Abstract
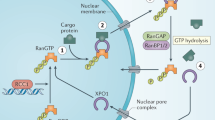
Exportin-1 (XPO1/CRM1) plays a central role in the nuclear-to-cytoplasmic transport of hundreds of proteins and contributes to other cellular processes, such as centrosome duplication. Small molecules targeting XPO1 induce cytotoxicity, and selinexor was approved by the Food and Drug Administration in 2019 as a cancer chemotherapy for relapsed multiple myeloma. Here, we describe a cell-type-dependent chromatin-binding function for XPO1 that is essential for the chromatin occupancy of NFAT transcription factors and thus the appropriate activation of T cells. Additionally, we establish a class of XPO1-targeting small molecules capable of disrupting the chromatin binding of XPO1 without perturbing nuclear export or inducing cytotoxicity. This work defines a broad transcription regulatory role for XPO1 that is essential for T cell activation as well as a new class of XPO1 modulators to enable therapeutic targeting of XPO1 beyond oncology including in T cell-driven autoimmune disorders.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All sequencing datasets generated in this study have been deposited in Gene Expression Omnibus under the SuperSeries accession code GSE228212 with SubSeries GSE228210 (RNA sequencing) and GSE228211 (CUT&RUN). Chemoproteomics data generated in this study have been deposited in the Proteomics Identification Database under the accession code PXD041193. Chemoproteomics data are supplied as part of the source data in this manuscript, and CUT&RUN peaks have been provided as supplementary data. Source data are provided with this paper.
Code availability
No code was generated for this study.
References
Nguyen, K. T., Holloway, M. P. & Altura, R. A. The CRM1 nuclear export protein in normal development and disease. Int. J. Biochem. Mol. Biol. 3, 137–151 (2012).
Camus, V., Miloudi, H., Taly, A., Sola, B. & Jardin, F. XPO1 in B cell hematological malignancies: from recurrent somatic mutations to targeted therapy. J. Hematol. Oncol. 10, 47 (2017).
Kudo, N. et al. Leptomycin B inactivates CRM1/exportin 1 by covalent modification at a cysteine residue in the central conserved region. Proc. Natl Acad. Sci. USA 96, 9112–9117 (1999).
Ferreira, B. I., Cautain, B., Grenho, I. & Link, W. Small molecule inhibitors of CRM1. Front. Pharm. 11, 625 (2020).
Chari, A. et al. Oral selinexor-dexamethasone for triple-class refractory multiple myeloma. N. Engl. J. Med. 381, 727–738 (2019).
Adachi, Y. & Yanagida, M. Higher order chromosome structure is affected by cold-sensitive mutations in a Schizosaccharomyces pombe gene crm1+ which encodes a 115-kD protein preferentially localized in the nucleus and its periphery. J. Cell Biol. 108, 1195–1207 (1989).
Wang, W., Budhu, A., Forgues, M. & Wang, X. W. Temporal and spatial control of nucleophosmin by the RAN–CRM1 complex in centrosome duplication. Nat. Cell Biol. 7, 823–830 (2005).
Knauer, S. K., Bier, C., Habtemichael, N. & Stauber, R. H. The survivin–CRM1 interaction is essential for chromosomal passenger complex localization and function. EMBO Rep. 7, 1259–1265 (2006).
Oka, M. et al. Chromatin-bound CRM1 recruits SET-Nup214 and NPM1c onto HOX clusters causing aberrant HOX expression in leukemia cells. eLife 8, e46667 (2019).
Oka, M. et al. Chromatin-prebound Crm1 recruits Nup98–HoxA9 fusion to induce aberrant expression of Hox cluster genes. eLife 5, e09540 (2016).
Sullivan, R. W. et al. 2-Chloro-4-(trifluoromethyl)pyrimidine-5-N-(3′,5′-bis(trifluoromethyl)phenyl)-carboxamide: a potent inhibitor of NF-κB- and AP-1-mediated gene expression identified using solution-phase combinatorial chemistry. J. Med. Chem. 41, 413–419 (1998).
Palanki, M. S. et al. The design and synthesis of novel orally active inhibitors of AP-1 and NF-κB mediated transcriptional activation. SAR of in vitro and in vivo studies. Bioorg. Med. Chem. Lett. 13, 4077–4080 (2003).
Ullman, K. S., Northrop, J. P., Verweij, C. L. & Crabtree, G. R. Transmission of signals from the T lymphocyte antigen receptor to the genes responsible for cell proliferation and immune function: the missing link. Annu. Rev. Immunol. 8, 421–452 (1990).
Serfling, E. et al. The role of NF-AT transcription factors in T cell activation and differentiation. Biochim. Biophys. Acta 1498, 1–18 (2000).
Chapman, N. M., Boothby, M. R. & Chi, H. Metabolic coordination of T cell quiescence and activation. Nat. Rev. Immunol. 20, 55–70 (2020).
Macian, F., Lopez-Rodriguez, C. & Rao, A. Partners in transcription: NFAT and AP-1. Oncogene 20, 2476–2489 (2001).
Zeiser, R. & Blazar, B. R. Acute graft-versus-host disease—biologic process, prevention, and therapy. N. Engl. J. Med. 377, 2167–2179 (2017).
Zhang, M. & Zhang, S. T cells in fibrosis and fibrotic diseases. Front. Immunol. 11, 1142 (2020).
Patocka, J., Nepovimova, E., Kuca, K. & Wu, W. Cyclosporine A: chemistry and toxicity—a review. Curr. Med. Chem. 28, 3925–3934 (2021).
Morikawa, M., Shorthouse, R. A., Suto, M. J., Goldman, M. E. & Morris, R. E. A novel inhibitor of nuclear factor-κB and activator protein-1 transcription factors in T cells suppresses host-versus-graft alloreactivity in vivo. Transpl. Proc. 29, 1269–1270 (1997).
Gerlag, D. M. et al. The effect of a T cell-specific NF-κB inhibitor on in vitro cytokine production and collagen-induced arthritis. J. Immunol. 165, 1652–1658 (2000).
Fujimoto, H. et al. Inhibition of nuclear factor-κB in T cells suppresses lung fibrosis. Am. J. Respir. Crit. Care Med. 176, 1251–1260 (2007).
Palanki, M. S. et al. Inhibitors of NF-κB and AP-1 gene expression: SAR studies on the pyrimidine portion of 2-chloro-4-trifluoromethylpyrimidine-5-[N-(3′,5′-bis(trifluoromethyl)phenyl)carboxamide]. J. Med. Chem. 43, 3995–4004 (2000).
Goldman, M. E. et al. SP100030 is a novel T-cell-specific transcription factor inhibitor that possesses immunosuppressive activity in vivo. Transpl. Proc. 28, 3106–3109 (1996).
Huang, T. J., Adcock, I. M. & Chung, K. F. A novel transcription factor inhibitor, SP100030, inhibits cytokine gene expression, but not airway eosinophilia or hyperresponsiveness in sensitized and allergen-exposed rat. Br. J. Pharmacol. 134, 1029–1036 (2001).
Ross, S. H. & Cantrell, D. A. Signaling and function of interleukin-2 in T lymphocytes. Annu. Rev. Immunol. 36, 411–433 (2018).
Neggers, J. E. et al. Heterozygous mutation of cysteine528 in XPO1 is sufficient for resistance to selective inhibitors of nuclear export. Oncotarget 7, 68842–68850 (2016).
Martin, J. G. et al. Chemoproteomic profiling of covalent XPO1 inhibitors to assess target engagement and selectivity. ChemBioChem 22, 2116–2123 (2021).
Niu, M., Chong, Y., Han, Y. & Liu, X. Novel reversible selective inhibitor of nuclear export shows that CRM1 is a target in colorectal cancer cells. Cancer Biol. Ther. 16, 1110–1118 (2015).
Freedy, A. M. & Liau, B. B. Discovering new biology with drug-resistance alleles. Nat. Chem. Biol. 17, 1219–1229 (2021).
Tai, Y. T. et al. CRM1 inhibition induces tumor cell cytotoxicity and impairs osteoclastogenesis in multiple myeloma: molecular mechanisms and therapeutic implications. Leukemia 28, 155–165 (2014).
Thakar, K., Karaca, S., Port, S. A., Urlaub, H. & Kehlenbach, R. H. Identification of CRM1-dependent nuclear export cargos using quantitative mass spectrometry. Mol. Cell Proteom. 12, 664–678 (2013).
Dai, J., Sultan, S., Taylor, S. S. & Higgins, J. M. The kinase haspin is required for mitotic histone H3 Thr 3 phosphorylation and normal metaphase chromosome alignment. Genes Dev. 19, 472–488 (2005).
Tokuyama, Y., Horn, H. F., Kawamura, K., Tarapore, P. & Fukasawa, K. Specific phosphorylation of nucleophosmin on Thr199 by cyclin-dependent kinase 2-cyclin E and its role in centrosome duplication. J. Biol. Chem. 276, 21529–21537 (2001).
Skene, P. J., Henikoff, J. G. & Henikoff, S. Targeted in situ genome-wide profiling with high efficiency for low cell numbers. Nat. Protoc. 13, 1006–1019 (2018).
Macian, F. NFAT proteins: key regulators of T-cell development and function. Nat. Rev. Immunol. 5, 472–484 (2005).
Schroeder, M. A. & DiPersio, J. F. Mouse models of graft-versus-host disease: advances and limitations. Dis. Model Mech. 4, 318–333 (2011).
Toubai, T. et al. Immunization with host-type CD8α+ dendritic cells reduces experimental acute GVHD in an IL-10-dependent manner. Blood 115, 724–735 (2010).
Gavriatopoulou, M. et al. Integrated safety profile of selinexor in multiple myeloma: experience from 437 patients enrolled in clinical trials. Leukemia 34, 2430–2440 (2020).
Monecke, T. et al. Structural basis for cooperativity of CRM1 export complex formation. Proc. Natl Acad. Sci. USA 110, 960–965 (2013).
Fu, S. C., Fung, H. Y. J., Cagatay, T., Baumhardt, J. & Chook, Y. M. Correlation of CRM1-NES affinity with nuclear export activity. Mol. Biol. Cell 29, 2037–2044 (2018).
Dong, X. et al. Structural basis for leucine-rich nuclear export signal recognition by CRM1. Nature 458, 1136–1141 (2009).
Crochiere, M. L. et al. A method for quantification of exportin-1 (XPO1) occupancy by selective inhibitor of nuclear export (SINE) compounds. Oncotarget 7, 1863–1877 (2016).
Zhu, J. & McKeon, F. NF-AT activation requires suppression of Crm1-dependent export by calcineurin. Nature 398, 256–260 (1999).
Mitrea, D. M., Mittasch, M., Gomes, B. F., Klein, I. A. & Murcko, M. A. Modulating biomolecular condensates: a novel approach to drug discovery. Nat. Rev. Drug Discov. 21, 841–862 (2022).
Bray, N. L., Pimentel, H., Melsted, P. & Pachter, L. Near-optimal probabilistic RNA-seq quantification. Nat. Biotechnol. 34, 525–527 (2016).
Robinson, M. D., McCarthy, D. J. & Smyth, G. K. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26, 139–140 (2010).
Barretina, J. et al. The cancer cell line encyclopedia enables predictive modelling of anticancer drug sensitivity. Nature 483, 603–607 (2012).
Savic, D. et al. CETCh-seq: CRISPR epitope tagging ChIP–seq of DNA-binding proteins. Genome Res. 25, 1581–1589 (2015).
Ramirez, F., Dundar, F., Diehl, S., Gruning, B. A. & Manke, T. deepTools: a flexible platform for exploring deep-sequencing data. Nucleic Acids Res. 42, W187–W191 (2014).
Meers, M. P., Tenenbaum, D. & Henikoff, S. Peak calling by sparse enrichment analysis for CUT&RUN chromatin profiling. Epigenetics Chromatin 12, 42 (2019).
McLean, C. Y. et al. GREAT improves functional interpretation of cis-regulatory regions. Nat. Biotechnol. 28, 495–501 (2010).
Quinlan, A. R. & Hall, I. M. BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics 26, 841–842 (2010).
Favorov, A. et al. Exploring massive, genome scale datasets with the GenometriCorr package. PLoS Comput. Biol. 8, e1002529 (2012).
Acknowledgements
This work was supported by National Institutes of Health grant AI171104 (D.J.A.), the Falk Medical Research Trust (D.J.A), the Harrington Discovery Institute (Y.F.C.) and support from the Case Western Reserve University School of Medicine and Thomas F. Peterson, Jr. Y.F.C., K.C.A. and J.L.S. were supported by the Case Western Reserve University Medical Scientist Training Program (NIH T32GM007250). Y.F.C. was also supported by NIH T32GM135081, and K.C.A. was also supported by NIH F30HD096784. Additional support was provided by the Small-Molecule Drug Development, Proteomics (S10OD028614), Genomics and Hematopoietic Biorepository and Cellular Therapy Shared Resources of the Case Comprehensive Cancer Center (P30CA043703) as well as the Genomics Shared Resource of the University of Colorado Cancer Center (P30CA046934). The graphical abstract was created with BioRender.com. We thank M. Pleshinger, P. Scacheri, B.-G. Kim, S. Chakrapani, I. Alam, A. Assaiya, M. Gallogly, F. Najm, A. Harris, P. Tesar, J. Klein, G. Trainor and B. Daugherty for technical assistance and discussion.
Author information
Authors and Affiliations
Contributions
Y.F.C., M.G., D.F.Y. and Y.F. tested and analyzed the effects of XPO1-targeting small molecules and genetic manipulations in cell-based assays. Y.F.C. performed RNA-sequencing and transcriptomic analyses. Y.F.C., J.L.S. and K.C.A. performed CUT&RUN experiments and epigenomics analyses. Y.F.C., S.L.T. and A.Y.H. obtained and analyzed primary mouse and human T cells. Y.F.C. and K.C. performed in vivo studies of XPO1 target engagement, and Y.F.C, B.A.C., F.N.P. and A.B.D. performed and analyzed all remaining in vivo experiments. R.S., R.M.F. and D.F.Y. synthesized and characterized XPO1-targeting small molecules. Y.F.C. and D.J.A. analyzed all data and wrote the manuscript. All authors provided intellectual input, edited and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
Y.F.C., R.S., D.F.Y. and D.J.A. are inventors on a provisional patent (63/627,975) filed by Case Western Reserve University on the synthesis of small molecules, their analogs and their derivatives that target XPO1 to suppress T cell activation with minimal cytotoxicity. The other authors declare no competing interests.
Peer review
Peer review information
Nature Chemical Biology thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 SP100030 and SPC-839 potently inhibit T cell activation.
a) Transcriptional activity of an NF-κB luciferase reporter construct in Jurkat T cells activated with PMA/Iono and treated with SP100030 and SPC-839 (SP100030, n = 3 independent experiments, SPC-839, n = 2 independent experiments, with at least 4 wells per concentration per experiment). b) Gene set enrichment analysis performed on transcripts suppressed by SP100030 treatment identified gene sets relating to T cell activation. c) Gene expression (TPM) of XCL2, TNF, IL3, and CSF2 following RNA sequencing of Jurkat cells treated as indicated (n = 3 biological replicates). d) IL2 expression assayed using RT-qPCR (SP100030, n = 8 wells, 2 experiments; SPC-839, n = 8 wells, 2 experiments) in Jurkat cells activated with anti-CD3/anti-CD28 and treated with SP100030 and SPC-839. e) IL2 and CSF2 expression assayed using RT-qPCR in primary mouse splenocytes activated with soluble anti-CD3/anti-CD28 and treated with SP100030 and SPC-839 (SP100030, n = 8 wells, 2 experiments; SPC-839, n = 8 wells, 2 experiments). p-values were calculated using the two-tailed Dunnett’s multiple comparisons test. f) Human primary PBMCs were activated with PMA/Iono and treated with SP100030 prior to measurement of IL2 mRNA via RT-qPCR (n = 4 wells from one experiment).
Extended Data Fig. 2 SP100030 and SPC-839 target XPO1.
a) Effects of SP-OH, a hydrolyzed analog of SP100030, on IL2 transcript levels (left, n = 4 wells) or the transcriptional activity of AP-1 and NF-κB luciferase reporter constructs (right, n = 4 wells) in Jurkat cells activated with PMA/Iono. b) Evaluation of analogs of SPC-839 that retain (CW0110) or reduce (CW0110-H2) the citraconimide electrophile. Analogs were evaluated for effects on IL2 transcript levels (n = 4 wells) in Jurkat cells activated with PMA/Iono. c, d) Transcriptional activity of AP-1 and NFAT luciferase reporter constructs in Jurkat cells activated with PMA/Iono and treated with SP-Alkyne (c, n = 2 independent experiments in each assay) and SPC-Alkyne (d, n = 4 wells in each assay). e) In-gel fluorescence detection of proteins covalently labeled following cellular treatment with Selinexor-Alkyne and SP100030 (10 μM) or SPC-839 (10 μM). Representative of 2 independent experiments. Yellow arrow, 120 kDa. f) In-gel fluorescence detection of proteins covalently labeled following cellular treatment with SP-Alkyne or SPC-Alkyne (1 μM) with and without Selinexor co-treatment (10 μM). Representative of 2 independent experiments. Yellow arrow, 120 kDa. Red asterisks indicate electrophilic site for covalent addition, and red ‘NONE’ indicates absence of electrophilic moiety.
Extended Data Fig. 3 XPO1 is the mechanistic target for suppressing T cell activation.
a) IL2 and XPO1 expression assayed using RT-qPCR following treatment with a second independent pool of XPO1-targeting siRNA (n = 2 independent experiments). p-value was calculated with the two-tailed Mann-Whitney test. b–d) IL-2 expression assayed using RT-qPCR (Selinexor and S109, n = 2 independent experiments; Leptomycin, n = 4 wells) and a luciferase reporter driven by the IL-2 promoter (Leptomycin, n = 4 wells; S109, n = 3 independent experiments; Selinexor, n = 2 independent experiments) as well as AP-1 luciferase reporter activity (n = 4 wells for each molecule) in Jurkat cells activated with PMA/Iono and treated with Selinexor (b), S109 (c) Leptomycin B (d). Red asterisks indicate electrophilic site for covalent addition. e) Transcriptional activity of an NFAT luciferase reporter construct in Jurkat T-ALL cells activated with PMA/Iono and treated with Selinexor, Leptomycin B, or S109 (n = 4 wells). f) Volcano plot highlighting differential effects observed in RNA-seq data of PMA/Iono-stimulated Jurkat cells treated with SP100030 or Selinexor. Only 4 genes were differentially expressed (log2FC > 1, p < 0.05). g, h) IL2 expression assayed using RT-qPCR in wild-type Jurkat cells (gray bars) or XPO1-C528S Jurkat cells (blue bars) activated with PMA/Iono and treated with the indicated concentrations of SP100030 (g) and SPC-839 (h).
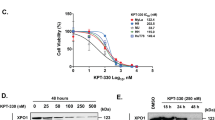
Extended Data Fig. 4 Structurally diverse XPO1 modulators drive divergent cellular activity profiles.
a) IL2 luciferase activity and cell viability following treatment with the SINE S109 in Jurkat cells (n = 3 independent experiments). b) Cell viability following treatment with the SINEs Selinexor, S109, and Leptomycin B in XPO1-C528S Jurkat cells (Selinexor, n = 3 independent experiments; SP100030, S109, Leptomycin B, n = 4 wells). c) IL2 RT-qPCR and cell viability following treatment with Selinexor or SP100030 in MOLT-4 (n = 4 wells). d, e) Cell viability following treatment with Selinexor and SP100030 for 72 h in U-2 OS (d) or 24 h in MM1.S cells (e) (n = 4 wells). f) Western blot for XPO1 following treatment for 24 h with the following XPO1-targeting small molecules: SP100030, Selinexor, S109, SPC-839, CW2158, CW1175, CW0134 (5 μM each, representative of 2 independent experiments) in Jurkat cells. g) Western blot for XPO1 following treatment with S109 (1 µM) and Selinexor (1 µM) for the indicated times in Jurkat cells (representative of 2 independent experiments). h) Subcellular localization using immunofluorescence detection of IκBα following treatment of U-2 OS cells for 6 h with SINEs and SITAs (n = 3 independent experiments per compound per concentration). i) Subcellular localization using immunofluorescence detection of RANBP1 following treatment of HeLa cells for 6 h with SINEs and SITAs (n = 2 independent experiments per compound per concentration). h and i are reported as the % nuclear export inhibition (DMSO = 0%, 3 nM Leptomycin B = 100%). j) Representative images of immunofluorescence staining for RANBP1 in HeLa cells, associated with panel i. Scale bar, 50 μm.
Extended Data Fig. 5 XPO1 associates with open chromatin.
a) Western blot of phospho-ERK and phospho-p38 after 6 h treatment with 1 μM of the indicated inhibitor (representative of 2 independent experiments). b) Electrophoretic mobility shift assay of Jurkat nuclear lysates binding to a fluorescent-tagged oligonucleotide of the consensus AP-1 binding sequence. Western blot of FOS provided as a loading normalization control (n = 1). c) Genome browser view of XPO1 in Jurkat cells (CUT&RUN) indicating overlap with chromatin marks H3K27Ac, H3K4Me3, CTCF (GSE68976), H3K9Me3 (GSE162605), and H3K27Me3 (GSE23080) (XPO1, n = 3; H3K27Ac, n = 2; H3K4Me3, n = 2; CTCF, H3K9Me3, HK27Me3, n = 1). CUT&RUN for FLAG in XPO1-FLAG Jurkat cells is also shown (n = 1). d) Gene ontology analysis of the most highly enriched biological processes for all XPO1 peaks identified in Jurkat cells and peaks identified uniquely in Jurkat cells. See Supplementary Data 1 full a full list of peaks.
Extended Data Fig. 6 XPO1 chromatin occupancy is associated with elevated transcriptional activity.
a) Genome browser view of XPO1 at the HOXB9 locus in Jurkat, CD3+ T cells, and Loucy cells (Jurkat, n = 3; CD3+ T cells, n = 2; Loucy, n = 1). b) Genome browser view at select cell-type specific loci in Jurkat (CD28), THP-1 (TLR2), U-2 OS (MYL2), and at a shared locus (eIF4E) profiled using CUT&RUN (HeLa, n = 1; THP-1, n = 1; U-2 OS, n = 1). c, d) RNA-Seq expression (obtained from the Cancer Cell Line Encyclopedia) of genes in THP-1 and U-2 OS stratified based on whether an XPO1 peak was present at each gene. p-value was calculated using the two-tailed Kruskal-Wallis multiple comparisons test. e) Cumulative density function of the correlation between XPO1 peaks with H3K4Me3, CTCF, or H3K27Me3 peaks (black line) compared to the expected density function (blue line). f) Venn diagram of overlap between XPO1 and H3K27Ac peaks in Jurkat and CD3+ T cells. See Supplementary Data 1 full a full list of peaks.
Extended Data Fig. 7 XPO1 chromatin occupancy overlaps extensively with transcription factors that drive T cell activation.
a–c) Genome browser view of the chromatin localization of XPO1, FOS, and NFAT1 at the IL2 locus in Jurkat cells (representative tracks from XPO1, n = 3; FOS, n = 2; NFAT1 + SP100030, n = 2; NFAT1 + Selinexor, n = 1), in CD3+ primary T cells (XPO1, n = 2; NFAT1, n = 2), and in Jurkat cells with homozygous expression of XPO1-C528S (XPO1, n = 1; NFAT1, n = 1). SP100030 and Selinexor were used at 1 µM. d) Global profiles of FOS, JUN, ATF2, RelA, NFAT2, and NFAT4 with and without 1 µM SP100030 treatment (n = 1 per transcription factor; FOS, n = 2). See Supplementary Data 1 full a full list of peaks.
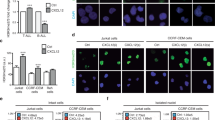
Extended Data Fig. 8 Targeting XPO1 disrupts NFAT chromatin occupancy to suppress T cell activation.
a) Gene ontology analysis of the most highly enriched biological processes associated with XPO1 peaks depleted by SP100030 treatment in CD3+ primary T cells. b) Genome-wide differential binding of NFAT1 peaks between cells activated with PMA/Iono and those that were also treated with SP100030. c) Venn diagram overlap of genes that were upregulated by SP100030 or Selinexor on RNA-Seq (log2FC > 1; p-adj. < 0.05) with NFAT1-responsive genes that were suppressed by PMA/Iono on RNA-Seq (log2FC < -1; p-adj. < 0.05). Enrichment was calculated as the percentage of genes upregulated by SP100030 or Selinexor that were NFAT1-responsive divided by the percentage of NFAT1-responsive genes across the genome. d) Expression (TPM) of all NFAT1-responsive genes from panel d that were suppressed by PMA/Iono treatment (log2FC < -1) under activated conditions, SP100030 treatment, or Selinexor treatment. p-value was calculated using two-tailed Dunnett’s multiple comparisons test.
Extended Data Fig. 9 In vivo targeting of XPO1 suppresses lung inflammation.
a) RT-qPCR of cytokines in the lungs associated with bleomycin-induced pulmonary fibrosis from two independent experiments (Vehicle, n = 11; 10 mg/kg SP100030, n = 11; 30 mg/kg SP100030, n = 6). False discovery rate was calculated using the two-stage step-up method following multiple two-tailed Mann-Whitney tests. b) Labeling of XPO1 in the lungs by alkynyl probes following five daily IP injections (n = 2 mice per condition across two independent experiments). c) In-gel fluorescence of spleen and lung samples for Selinexor-treated mice followed by ex vivo treatment of cellular suspensions with respective alkynyl probes (representative of 2 independent experiments).
Extended Data Fig. 10 SP100030 suppresses GvHD with minimal toxicity.
a-c) Quantification of CD4+ and CD8+ T cells in the bone marrow (a), spleen (b) (Naïve, n = 10; Vehicle, n = 10; SP100030, n = 11 across two independent experiments), and peripheral blood (c) (Naïve, n = 4; Vehicle, n = 5; SP100030, n = 4 from one experiment). d) Complete blood count measurements of total neutrophils and platelets in healthy C57BL/6 mice treated with vehicle, Selinexor, or SP100030 (n = 5 per group from one experiment). e) Serum chemistry measurement of alkaline phosphatase in healthy C57BL/6 mice treated with vehicle, Selinexor, or SP100030 (n = 5 per group from one experiment). All p-values were calculated using the two-tailed Mann-Whitney test.
Supplementary information
Supplementary Information
Chemical characterization data, Supplementary Table 1, Figs. 1–6 and source data for Supplementary Table 1 and Supplementary Figs. 1–6.
Supplementary Data 1
Statistical source data for Supplementary Figs. 1–6 and CUT&RUN peaks used in this study.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Uncropped western blots and gels.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Uncropped western blots and gels.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Uncropped western blots and gels.
Source Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 2
Uncropped western blots and gels.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 4
Uncropped western blots and gels.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 5
Uncropped western blots and gels.
Source Data Extended Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 8
Statistical source data.
Source Data Extended Data Fig. 9
Uncropped western blots and gels.
Source Data Extended Data Fig. 9
Statistical source data.
Source Data Extended Data Fig. 10
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, Y.F., Ghazala, M., Friedrich, R.M. et al. Targeting the chromatin binding of exportin-1 disrupts NFAT and T cell activation. Nat Chem Biol (2024). https://doi.org/10.1038/s41589-024-01586-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41589-024-01586-5