Abstract
The incidence of atherosclerosis is higher among patients with systemic lupus erythematosus (SLE); however, the mechanism by which an atherogenic environment affects autoimmunity remains unclear. We found that reconstitution of atherosclerosis-prone Apoe–/– and Ldlr–/– mice with bone marrow from lupus-prone BXD2 mice resulted in increased autoantibody production and glomerulonephritis. This enhanced disease was associated with an increase in CXCR3+ follicular helper T cells (TFH cells). TFH cells isolated from Apoe–/– mice had higher expression of genes associated with inflammatory responses and SLE and were more potent in inducing production of the immunoglobulin IgG2c. Mechanistically, the atherogenic environment induced the cytokine IL-27 from dendritic cells in a Toll-like receptor 4 (TLR4)-dependent manner, which in turn triggered the differentiation of CXCR3+ TFH cells while inhibiting the differentiation of follicular regulatory T cells. Blockade of IL-27 signals diminished the increased TFH cell responses in atherogenic mice. Thus, atherogenic dyslipidemia augments autoimmune TFH cell responses and subsequent IgG2c production in a TLR4- and IL-27-dependent manner.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Change history
19 June 2018
In the version of this article initially published, the third label along the horizontal axis of Fig. 4b (Il13a) and the middle label above each plot in Fig. 6k (Stat–/–) were incorrect, and the hash marks along the horizontal axis for Fig. 6i were spaced incorrectly. Also, the statistical results in the citation for Supplementary Fig. 5a (*P < 0.05, **P < 0.01 and ***P < 0.001 (unpaired Student’s t-test)) in the fifth subsection of Results were incorrect. The correct label for Fig. 4b is Il23a and for Fig. 6k is Stat1–/–, and the right hash mark along the horizontal axis for Fig. 6i should be beneath the data points at right. The correct citation of the statistical results is as follows: “(P < 0.05 and P < 0.01 (unpaired Student’s t-test); Supplementary Fig. 5a).” The errors have been corrected in the HTML and PDF version of the article.
References
Weber, C. & Noels, H. Atherosclerosis: current pathogenesis and therapeutic options. Nat. Med. 17, 1410–1422 (2011).
Hansson, G. K. & Libby, P. The immune response in atherosclerosis: a double-edged sword. Nat. Rev. Immunol. 6, 508–519 (2006).
Laurat, E. et al. In vivo downregulation of T helper cell 1 immune responses reduces atherogenesis in apolipoprotein E-knockout mice. Circulation 104, 197 (2001).
Danzaki, K. et al. Interleukin-17A deficiency accelerates unstable atherosclerotic plaque formation in apolipoprotein E-deficient mice. Arterioscler. Thromb. Vasc. Biol. 32, 273 (2012).
Goodson, N., Marks, J., Lunt, M. & Symmons, D. Cardiovascular admissions and mortality in an inception cohort of patients with rheumatoid arthritis with onset in the 1980s and 1990s. Ann. Rheum. Dis. 64, 1595–1601 (2005).
Kimball, A. B. et al. Cardiovascular disease and risk factors among psoriasis patients in two US healthcare databases, 2001–2002. Dermatology 217, 27–37 (2008).
Roman, M. J. et al. Prevalence and correlates of accelerated atherosclerosis in systemic lupus erythematosus. N. Engl. J. Med. 349, 2399–2406 (2003).
Yu, H.-H. et al. Statin reduces mortality and morbidity in systemic lupus erythematosus patients with hyperlipidemia: a nationwide population-based cohort study. Atherosclerosis 243, 11–18 (2015).
Ghazizadeh, R., Tosa, M. & Ghazizadeh, M. Clinical improvement in psoriasis with treatment of associated hyperlipidemia. Am. J. Med. Sci. 341, 394–398 (2011).
Cai, Y., Fleming, C. & Yan, J. New insights of T cells in the pathogenesis of psoriasis. Cell. Mol. Immunol. 9, 302–309 (2012).
Ryu, H. & Chung, Y. Regulation of IL-17 in atherosclerosis and related autoimmunity. Cytokine 74, 219–227 (2015).
Choi, J.-Y. et al. Circulating follicular helper–like T cells in systemic lupus erythematosus: association with disease activity. Arthritis Rheumatol. 67, 988–999 (2015).
McGeachy, M. J., Singh, D., Henkel, M. & Moreland, L. Th17/TfH cells in rheumatoid arthritis: correlations with disease activity and therapy response. J. Immunol. 196, 51.23 (2016).
Lim, H. et al. Proatherogenic conditions promote autoimmune T helper 17 cell responses in vivo. Immunity 40, 153–165 (2014).
Park, H.-J. et al. Insights into the role of follicular helper T cells in autoimmunity. Immune Netw. 14, 21–29 (2014).
Yuan, J., Li, L. I., Wang, Z., Song, W. & Zhang, Z. Dyslipidemia in patients with systemic lupus erythematosus: association with disease activity and B-type natriuretic peptide levels. Biomed. Rep. 4, 68–72 (2016).
Chung, C. P. et al. Inflammatory mechanisms affecting the lipid profile in patients with systemic lupus erythematosus. J. Rheumatol. 34, 1849 (2007).
Baudino, L., Azeredo da Silveira, S., Nakata, M. & Izui, S. Molecular and cellular basis for pathogenicity of autoantibodies: lessons from murine monoclonal autoantibodies. Springer Semin. Immunopathol. 28, 175–184 (2006).
Tangye, S. G., Ma, C. S., Brink, R. & Deenick, E. K. The good, the bad and the ugly—TFH cells in human health and disease. Nat. Rev. Immunol. 13, 412–426 (2013).
Shortman, K. & Liu, Y.-J. Mouse and human dendritic cell subtypes. Nat. Rev. Immunol. 2, 151–161 (2002).
Reynolds, C. M. et al. Dietary saturated fatty acids prime the NLRP3 inflammasome via TLR4 in dendritic cells—implications for diet-induced insulin resistance. Mol. Nutr. Food Res. 56, 1212–1222 (2012).
Hong, C. & Tontonoz, P. Liver X receptors in lipid metabolism: opportunities for drug discovery. Nat. Rev. Drug Discov. 13, 433 (2014).
Castrillo, A. et al. Crosstalk between LXR and Toll-like receptor signaling mediates bacterial and viral antagonism of cholesterol metabolism. Mol. Cell 12, 805–816 (2003).
Collins, J. L. et al. Identification of a nonsteroidal liver X receptor agonist through parallel array synthesis of tertiary amines. J. Med. Chem. 45, 1963–1966 (2002).
Stumhofer, J. S. et al. Interleukins 27 and 6 induce STAT3-mediated T cell production of interleukin 10. Nat. Immunol. 12, 1363–1371 (2007).
Snapper, C. M. et al. Induction of IgG3 secretion by interferon gamma: a model for T cell-independent class switching in response to T cell-independent type 2 antigens. J. Exp. Med. 175, 1367 (1992).
Mozaffarian, D. F. et al. Executive summary: heart disease and stroke statistics—2016 update: a report from the American Heart Association. Circulation 133, 447–454 (2016).
Batten, M. et al. IL-27 supports germinal center function by enhancing IL-21 production and the function of T follicular helper cells. J. Exp. Med. 207, 2895 (2010).
Nurieva, R. I. et al. Generation of T follicular helper cells is mediated by interleukin-21 but independent of T helper 1, 2, or 17 cell lineages. Immunity 29, 138–149 (2008).
Owaki, T. et al. A role for IL-27 in early regulation of Th1 differentiation. J. Immunol. 175, 2191 (2005).
Vijayan, D. et al. IL-27 directly enhances germinal center B cell activity and potentiates lupus in Sanroque mice. J. Immunol. 197, 3008 (2016).
Koch, M. et al. The transcription factor T-bet controls regulatory T cell homeostasis and function during type 1 inflammation. Nat. Immunol. 10, 595–602 (2009).
Barbi, J., Oghumu, S., Lezama-Davila, C. M. & Satoskar, A. R. IFN-γ and STAT1 are required for efficient induction of CXC chemokine receptor 3 (CXCR3) on CD4+ but not CD8+ T cells. Blood 110, 2215–2216 (2007).
Chung, Y. et al. Follicular regulatory T (Tfr) cells with dual Foxp3 and Bcl6 expression suppress germinal center reactions. Nat. Med. 17, 983–988 (2011).
Chang, J.-H. & Chung, Y. Regulatory T cells in B cell follicles. Immune Netw. 14, 227–236 (2014).
Lee, S. K. et al. Interferon-γ excess leads to pathogenic accumulation of follicular helper T cells and germinal centers. Immunity 37, 880–892 (2012).
Miyauchi, K. et al. Protective neutralizing influenza antibody response in the absence of T follicular helper cells. Nat. Immunol. 17, 1447 (2016).
Wang, L. et al. Selective depletion of CD11c+CD11b+ dendritic cells partially abrogates tolerogenic effects of intravenous MOG in murine EAE. Eur. J. Immunol. 46, 2454–2466 (2016).
Shin, C. et al. CD8α– dendritic cells induce antigen-specific T follicular helper cells generating efficient humoral immune responses. Cell Reports 11, 1929–1940 (2015).
Joseph, S. B., Castrillo, A., Laffitte, B. A., Mangelsdorf, D. J. & Tontonoz, P. Reciprocal regulation of inflammation and lipid metabolism by liver X receptors. Nat. Med. 9, 213 (2003).
Jin, W. et al. Elevated circulating interleukin-27 in patients with coronary artery disease is associated with dendritic cells, oxidized low-density lipoprotein, and severity of coronary artery stenosis. Mediators Inflamm. 2012, 10 (2012).
Leng, C.-H. et al. A recombinant lipoprotein containing an unsaturated fatty acid activates NF-κB through the TLR2 signaling pathway and induces a differential gene profile from a synthetic lipopeptide. Mol. Immunol. 47, 2015–2021 (2010).
Kim, H. S., Go, H., Akira, S. & Chung, D. H. TLR2-mediated production of IL-27 and chemokines by respiratory epithelial cells promotes bleomycin-induced pulmonary fibrosis in mice. J. Immunol. 187, 4007 (2011).
Gringhuis, S. I. et al. Fucose-based PAMPs prime dendritic cells for follicular T helper cell polarization via DC-SIGN-dependent IL-27 production. Nat. Commun. 5, 5074 (2014).
Mills, E. L., Kelly, B. & O’Neill, L. A. J. Mitochondria are the powerhouses of immunity. Nat. Immunol. 18, 488 (2017).
O’Neill, L. A. J. & Pearce, E. J. Immunometabolism governs dendritic cell and macrophage function. J. Exp. Med. 213, 15–23 (2016).
Dorosz, S. A. et al. Role of calprotectin as a modulator of the IL27-mediated proinflammatory effect on endothelial cells. Mediators Inflamm. 2015, 16 (2015).
Qiu, H.-N., Liu, B., Liu, W. & Liu, S. Interleukin-27 enhances TNF-α-mediated activation of human coronary artery endothelial cells. Mol. Cell. Biochem. 411, 1–10 (2016).
Xia, L. P., Li, B. F., Shen, H. & Lu, J. Interleukin-27 and interleukin-23 in patients with systemic lupus erythematosus: possible role in lupus nephritis. Scand. J. Rheumatol. 44, 200–205 (2015).
Pan, H.-F., Tao, J.-H. & Ye, D.-Q. Therapeutic potential of IL-27 in systemic lupus erythematosus. Expert Opin. Ther. Targets 14, 479–484 (2010).
Yoo, S.-A. et al. Arginine-Rich anti-vascular endothelial growth factor (anti-VEGF) hexapeptide inhibits collagen-induced arthritis and VEGF-stimulated productions of TNF-α and IL-6 by human monocytes. J. Immunol. 174, 5846 (2005).
Kim, Y. U., Lim, H., Jung, H. E., Wetsel, R. A. & Chung, Y. Kim, Y. U., Lim, H., Jung, H. E., Wetsel, R. A. & Chung, Y. Regulation of autoimmune germinal center reactions in lupus-prone BXD2 mice byfollicular helper T cells. PLoS One 10, e0120294 (2015).
Acknowledgements
We thank K.-W. Kim and S.-J. Bae for support in flow cytometry; S. Akira (Osaka University) and S-Y. Sung (Seoul National University) for Stat3fl/fl and Tlr4–/– mice; H. S. Kim (Ulsan University) for Stat1–/– mice; J.-H. Choi (Hanyang University) for Apoe–/– mice; S-A. Yoo (The Catholic University of Korea) for animal models; the entire Chung lab for suggestion and discussion; and J. Reynolds, G. Martinez and D.-S. Kuen for proofreading the manuscript. Supported by the National Research Foundation of Korea (research grants 2014R1A2A1A11054364, 2017R1A2B3007392 and 2017K1A1A2004511 to Y.C., and 2015R1D1A1A01059719 to M.C.).
Author information
Authors and Affiliations
Contributions
H.R., H.L., G.C. and H.N. performed the in vitro and in vivo experiments; Y.-J.P. generated Ebi3–/–Apoe–/– mice; M.C. generated the Tlr4–/– bone marrow chimeras; C.W.A. and Y.C.K. provided Ldlr–/– mice; H.R., W.-U.K. and Y.C. analyzed the data; S.-H.L. provided the human plasma samples and patient information; H.R. and Y.C. wrote the manuscript; and all authors complied in submission of the manuscript for publication.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Integrated supplementary information
Supplementary Figure 1 Apoe–/– recipients of BXD2 bone marrow did not exhibit augmented autoantibody production without HFD.
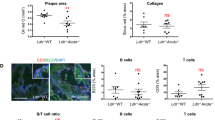
(a-b) Bone marrow chimeric mice were generated as described in Fig. 1 and were subjected to normal chow (NC). (a) ELISA of autoantibodies against dsDNA, and histone in the sera. (b) ELISA of autoreactive IgG subclass autoantibodies against dsDNA. (c) Gating strategy used for flow cytometry analysis of each cell types. (d-g) Representative flow cytometry data of germinal center B (d), plasma (e), TFH cells (f), and TFR (g) cells in the spleens of the bone marrow chimeric mice depicted in Fig. 2b,c. Data are one representative experiment of four independent experiments with n=5 per group.
Supplementary Figure 2 Germinal center reactions and TFH cell responses against exogenous antigen are augmented in atherogenic mice.
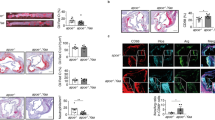
(a,b) Wild-type or Apoe–/– mice were subjected to HFD for four weeks before being immunized with chicken type II collagen in CFA. (a) Levels of collagen-specific antibodies in the sera of the indicated mice. (b) Flow cytometric analysis of germinal center B cells, plasma cells, and TFH cells in the lymph nodes of the indicated mice. Data are one representative experiment of two independent experiments with n=5 per group. (c-e) Wild-type or Apoe–/– mice were subjected to HFD for four weeks, immunized with OVA-NP and analyzed after seven days. (c) ELISA of NP-specific global affinity antibodies (NP29) in the sera of the indicated mice. (d) ELISA of NP-specific high affinity antibodies (NP7) in the sera of the indicated mice. (e) Flow cytometric analysis of germinal center B cells, plasma cells, and TFH cells in the lymph nodes of the indicated mice. Data are one representative experiment of four independent experiments with n=5 per group. *, p<0.05; **, p<0.01; ***, p<0.001 in comparison with WT (unpaired Student’s t-test, mean+SEM).
Supplementary Figure 3 CXCR3+ TFH cells are more potent in inducing IgG2c production in an IFNγ-dependent manner.
(a-c) CXCR3– or CXCR3+ TFH cells from HFD-fed Apoe–/– mice were flow-sorted and were co-cultured with naive B cells for seven days in the presence of anti-CD3 and anti-IgM. Amounts of IgG1 (a), IgG2c (b) or IgG2c/IgG1 (c) in the supernatant were determined. (d-f) CXCR3+ TFH cells were co-cultured with naive B cells as described above. Cells were treated with anti-IFNγ antibody or corresponding isotype control every other day. Amounts of IgG1 (d), IgG2c (e) or IgG2c/IgG1 (f) in the supernatant were determined. Data are representative of two independent experiments with n=4 per group. (g) Absolute numbers of TH1, TH17, and TFH cells of the indicated mice. (h) TFH cells or non-TFH (CD4+CD44+PD-1-CXCR5–) cells from HFD-fed Apoe–/– mice were flow-sorted and were co-cultured with naive B cells for seven days in the presence of anti-CD3 and anti-IgM. Amount of IgG2c in the supernatant were determined. (i) Heat map of the indicated genes that were differentially expressed subjected to RNA-seq analysis. Data are representative of two independent experiments with n=4 per group. *, p<0.05; **, p<0.01; ***, p<0.001 between the two groups (unpaired Student’s t-test, mean+SEM).
Supplementary Figure 4 Atherogenic condition promotes IL-27 production by CD11b+ dendritic cells through expression of pattern recognition receptors.
(a) Bone marrow-ablated wild-type or Apoe–/– mice were i.v. transferred with bone marrow cells from BXD2 mice, were subjected to normal chow (NC) or high fat diet (HFD). Levels of IL-27 in the sera of the indicated mice. Data are representative of two pooled independent experiments with n=5 per group. (b,c) Linear-regression analysis between serum IL-6 (b) or IL-27 (c) and TFH. Data are one representative experiment of four independent experiments with n=5 per group. (d-f) Bone marrow-ablated wild-type, Apoe–/– or Ebi3–/–Apoe–/– mice were i.v. transferred with bone marrow cells from congenic mice (WTSJL, ApoESJL, or Ebi3xApoESJL, respectively), and were subjected to high fat diet (HFD). (d) Levels of IL-27 in the sera of the indicated mice. (e,f) Flow cytometric analysis of lymphocytes (e) and dendritic cells (f). Data are one representative experiment of two independent experiments with n=4 per group. (g) Bone marrow chimeric mice were generated and treated as described in Fig. 1 (n=5). CD11c+ dendritic cells were isolated and quantitative RT-PCR analysis of the indicated genes were determined. Data are representative of two independent experiments with n=4 per group. *, p<0.05; **, p<0.01; ***, p<0.001 in comparison with WT (unpaired Student’s t-test, mean+SEM).
Supplementary Figure 5 Lipid accumulation in dendritic cells alters metabolic profile and differs in LXR expression.
(a) Bone marrow chimeric mice were generated as described in Fig. 5. The levels of NP-specific antibodies were analyzed (a). Data are one representative experiment of two independent experiments with n=4 per group. (b-f) CD11c+ dendritic cells were isolated from the indicated mice and were analyzed for ECAR profile (b), glycolysis, glycolytic capacity (c), OCR profile (d), basal respiration, ATP production, and maximal respiration (e). Energy map of dendritic cells isolated from the indicated mice (f). Data are representative of three independent experiments with two technical replicates (n=5 per group). (g) BODIPY staining of each cell types. Data are one representative experiment of three independent experiments with n=5 per group. (h-j) Splenocytes from indicated mice were enriched with CD11c (h) and flow-sorted (i). The expression of LXRβ was examined by Immunoblot analysis. Quantified values of the band intensities are presented at the bottom of each blot. Cropped blot images (h). Quantitative RT-PCR analysis of the indicated gene were determined (i). Original gel image from Supplementary Fig. 6h (j). Data are one representative experiment of three independent experiments with n=5 per group. *, p<0.05; **, p<0.01 between the two groups except in a. (a) *, p<0.05; **, p<0.01; ***, p<0.001 in comparison with ApoEWT. #, p<0.05; ##, p<0.01 in comparison with WTWT mice (unpaired Student’s t-test, mean+SEM).
Supplementary Figure 6 IL-27 plays an essential role on the antibody production.
(a) Mice were generated and treated as described in Fig. 6. Levels of total IgG, IgG1 and IgG2c in the sera of the indicated mice. (b,c) Mice were generated and treated as described in Supplementary Figure 2a,b. Levels of collagen-specific antibodies in the sera (b). Flow cytometric analysis of germinal center B cells, plasma cell and TFH cells in the lymph nodes of the indicated mice (c). Data are one representative experiment of three independent experiments with n=5 per group. (d,e) Quantitative RT-PCR analysis of selected genes from the RNA-seq (d) or TFH-related genes (e) in the sorted TFH cells. Data are one representative experiment of two independent experiments with n=4 per group. Box-and-whisker: center line, media; box limits, min to max. (f,g) Bone marrow-ablated Apoe–/– mice were i.v. transferred with bone marrow cells from mixture of congenic and Il27ra–/– mice, and were subjected to high fat diet. Flow cytometric analysis of TFH cells, each TFH subset (f) and TFR cells (g). (h-k) Apoe–/– mice were fed with HFD for four weeks, immunized with KLH and analyzed after seven days. Each group of mice were injected with control IgG or anti-IL-6 antibody intraperitoneally every other day. (h) ELISA of KLH-specific antibodies in the sera of the indicated mice. (i-k) Flow cytometric analysis of germinal center B cells, TFH cells (i), TFH subset (j), and indicated CD4+ T cell subsets (k) in the lymph nodes of the indicated mice. (l) Flow cytometric analysis of TFH cells in the lymph nodes of the mice as described in Fig. 6j. Data are one representative experiment of two independent experiments with n=5 per group. *, p<0.05; **, p<0.01; ***, p<0.001 between the two groups (unpaired Student’s t-test, mean+SEM).
Supplementary Figure 7 A graphical summary of the present study.
Our study demonstrates that atherogenic condition triggers the secretion of IL-27 and IL-6 from CD11b+ dendritic cells in a TLR4-dependent manner. IL-27 activates STAT1 and STAT3 signaling pathway in T cells and increases the numbers of CXCR3+ TFH cells while suppressing TFR cells. These CXCR3+ TFH cells, in turn, stimulate the differentiation of autoreactive B cells into IgG2c-secreting plasma cells, and pathogenic IgG2c autoantibodies exacerbate autoimmune lupus. Thus hyperlipidemia not only induces cardiovascular diseases but also promotes autoimmune lupus.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1-7 and Supplementary Tables 1-3
Rights and permissions
About this article
Cite this article
Ryu, H., Lim, H., Choi, G. et al. Atherogenic dyslipidemia promotes autoimmune follicular helper T cell responses via IL-27. Nat Immunol 19, 583–593 (2018). https://doi.org/10.1038/s41590-018-0102-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41590-018-0102-6
This article is cited by
-
The why and how of adaptive immune responses in ischemic cardiovascular disease
Nature Cardiovascular Research (2022)
-
How the immune system shapes atherosclerosis: roles of innate and adaptive immunity
Nature Reviews Immunology (2022)
-
Autoimmunity and organ damage in systemic lupus erythematosus
Nature Immunology (2020)
-
Immune metabolism regulation of the germinal center response
Experimental & Molecular Medicine (2020)
-
T cell subsets and functions in atherosclerosis
Nature Reviews Cardiology (2020)