Abstract
Stroke is a multiphasic process in which initial cerebral ischemia is followed by secondary injury from immune responses to ischemic brain components. Here we demonstrate that peripheral CD11b+CD45+ myeloid cells magnify stroke injury via activation of triggering receptor expressed on myeloid cells 1 (TREM1), an amplifier of proinflammatory innate immune responses. TREM1 was induced within hours after stroke peripherally in CD11b+CD45+ cells trafficking to ischemic brain. TREM1 inhibition genetically or pharmacologically improved outcome via protective antioxidant and anti-inflammatory mechanisms. Positron electron tomography imaging using radiolabeled antibody recognizing TREM1 revealed elevated TREM1 expression in spleen and, unexpectedly, in intestine. In the lamina propria, noradrenergic-dependent increases in gut permeability induced TREM1 on inflammatory Ly6C+MHCII+ macrophages, further increasing epithelial permeability and facilitating bacterial translocation across the gut barrier. Thus, following stroke, peripheral TREM1 induction amplifies proinflammatory responses to both brain-derived and intestinal-derived immunogenic components. Critically, targeting this specific innate immune pathway reduces cerebral injury.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon request.
References
Fang, M. C., Cutler, D. M. & Rosen, A. B. Trends in thrombolytic use for ischemic stroke in the United States. J. Hosp. Med. 5, 406–409 (2010).
Stevens, S. L. et al. The use of flow cytometry to evaluate temporal changes in inflammatory cells following focal cerebral ischemia in mice. Brain Res. 932, 110–119 (2002).
Frangogiannis, N. G. Chemokines in ischemia and reperfusion. Thromb. Haemost. 97, 738–747 (2007).
Wang, Q., Tang, X. N. & Yenari, M. A. The inflammatory response in stroke. J. Neuroimmunol. 184, 53–68 (2007).
Gelderblom, M. et al. Temporal and spatial dynamics of cerebral immune cell accumulation in stroke. Stroke 40, 1849–1857 (2009).
Iadecola, C. & Anrather, J. The immunology of stroke: from mechanisms to translation. Nat. Med. 17, 796–808 (2011).
Vendrame, M. et al. Cord blood rescues stroke-induced changes in splenocyte phenotype and function. Exp. Neurol. 199, 191–200 (2006).
Ajmo, C. T. Jr. et al. The spleen contributes to stroke-induced neurodegeneration. J. Neurosci. Res. 86, 2227–2234 (2008).
Seifert, H. A. et al. The spleen contributes to stroke induced neurodegeneration through interferon gamma signaling. Metab. Brain Dis. 27, 131–141 (2012).
Bao, Y., Kim, E., Bhosle, S., Mehta, H. & Cho, S. A role for spleen monocytes in post-ischemic brain inflammation and injury. J. Neuroinflammation 7, 92 (2010).
Dotson, A. L., Wang, J., Saugstad, J., Murphy, S. J. & Offner, H. Splenectomy reduces infarct volume and neuroinflammation in male but not female mice in experimental stroke. J. Neuroimmunol. 278, 289–298 (2015).
Lalancette-Hebert, M., Gowing, G., Simard, A., Weng, Y. C. & Kriz, J. Selective ablation of proliferating microglial cells exacerbates ischemic injury in the brain. J. Neurosci. 27, 2596–2605 (2007).
Gliem, M. et al. Macrophages prevent hemorrhagic infarct transformation in murine stroke models. Ann. Neurol. 71, 743–752 (2012).
Szalay, G. et al. Microglia protect against brain injury and their selective elimination dysregulates neuronal network activity after stroke. Nat. Commun. 7, 11499 (2016).
Bouchon, A., Dietrich, J. & Colonna, M. Cutting edge: inflammatory responses can be triggered by TREM-1, a novel receptor expressed on neutrophils and monocytes. J. Immunol. 164, 4991–4995 (2000).
Colonna, M. TREMs in the immune system and beyond. Nat. Rev. Immunol. 3, 445–453 (2003).
Bleharski, J. R. et al. A role for triggering receptor expressed on myeloid cells-1 in host defense during the early-induced and adaptive phases of the immune response. J. Immunol. 170, 3812–3818 (2003).
Radsak, M. P., Salih, H. R., Rammensee, H. G. & Schild, H. Triggering receptor expressed on myeloid cells-1 in neutrophil inflammatory responses: differential regulation of activation and survival. J. Immunol. 172, 4956–4963 (2004).
Schenk, M., Bouchon, A., Seibold, F. & Mueller, C. TREM-1-expressing intestinal macrophages crucially amplify chronic inflammation in experimental colitis and inflammatory bowel diseases. J. Clin. Invest. 117, 3097–3106 (2007).
Park, J. J. et al. Correlation of serum-soluble triggering receptor expressed on myeloid cells-1 with clinical disease activity in inflammatory bowel disease. Dig. Dis. Sci. 54, 1525–1531 (2009).
Bouchon, A., Facchetti, F., Weigand, M. A. & Colonna, M. TREM-1 amplifies inflammation and is a crucial mediator of septic shock. Nature 410, 1103–1107 (2001).
Knapp, S. et al. Cutting edge: expression patterns of surface and soluble triggering receptor expressed on myeloid cells-1 in human endotoxemia. J. Immunol. 173, 7131–7134 (2004).
Gibot, S. et al. Plasma level of a triggering receptor expressed on myeloid cells-1: its diagnostic accuracy in patients with suspected sepsis. Ann. Int. Med. 141, 9–15 (2004).
Weber, B. et al. TREM-1 deficiency can attenuate disease severity without affecting pathogen clearance. PLoS Pathog. 10, e1003900 (2014).
Collins, C. E. et al. Elevated synovial expression of triggering receptor expressed on myeloid cells 1 in patients with septic arthritis or rheumatoid arthritis. Ann. Rheum. Dis. 68, 1768–1774 (2009).
Yasuda, T. et al. Increased levels of soluble triggering receptor expressed on myeloid cells-1 in patients with acute pancreatitis. Crit. Care Med. 36, 2048–2053 (2008).
Zysset, D. et al. TREM-1 links dyslipidemia to inflammation and lipid deposition in atherosclerosis. Nat. Commun. 7, 13151 (2016).
Saurer, L. et al. TREM-1 promotes intestinal tumorigenesis. Sci. Rep. 7, 14870 (2017).
Nguyen-Lefebvre, A. T. et al. The innate immune receptor TREM-1 promotes liver injury and fibrosis. J. Clin. Invest. 128, 4870–4883 (2018).
Jung, S. et al. Analysis of fractalkine receptor CX3CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol. Cell. Biol. 20, 4106–4114 (2000).
Saederup, N. et al. Selective chemokine receptor usage by central nervous system myeloid cells in CCR2-red fluorescent protein knock-in mice. PLoS ONE 5, e13693 (2010).
Garcia-Bonilla, L. et al. Spatio-temporal profile, phenotypic diversity, and fate of recruited monocytes into the post-ischemic brain. J. Neuroinflammation 13, 285 (2016).
Chen, C., Ai, Q. D., Chu, S. F., Zhang, Z. & Chen, N. H. NK cells in cerebral ischemia. Biomed. Pharmacother. 109, 547–554 (2019).
Swirski, F. K. et al. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science 325, 612–616 (2009).
Herwig, R., Hardt, C., Lienhard, M. & Kamburov, A. Analyzing and interpreting genome data at the network level with ConsensusPathDB. Nat. Protoc. 11, 1889–1907 (2016).
Poliani, P. L. et al. TREM2 sustains microglial expansion during aging and response to demyelination. J. Clin. Invest. 125, 2161–2170 (2015).
Wang, Y. et al. TREM2 lipid sensing sustains the microglial response in an Alzheimer’s disease model. Cell 160, 1061–1071 (2015).
Jay, T. R. et al. TREM2 deficiency eliminates TREM2+ inflammatory macrophages and ameliorates pathology in Alzheimer’s disease mouse models. J. Exp. Med. 212, 287–295 (2015).
Kawabori, M. et al. Triggering receptor expressed on myeloid cells 2 (TREM2) deficiency attenuates phagocytic activities of microglia and exacerbates ischemic damage in experimental stroke. J. Neurosci. 35, 3384–3396 (2015).
Kleinberger, G. et al. TREM2 mutations implicated in neurodegeneration impair cell surface transport and phagocytosis. Sci. Transl. Med. 6, 243ra286 (2014).
Cantoni, C. et al. TREM2 regulates microglial cell activation in response to demyelination in vivo. Acta Neuropathol. 129, 429–447 (2015).
Mazaheri, F. et al. TREM2 deficiency impairs chemotaxis and microglial responses to neuronal injury. EMBO Rep. 18, 1186–1198 (2017).
Gibot, S. et al. Modulation of the triggering receptor expressed on the myeloid cell type 1 pathway in murine septic shock. Infect. Immun. 74, 2823–2830 (2006).
Gibot, S. et al. A soluble form of the triggering receptor expressed on myeloid cells-1 modulates the inflammatory response in murine sepsis. J. Exp. Med. 200, 1419–1426 (2004).
Tamoutounour, S. et al. CD64 distinguishes macrophages from dendritic cells in the gut and reveals the Th1-inducing role of mesenteric lymph node macrophages during colitis. Eur. J. Immunol. 42, 3150–3166 (2012).
Stanley, D. et al. Translocation and dissemination of commensal bacteria in post-stroke infection. Nat. Med. 22, 1277–1284 (2016).
Crapser, J. et al. Ischemic stroke induces gut permeability and enhances bacterial translocation leading to sepsis in aged mice. Aging (Albany NY) 8, 1049–1063 (2016).
Souto, F. O. et al. Essential role of CCR2 in neutrophil tissue infiltration and multiple organ dysfunction in sepsis. Am. J. Respir. Crit. Care Med. 183, 234–242 (2011).
Johnston, B. et al. Chronic inflammation upregulates chemokine receptors and induces neutrophil migration to monocyte chemoattractant protein-1. J. Clin. Invest. 103, 1269–1276 (1999).
Read, C. B. et al. Cutting edge: identification of neutrophil PGLYRP1 as a ligand for TREM-1. J. Immunol. 194, 1417–1421 (2015).
Schenk, M., Bouchon, A., Birrer, S., Colonna, M. & Mueller, C. Macrophages expressing triggering receptor expressed on myeloid cells-1 are underrepresented in the human intestine. J. Immunol. 174, 517–524 (2005).
Batarseh, A. & Papadopoulos, V. Regulation of translocator protein 18 kDa (TSPO) expression in health and disease states. Mol. Cell. Endocrin. 327, 1–12 (2010).
Chen, M. K. & Guilarte, T. R. Translocator protein 18 kDa (TSPO): molecular sensor of brain injury and repair. Pharmacol. Ther. 118, 1–17 (2008).
Ching, A. S. et al. Current paradigm of the 18-kDa translocator protein (TSPO) as a molecular target for PET imaging in neuroinflammation and neurodegenerative diseases. Insights Imaging 3, 111–119 (2012).
McCullough, L. et al. Neuroprotective function of the PGE2 EP2 receptor in cerebral ischemia. J. Neurosci. 24, 257–268 (2004).
Liang, X. et al. Neuronal and vascular protection by the prostaglandin E2 EP4 receptor in a mouse model of cerebral ischemia. J. Clin. Invest. 121, 4362–4371 (2011).
Longa, E. Z., Weinstein, P. R., Carlson, S. & Cummins, R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke 1, 84–91 (1989).
Swanson, R. A. et al. A semiautomated method for measuring brain infarct volume. J. Cereb. Blood Flow Metab. 10, 290–293 (1990).
Zhang, Z., Chen, L., Liu, L., Su, X. & Rabinowitz, J. D. Chemical basis for deuterium labeling of fat and NADPH. J. Am. Chem. Soc. 139, 14368–14371 (2017).
Lin, B., Levy, S., Raval, A. P., Perez-Pinzon, M. A. & Defazio, R. A. Forebrain ischemia triggers GABAergic system degeneration in substantia nigra at chronic stages in rats. Cardiovasc. Psychiatry Neurol. 2010, 506952 (2010).
Southwell, A. L., Ko, J. & Patterson, P. H. Intrabody gene therapy ameliorates motor, cognitive, and neuropathological symptoms in multiple mouse models of Huntington’s disease. J. Neurosci. 29, 13589–13602 (2009).
Carter, R. J., Morton, J. & Dunnett, S. B. Motor coordination and balance in rodents. Curr. Protoc. Neurosci. 15, 8.12.1–8.12.14 (2001).
Acknowledgements
This work was supported by National Institutes of Health (NIH) grant (nos. R01NS045727, R21NS087639, R01NS100180 and RO1AG053001 to K.I.A.), The Paul and Daisy Soros Fellowship for New Americans (to P.S.M.), the Gerald J. Lieberman Fellowship (to P.S.M.) and Stanford Medicine Dean’s Fellowship (to E.N.W.). The authors would like to thank W. Lu for LC–MS expertise, the Stanford Shared FACS Facility, the Stanford Human Immune Monitoring Center, and the Protein and Nucleic Acid Facility.
Author information
Authors and Affiliations
Contributions
Q.L., E.M.J., R.K.L, Q.W., E.N.W., P.S.M., M.S.S., S.S.M. and X.Y. designed and performed the experiments and analyzed the data. H.B.Y., S.T. and J.W. performed the experiments. M.S., S.S.Y and C.M. provided advice. J.D.R. and L.L. designed and performed metabolic measurements and analyzed the data. E.M.J. and M.L.J. designed and performed PET measurements and analyzed the data. Q.L., M.L.J. and K.I.A. conceived and supervised the project, designed the experiments, interpreted the data and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interest
The authors declare no competing interests.
Additional information
Peer review information: Laurie Dempsey was the primary editor on this article and managed its editorial process and peer review in collaboration with the rest of the editorial team.
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Integrated supplementary information
Supplementary Figure 1 Temporal dynamics of myeloid cells and TREM1 expression in ipsilateral (IL) hemisphere after MCAo.
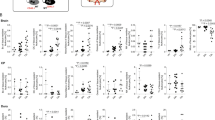
Flow cytometry gating strategy of brain myeloid cells in the ischemic hemisphere 48 h after MCAo. Time courses were carried out over 7 days post MCAo in C56Bl/6J 2–3 mo male mice. Representative plots of CD11b+CD45+ myeloid cell populations at day 0 before MCAo, and at 2 and 6 days after MCAo. Time course of percents of macrophages, neutrophils, and microglia in ischemic hemisphere out to Day 7 post MCAo; n=6 biologically independent samples at days 0 and 7, and n=7 at days 2,4,and 6, mean +/- SEM;1 way ANOVA for each cell type: microglia P=0.015, Mo/MΦ P<0.0001, PMNs P<0.0001. TREM1 surface expression on CD11b+CD45hi Mo/MΦ and CD11b+CD45hiLy6G+ PMNs 2 days after MCAo compared to isotype control antibody. Representative plots of CD11b+CD45+TREM1+ cells at day 2 and day 6 after MCAo in ischemic ipsilateral (IL) and non-infarcted contralateral (CL) hemispheres. Percentages of TREM1+ macrophages, neutrophils, and microglia in IL and CL hemispheres and in sham IL and CL hemispheres at day 2 and day 6 (n= 6 biologically independent samples for sham, n=7 for MCAo, mean +/- SEM; two-way ANOVA for IL MCAo vs IL sham hemispheres: for macrophages, effect of MCAo P <0.0001, effect of time P <0.01, effect of interaction, P <0.05; Bonferroni post-hoc day 2 IL MCAo vs IL sham **** P <0.0001; post-hoc day 6 IL MCAo vs day 6 IL sham hemisphere ## P <0.01; for microglia, effect of MCAo ** P =0.006).
Supplementary Figure 2 TREM1 surface expression is induced early after MCAo in peripheral Mo/MΦ cells.
TREM1 surface expression was quantified in blood at 0h before sham or MCAo, and 1h, 4.5h, 48h, and 144h (6 days) after sham surgery or MCAo. Percents of TREM1+ Mo/MΦ and PMNs in blood are shown (n=3–12 biologically independent samples per time point, mean +/- SEM; two-way ANOVA for macrophages, effects of MCAo, time, and interaction P<0.0001; Bonferroni post hoc **** P <0.0001 at 4.5h for MCAo vs sham). TREM1 surface expression was quantified in spleen at 0h before sham or MCAo, and 1h, 4.5h, 48h, and 144h (6 days) after sham surgery or after MCAo. Percents TREM1+ Mo/MΦ and PMNs in spleen are shown (n=3–12 biologically independent samples per time point, represented as mean +/- SEM; two-way ANOVA for macrophages, effect of MCAo and time, P<0.0001; effect of interaction P<0.001, Bonferroni post hoc **** P <0.0001 at 4.5h for MCAo vs sham). TREM1 MFI in blood and IL hemisphere Mo/MΦ subsets 2 days (n=5–7 biologically independent samples per group, mean +/- SEM) and 6 days (n=4–7 biologically independent samples per group, mean +/- SEM) after MCAo (Student’s two tailed t-test, ** P <0.01).
Supplementary Figure 3 Genetic ablation of Trem1 improves outcome after MCAo.
Percent TREM1 expression in CD11b+CD45hiLy6Ghi PMNs, CD11b+CD45hi Mo/MΦ, and CD11b+CD45int microglia 2 days after MCAo from Trem1+/+, Trem1+/-, and Trem1-/- ischemic hemispheres (n=5–10 biologically independent samples per cell type per genotype, mean +/- SEM). Representative plots of CD11b+CD45hiLy6Ghi PMNs, CD11b+CD45hi Mo/MΦ, and CD11b+CD45int microglia that were sorted and isolated for transcriptomic analysis at 2 days after MCAo. Top three Gene Ontology pathways (FDR<0.05, absolute fold ≥2). KEGG lysosomal genes are induced in Trem1-/- vs Trem1+/+ PMNs (note log10 scale). Heat map of macrophage genes differentially regulated and FDR corrected (P<0.05) in Trem1+/+ vs Trem1-/- ischemic hemispheres at 2 days after MCAo (absolute fold ≥2). Histogram of TREM2 antibody vs isotype control. BV2 microglia were stimulated with LPS 10 ng/ml and surface expression of TREM1 determined at 2, 6, 10, and 20 h after stimulation. Raw macrophages and BV2 microglia were stimulated with LPS 10 ng/ml and qRT-PCR performed for TREM1 and TREM2 expression at 0, 4, and 20 h (n=3 biologically independent samples per time point per group, mean +/- SEM; 1-way ANOVA, P values for TREM1 in red and TREM2 in blue). Percent CD11b+CD45+ myeloid cells in Trem1+/+, Trem1+/-, and Trem1-/- IL and CL hemispheres 2 days after MCAo (n=8 biologically independent samples per genotype for Trem1+/+ and Trem1+/- mice and n=9 for Trem1-/- mice, mean +/- SEM; two-way ANOVA, effect of genotype P <0.0001, effect of hemisphere P <0.05; post-hoc * P <0.05 and *** P <0.001). Percent CD11b+CD45hiLy6Ghi PMNs, CD11b+CD45hi Mo/MΦ, and CD11b+CD45int microglia in Trem1+/+, Trem1+/-, and Trem1-/- IL and CL hemispheres 2 days after MCAo (n=8 biologically independent samples for Trem1+/+ and Trem1+/- mice and n=9 samples for Trem1-/- mice, mean +/- SEM; two-way ANOVA, effect of hemisphere P <0.0001 all three cell types, effect of genotype P <0.01 for Mo/MΦ only; post-hoc *** P <0.001).
Supplementary Figure 4 Administration of LP17 to Trem1-/- mice.
LP17 reduces mortality post-stroke (n=26 mice per group; Log-rank test * P =0.011). LP17 administered at 4.5h after MCAo does not affect survival (n=28–36 mice per group). Neuroscores of Trem1-/- mice that underwent MCAo and received LP17 or scrambled peptide at the time of reperfusion (n=8 mice per group; mean +/- SEM). Percent infarct volume in Trem1-/- mice treated with LP17 or scrambled peptide (n= 8 mice per group, mean +/- SEM). Representative histogram of TREM2 expression on CD11b+CD45+Ly6G+ PMNs 2 days after MCAo +/- LP17 or scrambled peptide treatment at time of reperfusion. Body weights of sham and MCAo mice from Fig. 4m-n. Number of left front and left hind paw slips in beam-tested mice (n=5 sham, n=8 scrambled, n=11 LP17).
Supplementary Figure 5 TREM1 signal is increased in peripheral myeloid tissues after MCAo.
64Cu-labeled anti-TREM1-mAb (that is, [64Cu]TREM1-mAb) was generated with high specific radioactivity (>0.400 MBq/μg), radiochemical purity (>99%), and labeling efficiency (70–95%), and formulated in phosphate-buffered saline [0.1 mol/L NaCl, 0.05 mol/L sodium phosphate (pH 7.4)] (see Methods). HEK293 cells transiently expressing murine Trem1 cDNA and control empty vector transfected cells were assayed for binding of [64Cu]TREM1-mAb at 1 hour (n=3–4 biologically independent samples per group, mean +/- SEM). Unlabeled TREM1 antibody was used to block binding. Quantification of PET signal in peripheral organs from MCAo and sham mice (36 h post-MCAo; n=9 biologically independent samples per group, mean +/- SEM). Quantification of TREM1 signal from ex vivo biodistribution studies of blood, heart, liver and lungs (n=8–15 biologically independent samples per group, mean +/- SEM). Quantification of spleen TREM1 signal from ex vivo biodistribution studies shows higher uptake of [64Cu]TREM1-mAb in MCAo mice compared to shams and compared to MCAo and sham mice injected with [64Cu]Isotype control (n=12, MCAo-[64Cu]TREM1; n=10, sham-[64Cu]TREM1; n=8, MCAo-[64Cu]ISO-Ctrl; n=3, Sham-[64Cu]ISO-Ctrl) mice, mean +/- SEM). Ex vivo biodistribution of brain hemispheres corroborates brain PET imaging findings (n=10 MCAo-[64Cu]TREM1, n=9 sham-[64Cu]TREM1 biologically independent samples, mean +/- SEM). All comparisons are two-tailed unpaired Student’s t-test **** P <0.001, ** P <0.01, * P <0.05.
Supplementary Figure 6 TREM1 is induced in the intestinal inflammatory Mo/MΦ subset after MCAo.
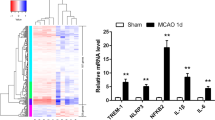
ß-adrenergic inhibition with propranolol (ppl) has no effect on neutrophil TREM1 expression after MCAo. TREM1 MFI in neutrophils in sham, MCAo, and MCAo treated with propranolol (n=5 sham, n=8 MCAo and n=9 MCAo+ppl mice, mean +/- SEM). Percent change in volume of IL hemisphere in sham and MCAo mice +/- ppl 4.5h after MCAo (n=5 sham, n=9 MCAo and n=9 MCAo+ppl, mean +/- SEM). Immune factor changes at 4.5h in small intestine lamina propria (n=3–6 biologically independent samples per group, mean +/- SEM.; ** P <0.01 Student’s two tailed t-test). Quantification of bacterial colonies that grew out of blood collected from Trem1+/+ and Trem1-/- mice at 4.5h after MCAo (n=3 sham Trem1+/+, n=6 MCAo Trem1+/+ and n=4 MCAo Trem1-/- mice, mean +/- SEM).
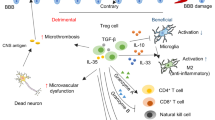
Supplementary Figure 7 Model of dual TREM1 amplification of immune responses after cerebral ischemia.
(Left side) Cerebral injury activates the sympathetic nervous system (SNS) within hours of MCAo, leading to early disruption of the gut barrier and translocation of bacterial PAMPs across the epithelial barrier. There, PAMPs induce and activate TREM1 signaling in lamina propria Mo/MΦ subsets, amplifying the innate immune response and further disrupting gut barrier integrity and facilitating translocation of bacteria to the periphery. (Right side): Cerebral infarction induces the release of sterile DAMPs that activate TREM1 on circulating peripheral and splenic myeloid cells. Thus, TREM1 is induced in myeloid cells in two spatially distinct processes after MCAo. Dual brain-derived and intestinal-derived TREM1 responses converge and amplify the post-stroke innate immune response, increasing cerebral injury.
Supplementary information
Supplementary Information
Supplementary Figs. 1–7
Supplementary Video 1
PET imaging of TREM1
Rights and permissions
About this article
Cite this article
Liu, Q., Johnson, E.M., Lam, R.K. et al. Peripheral TREM1 responses to brain and intestinal immunogens amplify stroke severity. Nat Immunol 20, 1023–1034 (2019). https://doi.org/10.1038/s41590-019-0421-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41590-019-0421-2
This article is cited by
-
Salvianolic acid C attenuates cerebral ischemic injury through inhibiting neuroinflammation via the TLR4-TREM1-NF-κB pathway
Chinese Medicine (2024)
-
Cross-talk between disulfidptosis and immune check point genes defines the tumor microenvironment for the prediction of prognosis and immunotherapies in glioblastoma
Scientific Reports (2024)
-
The crosstalk between enteric nervous system and immune system in intestinal development, homeostasis and diseases
Science China Life Sciences (2024)
-
TREM1 disrupts myeloid bioenergetics and cognitive function in aging and Alzheimer disease mouse models
Nature Neuroscience (2024)
-
Receptor-interacting protein kinase 2 (RIPK2) profoundly contributes to post-stroke neuroinflammation and behavioral deficits with microglia as unique perpetrators
Journal of Neuroinflammation (2023)