An amendment to this paper has been published and can be accessed via a link at the top of the paper.
References
Kyi, C. & Postow, M. A. Checkpoint blocking antibodies in cancer immunotherapy. FEBS Lett. 588, 368–376 (2014).
Topalian, S. L., Drake, C. G. & Pardoll, D. M. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell 27, 450–461 (2015).
Dong, H. et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat. Med. 8, 793–800 (2002).
Iwai, Y. et al. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc. Natl Acad. Sci. USA 99, 12293–12297 (2002).
Page, D. B., Postow, M. A., Callahan, M. K., Allison, J. P. & Wolchok, J. D. Immune modulation in cancer with antibodies. Annu. Rev. Med. 65, 185–202 (2014).
Zou, W., Wolchok, J. D. & Chen, L. PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: mechanisms, response biomarkers, and combinations. Sci. Transl. Med. 8, 328rv4 (2016).
Kleponis, J., Skelton, R. & Zheng, L. Fueling the engine and releasing the break: combinational therapy of cancer vaccines and immune checkpoint inhibitors. Cancer Biol. Med. 12, 201–208 (2015).
Sharma, P., Hu-Lieskovan, S., Wargo, J. A. & Ribas, A. Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell 168, 707–723 (2017).
Mkrtichyan, M. et al. Anti-PD-1 antibody significantly increases therapeutic efficacy of Listeria monocytogenes (Lm)-LLO immunotherapy. J. Immunother. Cancer 1, 15 (2013).
Mkrtichyan, M. et al. Anti-PD-1 synergizes with cyclophosphamide to induce potent anti-tumor vaccine effects through novel mechanisms. Eur. J. Immunol. 41, 2977–2986 (2011).
Mkrtichyan, M. et al. B7-DC-Ig enhances vaccine effect by a novel mechanism dependent on PD-1 expression level on T cell subsets. J. Immunol. 189, 2338–2347 (2012).
Karyampudi, L. et al. Accumulation of memory precursor CD8 T cells in regressing tumors following combination therapy with vaccine and anti-PD-1 antibody. Cancer Res. 74, 2974–2985 (2014).
Soares, K. C. et al. PD-1/PD-L1 blockade together with vaccine therapy facilitates effector T-cell infiltration into pancreatic tumors. J. Immunother. 38, 1–11 (2015).
Huang, A. C. et al. T-cell invigoration to tumour burden ratio associated with anti-PD-1 response. Nature 545, 60–65 (2017).
Patsoukis, N. et al. Immunometabolic regulations mediated by coinhibitory receptors and their impact on T cell immune responses. Front. Immunol. 8, 330 (2017).
Boussiotis, V. A. Molecular and biochemical aspects of the PD-1 checkpoint pathway. N. Engl. J. Med. 375, 1767–1778 (2016).
Zaretsky, J. M. et al. Mutations associated with acquired resistance to PD-1 blockade in melanoma. N. Engl. J. Med. 375, 819–829 (2016).
Philip, M. et al. Chromatin states define tumour-specific T cell dysfunction and reprogramming. Nature 545, 452–456 (2017).
Shrimali, R. K. et al. Concurrent PD-1 blockade negates the effects of OX40 agonist antibody in combination immunotherapy through inducing T-cell apoptosis. Cancer Immunol. Res. 5, 755–766 (2017).
Trautmann, L. et al. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat. Med. 12, 1198–1202 (2006).
Day, C. L. et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature 443, 350–354 (2006).
Latchman, Y. et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat. Immunol. 2, 261–268 (2001).
Refaeli, Y., Van Parijs, L., Alexander, S. I. & Abbas, A. K. Interferon γ is required for activation-induced death of T lymphocytes. J. Exp. Med. 196, 999–1005 (2002).
Lohman, B. L. & Welsh, R. M. Apoptotic regulation of T cells and absence of immune deficiency in virus-infected gamma interferon receptor knockout mice. J. Virol. 72, 7815–7821 (1998).
Overwijk, W. W. et al. gp100/pmel 17 is a murine tumor rejection antigen: induction of “self”-reactive, tumoricidal T cells using high-affinity, altered peptide ligand. J. Exp. Med. 188, 277–286 (1998).
Chen, L. & Flies, D. B. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat. Rev. Immunol. 13, 227–242 (2013).
Rosette, C. et al. The impact of duration versus extent of TCR occupancy on T cell activation: a revision of the kinetic proofreading model. Immunity 15, 59–70 (2001).
Vonderheide, R. H. The immune revolution: a case for priming, not checkpoint. Cancer Cell 33, 563–569 (2018).
Vonderheide, R. H., Domchek, S. M. & Clark, A. S. Immunotherapy for breast cancer: what are we missing? Clin. Cancer Res. 23, 2640–2646 (2017).
Sade-Feldman, M. et al. Defining T Cell states associated with response to checkpoint immunotherapy in melanoma. Cell 175, 998–1013 e20 (2018).
Frey, A. B. & Monu, N. Signaling defects in anti-tumor T cells. Immunol. Rev. 222, 192–205 (2008).
Rizvi, N. A. et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 348, 124–128 (2015).
Eroglu, Z. et al. High response rate to PD-1 blockade in desmoplastic melanomas. Nature 553, 347–350 (2018).
Banerjea, A., Bustin, S. A. & Dorudi, S. The immunogenicity of colorectal cancers with high-degree microsatellite instability. World J. Surg. Oncol. 3, 26 (2005).
Vandeven, N. A. & Nghiem, P. Merkel cell carcinoma: an unusually immunogenic cancer proves ripe for immune therapy. J. Oncol. Pract. 12, 649–650 (2016).
Yokosuka, T. et al. Programmed cell death 1 forms negative costimulatory microclusters that directly inhibit T cell receptor signaling by recruiting phosphatase SHP2. J. Exp. Med. 209, 1201–1217 (2012).
Acuto, O., Di Bartolo, V. & Michel, F. Tailoring T-cell receptor signals by proximal negative feedback mechanisms. Nat. Rev. Immunol. 8, 699–712 (2008).
Poltorak, M. et al. TCR activation kinetics and feedback regulation in primary human T cells. Cell Commun. Signal. 11, 4 (2013).
Pageon, S. V. et al. Functional role of T-cell receptor nanoclusters in signal initiation and antigen discrimination. Proc. Natl Acad. Sci. USA 113, E5454–E5463 (2016).
Lee, J., Ahn, E., Kissick, H. T. & Ahmed, R. Reinvigorating exhausted T Cells by blockade of the PD-1 pathway. For. Immunopathol. Dis. Therap. 6, 7–17 (2015).
Schietinger, A. et al. Tumor-specific T cell dysfunction is a dynamic antigen-driven differentiation program initiated early during tumorigenesis. Immunity 45, 389–401 (2016).
Chatterjee, S. et al. CD38-NAD+ axis regulates immunotherapeutic anti-tumor T cell response. Cell Metab. 27, 85–100.e8 (2018).
Mason, E. F. & Rathmell, J. C. Cell metabolism: an essential link between cell growth and apoptosis. Biochim. Biophys. Acta 1813, 645–654 (2011).
Thommen, D. S. et al. A transcriptionally and functionally distinct PD-1+ CD8+ T cell pool with predictive potential in non-small-cell lung cancer treated with PD-1 blockade. Nat. Med. 24, 994–1004 (2018).
Blackburn, S. D., Shin, H., Freeman, G. J. & Wherry, E. J. Selective expansion of a subset of exhausted CD8 T cells by αPD-L1 blockade. Proc. Natl Acad. Sci. USA 105, 15016–15021 (2008).
Patel, S. P. & Kurzrock, R. PD-L1 expression as a predictive biomarker in cancer immunotherapy. Mol. Cancer Ther. 14, 847–856 (2015).
Li, Y. et al. Prognostic impact of programed cell death-1 (PD-1) and PD-ligand 1 (PD-L1) expression in cancer cells and tumor infiltrating lymphocytes in colorectal cancer. Mol. Cancer 15, 55 (2016).
Sabatier, R. et al. Prognostic and predictive value of PDL1 expression in breast cancer. Oncotarget 6, 5449–5464 (2015).
Jiang, P. et al. Signatures of T cell dysfunction and exclusion predict cancer immunotherapy response. Nat. Med. 24, 1550–1558 (2018).
Buchwald, Z. S. et al. Radiation, immune checkpoint blockade and the abscopal effect: a critical review on timing, dose and fractionation. Front. Oncol. 8, 612 (2018).
Eisenhauer, E. A. et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur. J. Cancer 45, 228–247 (2009).
Villani, A. C. Single-cell RNA-seq reveals new types of human blood dendritic cells, monocytes, and progenitors. Science 356, eaah4573 (2017).
Ji, Y. et al. Identification of the genomic insertion site of Pmel-1 TCR α and β transgenes by next-generation sequencing. PLoS ONE 9, e96650 (2014).
Lin, K. Y. et al. Treatment of established tumors with a novel vaccine that enhances major histocompatibility class II presentation of tumor antigen. Cancer Res. 56, 21–26 (1996).
Abu Eid, R. et al. Akt1 and -2 inhibition diminishes terminal differentiation and enhances central memory CD8+ T-cell proliferation and survival. Oncoimmunology 4, e1005448 (2015).
Acknowledgements
We acknowledge the Georgia Cancer Center, Augusta University internal support grant to S.N.K. We acknowledge the Lombardi Comprehensive Cancer Center support grant to the Biostatistics and Bioinformatics shared service (P30 CA 051008). We acknowledge the Ludwig Center for Cancer Immunotherapy for financial support for the Immune Monitoring Core Facility at the MSKCC. This research was funded in part through the National Institutes of Health (NIH)/National Cancer Institute (NCI) Cancer Center Support grant no. P30 CA008748, grant no. NIH/NCI R01 CA056821, the Swim Across America, Ludwig Institute for Cancer Research, Parker Institute for Cancer Immunotherapy and Virginia B. Squiers Foundation to J.W. and T.M. The research related to the human tumor samples was supported by the Cancer Research Institute (N.H.), Adelson Medical Research Foundation (N.H.) and NIH/NCI grant no. R01CA208756 (N.H.). We thank R. Ibrahim, Parker Institute for Cancer Immunotherapy, for reviewing the manuscript.
Author information
Authors and Affiliations
Contributions
V.V., S.G. and S.N.K. were the main investigators and take primary responsibility for the paper. V.V., S.G. and S.N.K. were involved in the conception and design of the study, development of the methodology, analysis and interpretation of the data, administrative, technical or material support and writing and reviewing the manuscript. V.V. performed the experiments with assistance from R.K.S., S.A., W.D., H.W., S.L., R.N., P.G. and J.L. J.E.J. and M.M. helped review the manuscript. The acquisition of human tumor samples and their analysis were performed by M.S.-F., K.Y., S.L.B., K.T.F., J.A.W., G.M.B., R.J.S., G.G. and N.H. The acquisition of human PBMC samples and their analysis were performed by J.Q., P.W., T.M. and J.W. M.T. performed the statistical analysis of the human data. S.A.H. provided the anti-PD-1 antibody.
Corresponding author
Ethics declarations
Competing interests
S.N.K., S.G. and V.V. are inventors on patent application related to work on the methods for detecting and reversing immune therapy resistance and the development of PD-1+CD38+ CD8+ T cells as a predictive and therapeutic biomarker for response/resistance to immune checkpoint blockade therapy. S.N.K. reports an honorarium from Syndax, IO Biotech, BioLine, Northwest Biotherapeutics, Advaxis, EMD Serono, GSK, UbiVac, McKinsey, AstraZeneca and Lycera. S.N.K. reports stocks or ownership interest in Advaxis, GeorgiaImmune, IO Biotech and Northwest Therapeutics. S.N.K. is a consultant for Syndax, IO Biotech, BioLine, Kahr, PDS Biotechnology, AstraZeneca, CytomX, NewLink Genetics, AratingaBio, CanImGuide and Lycera. S.N.K. is a board member for Advaxis. S.N.K. has research contracts with Syndax, IO Biotech, BioLine, AstraZeneca, MedImmune and Lycera. T.M. is a consultant for Leap Therapeutics, Immunos Therapeutics and Pfizer and co-founder of Imvaq Therapeutics. T.M. has equity in Imvaq Therapeutics. T.M. reports grants from Bristol-Myers Squibb, Surface Oncology, Kyn Therapeutics, Infinity Pharmaceuticals, Peregrine Pharmaceuticals, Adaptive Biotechnologies, Leap Therapeutics and Aprea Therapeutics. T.M. is an inventor on patent applications related to work on oncolytic viral therapy, alphavirus-based vaccines, neoantigen modeling, CD40, glucocorticoid-induced TNFR-related protein (GITR), OX40, PD-1 and CTLA-4. J.W. is a consultant for Adaptive Biotechnologies, Advaxis, Amgen, Apricity, Array BioPharma, Ascentage Pharma, Astellas Pharma, Bayer, BeiGene, Bristol-Myers Squibb, Celgene, Chugai Pharmaceutical, Elucida Oncology, Eli Lilly, F Star, Genentech, Imvaq Therapeutics, Janssen, Kleo Pharmaceuticals, Linneaus, MedImmune, Merck, Neon Therapeutics, Ono Pharmaceutical, Polaris Pharma, Polynoma, PsiOxus Therapeutics, PureTech Health, Recepta Biopharma, Sellas Life Sciences, Serametrix, Surface Oncology and Syndax. J.W. reports an honorarium from Esanex and grants/research support from Bristol-Myers Squibb, MedImmune and Genentech. J.W. has equity in Potenza Therapeutics, Tizona Therapeutics, Adaptive Biotechnologies, Elucida Oncology, Imvaq Therapeutics, BeiGene, Trieza Therapeutics, Serametrix and Linneaus. J.W. is an inventor on patent applications related to work on xenogeneic DNA vaccines, alphavirus replicon particles expressing tyrosinase-related protein-2, myeloid-derived suppressor cell assay, Newcastle disease viruses for cancer therapy, genomic signature to identify responders to ipilimumab in melanoma, engineered vaccinia viruses for cancer immunotherapy, anti-CD40 agonist monoclonal antibody fused to monophosphoryl lipid A for cancer therapy, CAR+ T cells targeting differentiation antigens as means to treat cancer, anti-PD-1 antibody, anti-CTLA-4 antibodies, anti-GITR antibodies and methods of use thereof. P.W. is a consultant for Leap Therapeutics. G.M.B. reports paid lecturing from Novartis, Takeda Oncology; sponsored research agreements with Takeda Oncology; and consulting with NW Biotherapeutics. R.J.S. reports personal fees from Amgen, Merck, Genentech and Novartis; research grants from Amgen and Merck; and clinical trial support from Merck, Tesaro, Sanofi, Genentech and Novartis during the conduct of the study; and personal fees from Compugen, Replimmune, Array and Syndax outside the submitted work. J.A.W. is an inventor on a US patent application (PCT/US17/53.717) submitted by the University of Texas MD Anderson Cancer Center that covers methods to enhance immune checkpoint blockade responses by modulating the microbiome; reports compensation for speaker’s bureau and honoraria from Imedex, Dava Oncology, Omniprex, Illumina, Gilead, PeerView, Physician Education Resource, MedImmune and Bristol-Myers Squibb; serves as a consultant or advisory board member for Roche/Genentech, Novartis, AstraZeneca, GlaxoSmithKline, Bristol-Myers Squibb, Merck, Biothera Pharmaceuticals and Microbiome DX; and receives research support from GlaxoSmithKline, Roche/Genentech, Bristol-Myers Squibb and Novartis. K.T.F. owns equity in Shattuck Labs, Checkmate, X4 Pharmaceuticals; consults for Novartis, Genentech, BMS, Merck, Takeda, Verastem, Checkmate, X4 Pharmaceuticals, Sanofi, Amgen, Incyte, Adaptimmune, Shattuck Labs, Arch Oncology and Apricity; and receives research support from Novartis, Genentech, Sanofi and Amgen. N.H. is a founder and science advisory board member of Neon Therapeutics. All other authors declare no competing interests.
Additional information
Peer review information Zoltan Fehervari was the primary editor on this article and managed its editorial process and peer review in collaboration with the rest of the editorial team.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Integrated supplementary information
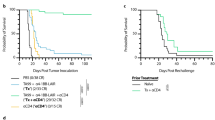
Supplementary Figure 1 Anti-PD-1 prior to antigenic stimulation abrogates the anti-tumor effects of Vax + αPD-1.
Tumor growth profiles of individual mice after various treatments in TC-1 (a) and B16 (b) tumor models. Experiments were repeated twice with an indicated number of mice/group.
Supplementary Figure 2 Prior PD-1 blockade abrogates vaccine-induced tumor-specific immune responses early during the course of treatment.
Tumor tissues were harvested 3 days after priming (D13) or three days after boosting (D20) from B16 melanoma-bearing mice. a-d. Numbers of total (a & c) and antigen-specific CD8+ T cells (b & d) at D20 (a-b) and at D13 (c-d). e-h. Frequencies of Annexin V+ total (e & g) and antigen-specific CD8+ T cells (f & h) in the TME at D20 (e-f) and at D13 (g-h) as indicated. i-l. Frequencies of CD40L+ (i and k) and IFN-γ+ (j & l) CD8+ T cells in the TME at D20 (i-j) and at D13 (k-l) as indicated. Flow cytometry data are the average of two independent experiments. Each dot corresponds to one mouse with the indicated number of mice per group given in parentheses. For comparison purposes, an unpaired, one-tailed Student’s t-test was used. The error bars indicate the s.e.m. NSnon-significant (a) *(lower) p=0.0228, *(middle) p=0.0277, *(upper) p=0.0286, **(left) p=0.0012, **(right) p=0.0014; (b) *(lower) p=0.0333, *(middle) p=0.042, *(upper) p=0.0472, ****p≤0.0001; (d) *(lower) p=0.018, *(upper) p=0.0207, **p=0.0072, ***p=0.0004, ****p≤0.0001; (e) *(lower) p=0.05, *(upper) p=0.03, **p=0.0041; (f) *(lower) p=0.0462, *(upper) p=0.0181, **p=0.0094; (g) *(lower) p=0.0157, *(upper) p=0.0469, ***p=0.0006; (h) *(lower) p=0.0265, *(upper) p=0.0152, **p=0.0062 (i) *(left) p=0.0384, *(middle) p=0.0399, *(right) p=0.0165; (j) *(lower) p=0.0414, *(upper) p=0.0260, **p=0.0013, ***p=0.0007; (k) *p=0.0343, **p=0.006, ****p≤0.0001; (l) *p=0.0156. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001; ****P ≤ 0.0001.
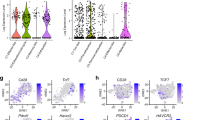
Supplementary Figure 3 PD-1 blockade prior to antigenic stimulation generates dysfunctional PD-1+CD38+ CD8+ T cells.
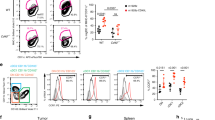
a. Gating strategy. b. FACS contour plots showing frequency of PD-1+CD38+ cells in total CD8+ T cells in the tumors (at D13) following various treatments.
Supplementary Figure 4 PD-1+CD38hi CD8+ T cells induced as a result of anti-PD-1 pre-treatment are dysfunctional.
a-b. Frequency of CD40L+ (a) and IFN-γ+ (b) T cells in PD-1+CD38hi CD8+ T cell population. c-d. Frequencies of Annexin V+ PD-1+CD38hi cells in total (c) and antigen-specific (d) CD8+ T cells at D13 post-B16 tumor implantation. Data are the average of two independent experiments. Each dot corresponds to one mouse with the indicated number of mice per group given in parentheses. The aerror bars indicate the s.e.m. For comparison purposes, an unpaired, one-tailed Student’s t-test was used. NSnon-significant (a) *(lower) p=0.0463, *(upper) p=0.0496, **p=0.0086, ***p=0.0009; (b) *(lower) p=0.018, *(upper) p=0.0441, **p=0.01, ****p≤0.0001; (c) vs. UT: *(lower) p=0.0327 and *(upper) p=0.0275, vs. Vax: *(lower) p=0.0143 and *(upper) p=0.0273, ****p≤0.0001; (d) *(lower) p=0.026, *(upper) p=0.0382, **p=0.01, ****p≤0.0001. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001; ****P ≤ 0.0001.
Supplementary Figure 5 Depletion of PD-1+CD38hi CD8+ T cells results in strong anti-tumor response.
a. Tumor growth of variously treated B16-bearing Rag1–/– mice following transfer of either total or PD-1+CD38+ depleted, in vitro activated CD8+ T cells (with an indicated number of mice per group given in parentheses). b. The full scan of the blot showing the expression of CD38 and β-actin in flow-sorted PD-1+CD38+ T cells transfected either with scrambled RNA (scRNA) or CD38 siRNA.
Supplementary Figure 6 ROC analysis to measure the predictive power of CD38+ fraction of PD1+CD8+ T-cells pre- and post- anti-PD-1 therapy in the human tumor and PBMC samples.
a-c. The ROC curves were generated using R version 3.5.1 software in post-therapy tumors with 4% cut-off (a), pre-therapy tumors with 10% cut-off (b), and PBMCs with 5% cut-off (c). AUC and 95% confidence interval (CI) were determined using Delong method with R version 3.5.1 statistical software. The diagnostic tables used for generating each ROC curve as well as the formulae used to calculate sensitivity, specificity, PPV and NPV are provided. Comparison of responding vs. non-responding tumor lesions that had more than 4%# or 10%^ PD-1+CD38+ cells in the CD8+ population in the TME. *For human PBMC data, the CD38+ fraction of PD-1+CD8+ T cells that showed more than 5% decline at 9 weeks when compared with 3 weeks were compared between responders and non-responders post-therapy. AUC: Area Under the Receiver Operating Characteristics (ROC) Curve; AUC 95% CI: There is 95% of confidence that the interval contains the true AUC. For example, there is 95% confidence that (0.679,1) contains the true value of AUC for 5% cut-off; PPP: Positive predictive value; NPV: Negative predictive value.
Supplementary information
Supplementary Information
Supplementary Figs. 1–6
Rights and permissions
About this article
Cite this article
Verma, V., Shrimali, R.K., Ahmad, S. et al. PD-1 blockade in subprimed CD8 cells induces dysfunctional PD-1+CD38hi cells and anti-PD-1 resistance. Nat Immunol 20, 1231–1243 (2019). https://doi.org/10.1038/s41590-019-0441-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41590-019-0441-y
This article is cited by
-
Single-cell combined bioinformatics analysis: construction of immune cluster and risk prognostic model in kidney renal clear cells based on CD8+ T cell-associated genes
European Journal of Medical Research (2024)
-
Multipeptide vaccines for melanoma in the adjuvant setting: long-term survival outcomes and post-hoc analysis of a randomized phase II trial
Nature Communications (2024)
-
Targeting aging and age-related diseases with vaccines
Nature Aging (2024)
-
Nivolumab for mismatch-repair-deficient or hypermutated gynecologic cancers: a phase 2 trial with biomarker analyses
Nature Medicine (2024)
-
The strategies to cure cancer patients by eradicating cancer stem-like cells
Molecular Cancer (2023)