Abstract
Mucin-type O-glycosylation is a post-translational modification present at the interface between cells where it has important roles in cellular communication. However, deciphering the function of O-glycoproteins and O-glycans can be challenging, especially as few enzymes are available for their assembly or selective degradation. Here, to address this deficiency, we developed a genetically encoded screening methodology for the discovery and engineering of the diverse classes of enzymes that act on O-glycoproteins. The method uses Escherichia coli that have been engineered to produce an O-glycosylated fluorescence resonance energy transfer probe that can be used to screen for O-glycopeptidase activity. Subsequent cleavage of the substrate by O-glycopeptidases provides a read-out of the glycosylation state of the probe, allowing the method to also be used to assay glycosidases and glycosyltransferases. We further show the potential of this methodology in the first ultrahigh-throughput-directed evolution of an O-glycopeptidase.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The authors declare that the data necessary to support the findings of this study are available within the main text, extended data and supplementary information. Source data for the information presented in the main text and extended data are available. Sequence data for enzymes used in this study are available online: IMPa (UniProt accession code Q9I5W4), ZmpB (Q0TR08), BT4244 (Q89ZX7), NedA (Q02834), EngCP (BAB80399.1) and BpGH31 (Genbank: UJQ44068.1). Sequence data for probes are provided in Supplementary Table 5. Source data are provided with this paper.
References
Schjoldager, K. T., Narimatsu, Y., Joshi, H. J. & Clausen, H. Global view of human protein glycosylation pathways and functions. Nat. Rev. Mol. Cell Biol. 21, 729–749 (2020).
Brockhausen, I., Wandall, H. H., Ten Hagen, K. G. & Stanley, P. Essentials of Glycobiology (Cold Spring Harbor Laboratory Press, 2022).
Xiao, H., Woods, E. C., Vukojicic, P. & Bertozzi, C. R. Precision glycocalyx editing as a strategy for cancer immunotherapy. Proc. Natl Acad. Sci. USA 113, 10304–10309 (2016).
Woods, E. C. et al. A bulky glycocalyx fosters metastasis formation by promoting g1 cell cycle progression. eLife 6, e25752 (2017).
Wang, L. X. & Davis, B. G. Realizing the promise of chemical glycobiology. Chem. Sci. 4, 3381 (2013).
Wardman, J. F., Bains, R. K., Rahfeld, P. & Withers, S. G. Carbohydrate-active enzymes (CAZymes) in the gut microbiome. Nat. Rev. Microbiol. 20, 542–556 (2022).
Malaker, S. A. et al. Revealing the human mucinome. Nat. Commun. 13, 3542 (2022).
Malaker, S. A. et al. The mucin-selective protease StcE enables molecular and functional analysis of human cancer-associated mucins. Proc. Natl Acad. Sci. USA 116, 7278–7287 (2019).
Shon, D. J. et al. An enzymatic toolkit for selective proteolysis, detection, and visualization of mucin-domain glycoproteins. Proc. Natl Acad. Sci. USA 117, 21299–21307 (2020).
Vainauskas, S. et al. A broad-specificity O-glycoprotease that enables improved analysis of glycoproteins and glycopeptides containing intact complex O-glycans. Anal. Chem. 94, 1060–1069 (2022).
Rodems, S. M. et al. A FRET-based assay platform for ultra-high density drug screening of protein kinases and phosphatases. Assay. Drug Dev. Technol. 1, 9–19 (2002).
Gross, B. J., Swoboda, J. G. & Walker, S. A strategy to discover inhibitors of O-linked glycosylation. J. Am. Chem. Soc. 130, 440–441 (2008).
Rahfeld, P. et al. Prospecting for microbial α-N-acetylgalactosaminidases yields a new class of GH31 O-glycanase. J. Biol. Chem. 294, 16400–16415 (2019).
Wardman, J. F. et al. Discovery and development of promiscuous O-glycan hydrolases for removal of intact sialyl T-antigen. ACS Chem. Biol. 16, 2004–2015 (2021).
Aharoni, A. et al. High-throughput screening methodology for the directed evolution of glycosyltransferases. Nat. Methods 3, 609–614 (2006).
Du, T. et al. A bacterial expression platform for production of therapeutic proteins containing human-like O-linked glycans. Cell Chem. Biol. 26, 203–212 (2019).
Sim, L., Thompson, N., Geissner, A., Withers, S. G. & Wakarchuk, W. W. Mammalian sialyltransferases allow efficient Escherichia coli-based production of mucin-type O-glycoproteins but can also transfer Kdo. Glycobiology 32, 429–440 (2022).
Kimura, R. H., Steenblock, E. R. & Camarero, J. A. Development of a cell-based fluorescence resonance energy transfer reporter for Bacillus anthracis lethal factor protease. Anal. Biochem. 369, 60–70 (2007).
Guerrero, J. L., O’Malley, M. A. & Daugherty, P. S. Intracellular FRET-based screen for redesigning the specificity of secreted proteases. ACS Chem. Biol. 11, 961–970 (2016).
Bajar, B. T. et al. Improving brightness and photostability of green and red fluorescent proteins for live cell imaging and FRET reporting. Sci. Rep. 6, 20889 (2016).
Bardoel, B. W. et al. Identification of an immunomodulating metalloprotease of Pseudomonas aeruginosa (IMPa). Cell. Microbiol. 14, 902–913 (2012).
Noach, I. et al. Recognition of protein-linked glycans as a determinant of peptidase activity. Proc. Natl Acad. Sci. USA 114, E679–E688 (2017).
Mohl, J. E., Gerken, T. A. & Leung, M. Y. ISOGlyP: de novo prediction of isoform-specific mucin-type O-glycosylation. Glycobiology 31, 168–172 (2021).
Riley, N. M. & Bertozzi, C. R. Deciphering O-glycoprotease substrate preferences with O-Pair Search. Mol. Omics 18, 908–922 (2022).
Haurat, M. F. et al. The glycoprotease CpaA secreted by medically relevant Acinetobacter species targets multiple O-linked host glycoproteins. mBio 11, e02033-20 (2020).
Pluvinage, B. et al. Architecturally complex O-glycopeptidases are customized for mucin recognition and hydrolysis. Proc. Natl Acad. Sci. USA 118, e2019220118 (2021).
Brown, A. S., Ackerley, D. F. & Calcott, M. J. High-throughput screening for inhibitors of the SARS-CoV-2 protease using a FRET-biosensor. Molecules 25, 4666 (2020).
Thomas, D. A. et al. A broad-spectrum fluorescence-based peptide library for the rapid identification of protease substrates. Proteomics 6, 2112–2120 (2006).
Cummings, R. T. et al. A peptide-based fluorescence resonance energy transfer assay for Bacillus anthracis lethal factor protease. Proc. Natl Acad. Sci. USA 99, 6603–6606 (2002).
Zhang, J.-H., Chung, T. D. Y. & Oldenburg, K. R. A simple statistical parameter for use in evaluation and validation of high throughput screening assays. J. Biomol. Screen. 4, 67–73 (1999).
Shon, D. J., Kuo, A., Ferracane, M. J. & Malaker, S. A. Classification, structural biology, and applications of mucin domain-targeting proteases. Biochem. J. 478, 1585–1603 (2021).
Wisnovsky, S. et al. Genome-wide CRISPR screens reveal a specific ligand for the glycan-binding immune checkpoint receptor Siglec-7. Proc. Natl Acad. Sci. USA 118, e2015024118 (2021).
Luo, Q. et al. Enterotoxigenic Escherichia coli secretes a highly conserved mucin-degrading metalloprotease to effectively engage intestinal epithelial cells. Infect. Immun. 82, 509–521 (2014).
Hews, C. L. et al. The StcE metalloprotease of enterohaemorrhagic Escherichia coli reduces the inner mucus layer and promotes adherence to human colonic epithelium ex vivo. Cell. Microbiol. 19, e12717 (2017).
Bachert, C. & Linstedt, A. D. A sensor of protein O-glycosylation based on sequential processing in the Golgi apparatus. Traffic 14, 47–56 (2013).
Natarajan, A. et al. Engineering orthogonal human O-linked glycoprotein biosynthesis in bacteria. Nat. Chem. Biol. 16, 1062–1070 (2020).
Shon, D. J., Fernandez, D., Riley, N. M., Ferracane, M. J. & Bertozzi, C. R. Structure-guided mutagenesis of a mucin-selective metalloprotease from Akkermansia muciniphila alters substrate preferences. J. Biol. Chem. 298, 101917 (2022).
Nason, R. et al. Display of the human mucinome with defined O-glycans by gene engineered cells. Nat. Commun. 12, 4070 (2021).
Sanchez, M. I. & Ting, A. Y. Directed evolution improves the catalytic efficiency of TEV protease. Nat. Methods 17, 167–174 (2020).
Denard, C. A. et al. YESS 2.0, a tunable platform for enzyme evolution, yields highly active TEV protease variants. ACS Synth. Biol. 10, 63–71 (2021).
Holstein, J. M., Gylstorff, C. & Hollfelder, F. Cell-free directed evolution of a protease in microdroplets at ultrahigh throughput. ACS Synth. Biol. 10, 252–257 (2021).
Dyer, R. P. & Weiss, G. A. Making the cut with protease engineering. Cell Chem. Biol. 29, 177–190 (2022).
Pedram, K. et al. Design of a mucin-selective protease for targeted degradation of cancer-associated mucins. Preprint at bioRxiv https://doi.org/10.1101/2022.05.20.492748 (2022).
Cioce, A. et al. Optimization of metabolic oligosaccharide engineering with Ac4GalNAlk and Ac4GlcNAlk by an engineered pyrophosphorylase. ACS Chem. Biol. 16, 1961–1967 (2021).
Choi, J. et al. Engineering orthogonal polypeptide GalNAc-transferase and UDP-sugar pairs. J. Am. Chem. Soc. 141, 13442–13453 (2019).
Mayer, C. et al. Directed evolution of new glycosynthases from Agrobacterium β-glucosidase: a general screen to detect enzymes for oligosaccharide synthesis. Chem. Biol. 8, 437–443 (2001).
Doores, K. J. & Davis, B. G. ‘Polar patch’ proteases as glycopeptiligases. Chem. Comm. 1, 168–170 (2005).
Berman, H. M. et al. The protein data bank. Nucleic Acids Res. 28, 235–242 (2000).
Studier, W. F. Protein production by auto-induction in high-density shaking cultures. Protein Expr. Purif. 41, 207–234 (2005).
Vocadlo, D. J., Wicki, J., Rupitz, K. & Withers, S. G. Mechanism of Thermoanaerobacterium saccharolyticum β-xylosidase: kinetic studies. Biochemistry 41, 9727–9735 (2002).
Marty, M. T. et al. Bayesian deconvolution of mass and ion mobility spectra: from binary interactions to polydisperse ensembles. Anal. Chem. 87, 4370–4376 (2015).
Engler, C., Kandzia, R. & Marillonnet, S. A one pot, one step, precision cloning method with high throughput capability. PLoS ONE 3, e3647 (2008).
Engler, C., Gruetzner, R., Kandzia, R. & Marillonnet, S. Golden gate shuffling: a one-pot DNA shuffling method based on type IIs restriction enzymes. PLoS ONE 4, e5553 (2009).
Acknowledgements
This work was supported by the Frederick Banting and Charles Best doctoral research award from the Canadian Institutes of Health Research (CIHR) (funding reference 165747 to J.F.W. and grant 148458 to S.G.W.), the Natural Sciences and Engineering Research Council (NSERC) of Canada (grant 05131 to S.G.W.), and the Canada Foundation for Innovation (to S.G.W.). The authors would like to acknowledge the help and experience of A. Johnson and J. Wong at the UBC Flow Cytometry Facility, and J. Rogalski from the UBC MS core facility. We also thank E. Jan and M. Roberge (University of British Columbia) for their gift of a plasmid containing mNeonGreen and mRuby3, and H. Brumer (University of British Columbia) for use of his ESI–TOF MS. The graphical abstract, Extended Data Fig. 2, Extended Data Fig. 9 and portions of other figures were created with BioRender.com.
Author information
Authors and Affiliations
Contributions
J.F.W. conceived the methodology, developed it with S.G.W. and carried out the majority of the work and analysis. L.S. assisted in work on the OGOs and carried out the mass spectrometry with A.G. J.L., T.A.H. and P.M.D. carried out and assisted in some of the cloning and probe characterization. W.W.W. and S.G.W. supervised the research and assisted in analysis. A.B.B. assisted in the analysis. The paper was written by J.F.W. and S.G.W. with input from all authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemical Biology thanks Sabine Flitsch and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
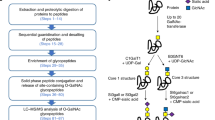
Extended Data Fig. 2 Workflows for probe production, determination of O-glycopeptidase activity, and assaying for GH activity.
a, Origami2(DE3) cells (or the OG2neu+ strain) are transformed with the probe plasmid and then the OGO of choice. Protein expression is carried out by IPTG induction. The probe can then be obtained in sufficient purity via Ni-NTA chromatography, with subsequent completion of chromophore maturation being carried out by incubation at 37 °C as needed. b, Kinetic characterization of O-glycopeptidases using purified probe. Briefly, v0 values are determined for a set of different substrate concentrations. A corresponding standard curve with the expected changes in FRET then enables unit conversion to M/s or mg/mL/s. c, Screening for GH activity in plates using MELiORA. E. coli carrying a plasmid encoding the GH of interest are grown and induced in supplemented M9 media with IPTG. They are then lysed in the presence of the probe and incubated to allow for the GHs to act on the probe. Following this, the glycosylation status of the probe is determined by addition of an O-glycopeptidase with subsequent read-out of the FRET in a plate reader.
Extended Data Fig. 3 Metal dependence of IMPa for activity against 1minOGO-1.
a, IMPa was assayed for activity against 1minOGO-1 across a range of concentrations of ZnCl2 in 100 mM MES pH 6.5. kcat/KM was determined via the substrate depletion method as previously described6 and/or using the initial reaction velocity as indicated. Data points indicate mean of three technical replicates. Error bars represent the standard error of the mean. b, Metal-dependence of IMPa was further investigated by assaying activity of IMPa where metals or EDTA were added to a final concentration of 100 μM. Values shown are technical replicates relative to the activity in the presence of 100 μM ZnCl2.
Extended Data Fig. 4 Cleavage of different glycoforms of probe 1 by BT4244.
Probe 1 was expressed with different OGOs in order to produce a variety of different glycoforms. BT4244 was then assayed for activity against these different glycoforms. This was observed through (a) the change in FRET over time and (b) SDS-PAGE followed by Coomassie staining. Data points shown in panel a correspond to four technical replicates for +BT4244 samples and three technical replicates for Buffer Control samples. For panel b, the label ‘m.’ indicates probe co-expressed with minOGO-1.
Extended Data Fig. 5 Metal dependence of BT4244 for activity against 1minOGO-1.
a, BT4244 was assayed for activity against 1minOGO-1 across a range of concentrations of ZnCl2 in 100 mM MES pH 6.5. kcat/KM was determined from the initial reaction velocity. Data points indicate the average of three technical replicates. Error bars represent standard error of the mean. b, Metal-dependence of BT4244 was further investigated by assaying activity of BT4244 where metals or EDTA were added to a final concentration of 100 μM. Values shown are technical replicates relative to the activity in the presence of 100 μM ZnCl2.
Extended Data Fig. 6 Cleavage of different glycoforms of probe 1 by ZmpB.
Probe 1 was expressed with different OGOs in order to produce a variety of different glycoforms. ZmpB was then assayed for activity against these different glycoforms. This was observed through (a) the change in FRET over time and (b) SDS-PAGE followed by in-gel fluorescence. Data points shown in panel a correspond to four technical replicates for +ZmpB samples and three technical replicates for Buffer control samples. Panel c shows the same gel as in panel b but with an extended exposure in order to make the formation of weaker bands more evident. For panels b and c, the label ‘m.’ indicates probe co-expressed with minOGO-1.
Extended Data Fig. 7 Metal dependence of ZmpB for activity against 1OGO-9.
a, ZmpB was assayed for activity against 1minOGO-1 across a range of concentrations of ZnCl2 in 100 mM MES pH 6.5. Data points indicate mean of three (for 1μΜ and 25 nM data points) or six technical replicates (for all other data points). Error bars indicate standard error of the mean. b, Metal-dependence of ZmpB was further investigated by assaying activity of ZmpB where metals or EDTA were added to a final concentration of 25 μM. Values shown are technical replicates relative to the activity of ZmpB in the presence of 25 μM ZnCl2.
Extended Data Fig. 8 Model sort to show efficacy of FACS-based enrichment for clones with active O-glycopeptidase and O-glycosylation machinery.
Cells expressing the probe, BT4244 and minOGO-1 were diluted 1:100 fold in cells expressing the probe, BT4244, and the equivalent empty vector to minOGO-1 (such that no glycosylation occurs). a, These were then sorted by FACS. Shown is the data for the separate populations (that is, without dilution of one set of cells into the other) as well as the gate used for sorting. Label indicates % of cells within the sorting gate. b, Ten recovered clones were then tested for functional glycosylation and O-glycopeptidase activity by individual culturing, expression, lysis, and then analysis by SDS-PAGE followed by detection using in gel fluorescence.
Extended Data Fig. 9 Workflow for screening for O-glycopeptidase activity using MELiORA by FACS and then in 96-well plates.
a, Origami2(DE3) cells (or the OG2neu+ strain) are transformed with the plasmid library encoding variants of the O-glycopeptidase of interest. They are then made electrocompetent and transformed with a plasmid encoding the OGO and probe. The plates are then scraped, diluted into media, and induced with IPTG overnight. A small volume of the culture (typically <100 μL) is then washed and sorted for low FRET via FACS. This process can then be repeated as needed. Once cells of interest are of sufficient purity, one can proceed into plate-based screening. To prevent carryover of the OGO plasmid it will likely be necessary to create a new library using DNA obtained from the final sort. This new library can then be transformed into the cell line of choice. b, In a 96-well plate, expression of the O-glycopeptidase can be induced by growth in Supplemented M9 + IPTG. The cells are then lysed thoroughly. To initiate the reaction, the glycosylated probe is added and the FRET is read-out at various time points in order to identify improved clones.
Extended Data Fig. 10 Cleavage of probe sequon variants co-expressed with OGO-6 by ZmpB mutants.
ZmpB mutants were incubated with 2OGO-6 and analyzed at (a) 1.5 hours and (b) 8 hours via SDS-PAGE and in-gel fluorescence, or 4OGO-6 before analysis again at (c) 1.5 hours and (d) 8 hours. Panels e and f show the reaction time course as monitored by densitometry for M6 (white data points) against (e) 2OGO-6 and (f) 4OGO-6. Data from the WT (black data points) is also included to serve as a reference. Note that for these reactions a high concentration of probe (2.5 mg/mL) and ZmpB (0.5 mg/mL) was used in order to better enable visualization of the cleavage products. Gels shown have been overexposed to better show detail and were not used for densitometry calculation. Data points shown are technical replicates.
Supplementary information
Supplementary Information
Supplementary Tables 1–5, Figs. 1–16 and References.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 1
Uncropped Coomassie-stained gel.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 2
Uncropped SDS–PAGE visualized by in-gel fluorescence.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 3
Uncropped SDS–PAGE visualized by in-gel fluorescence.
Source Data Table 1
Statistical source data.
Source Data Table 2
Statistical source data.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 4
Uncropped Coomassie-stained gel.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 6
Uncropped SDS–PAGE visualized by in-gel fluorescence.
Source Data Extended Data Fig. 7
Statistical source data.
Source Data Extended Data Fig. 8
Uncropped SDS–PAGE visualized by in-gel fluorescence.
Source Data Extended Data Fig. 10
Uncropped SDS–PAGE visualized by in-gel fluorescence.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wardman, J.F., Sim, L., Liu, J. et al. A high-throughput screening platform for enzymes active on mucin-type O-glycoproteins. Nat Chem Biol 19, 1246–1255 (2023). https://doi.org/10.1038/s41589-023-01405-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41589-023-01405-3