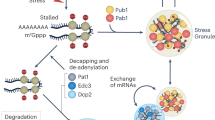
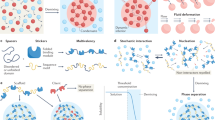
Abstract
Cells are exquisitely compartmentalized to achieve precise spatiotemporal regulation of myriad processes and pathways. Phase separation offers one way to achieve territorial organization in the cellular context, via the creation of membrane-less organelles (MLOs). MLOs formed through phase separation are associated with numerous critical biological functions. Although hundreds of publications on related topics are produced each year, robust criteria for the determination of biologically meaningful phase separation are yet to be well established. Here we present some principles and propose a few guidelines for phase-separation studies in biology. Specifically, we provide an in-depth experiment pipeline for phase-separation studies, including mechanisms of the molecular driving forces, ways to correlate in vivo and in vitro observations, and strategies to relate the phase-separation phenomenon to biological functions. We also intend to contribute to streamlining the aforementioned diagnostic criteria by further stressing a few common caveats in the field.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Banani, S. F., Lee, H. O., Hyman, A. A. & Rosen, M. K. Biomolecular condensates: organizers of cellular biochemistry. Nat. Rev. Mol. Cell Biol. 18, 285–298 (2017). This is a comprehensive review of phase-separated cellular membrane-less organelles.
Brangwynne, C. P. et al. Germline P granules are liquid droplets that localize by controlled dissolution/condensation. Science 324, 1729–1732 (2009). This paper describes that P-granules exhibit liquid-like behaviors.
Kato, M. et al. Cell-free formation of RNA granules: low complexity sequence domains form dynamic fibers within hydrogels. Cell 149, 753–767 (2012). This paper reconstituted RNA granules in vitro and revealed the role of LCDs in driving liquid-to-solid phase separation.
Bose, M., Lampe, M., Mahamid, J. & Ephrussi, A. Liquid-to-solid phase transition of oskar ribonucleoprotein granules is essential for their function in Drosophila embryonic development. Cell 185, 1308–1324 (2022).
Liu, Q. et al. Glycogen accumulation and phase separation drives liver tumor initiation. Cell 184, 5559–5576 (2021).
Fong, K.-w et al. Whole-genome screening identifies proteins localized to distinct nuclear bodies. J. Cell Biol. 203, 149–164 (2013).
Ditlev, J. A., Case, L. B. & Rosen, M. K. Who’s in and who’s out—compositional control of biomolecular condensates. J. Mol. Biol. 430, 4666–4684 (2018).
Acuna, C., Liu, X. & Südhof, T. C. How to make an active zone: unexpected universal functional redundancy between RIMs and RIM-BPs. Neuron 91, 792–807 (2016).
Wu, X. et al. RIM and RIM-BP form presynaptic active-zone-like condensates via phase separation. Mol. Cell 73, 971–984 (2019).
Banani, S. F. et al. Compositional control of phase-separated cellular bodies. Cell 166, 651–663 (2016).
Letunic, I., Khedkar, S. & Bork, P. SMART: recent updates, new developments and status in 2020. Nucleic Acids Res. 49, D458–D460 (2021).
Rao, J., Lahiri, J., Isaacs, L., Weis Robert, M. & Whitesides George, M. A trivalent system from vancomycin·D-Ala-D-Ala with higher affinity than avidin·biotin. Science 280, 708–711 (1998).
Li, P. et al. Phase transitions in the assembly of multivalent signalling proteins. Nature 483, 336–340 (2012). This is the leading paper that reconstituted liquid–liquid phase separation in vitro and demonstrated that LLPS is driven by multivalency.
Flory, P. J. Principles of Polymer Chemistry (Cornell Univ. Press, 1953).
Cohen, R. J. & Benedek, G. B. Equilibrium and kinetic theory of polymerization and the sol-gel transition. J. Phys. Chem. 86, 3696–3714 (1982).
Lehn, J.-M. Supramolecular polymer chemistry—scope and perspectives. Polym. Int. 51, 825–839 (2002).
Lin, Y. et al. Formation and maturation of phase-separated liquid droplets by RNA-binding proteins. Mol. Cell 60, 208–219 (2015).
Zeng, M. et al. Phase transition in postsynaptic densities underlies formation of synaptic complexes and synaptic plasticity. Cell 166, 1163–1175 (2016). This paper reported a model that phase separation from the multivalency of SynGAP/PSD-95 drives the formation of post-synaptic densities.
Sun, D., Wu, R., Zheng, J., Li, P. & Yu, L. Polyubiquitin chain-induced p62 phase separation drives autophagic cargo segregation. Cell Res. 28, 405–415 (2018).
Bouchard, J. J. et al. Cancer mutations of the tumor suppressor SPOP disrupt the formation of active, phase-separated compartments. Mol. Cell 72, 19–36 (2018).
Shen, B. et al. Computational screening of phase-separating proteins. Genomics Proteom. Bioinformatics 19, 13–24 (2021).
Cascarina, S. M., Elder, M. R. & Ross, E. D. Atypical structural tendencies among low-complexity domains in the Protein Data Bank proteome. PLoS Comput. Biol. 16, e1007487 (2020).
Frey, S., Richter Ralf, P. & Görlich, D. FG-rich repeats of nuclear pore proteins form a three-dimensional meshwork with hydrogel-like properties. Science 314, 815–817 (2006). This paper describes that LCD in nucleoporin forms reversible hydrogel, which is required for yeast viability.
Kato, M. & McKnight, S. L. A solid-state conceptualization of information transfer from gene to message to protein. Annu. Rev. Biochem. 87, 351–390 (2018).
Kwon, I. et al. Phosphorylation-regulated binding of RNA polymerase II to fibrous polymers of low-complexity domains. Cell 155, 1049–1060 (2013).
Yamazaki, H., Takagi, M., Kosako, H., Hirano, T. & Yoshimura, S. H. Cell cycle-specific phase separation regulated by protein charge blockiness. Nat. Cell Biol. 24, 625–632 (2022).
Wang, J. et al. Phase separation of OCT4 controls TAD reorganization to promote cell fate transitions. Cell Stem Cell 28, 1868–1883.e1811 (2021).
Mier, P. et al. Disentangling the complexity of low complexity proteins. Brief. Bioinformatics 21, 458–472 (2020).
Xiang, S. et al. The LC Domain of hnRNPA2 adopts similar conformations in hydrogel polymers, liquid-like droplets, and nuclei. Cell 163, 829–839 (2015).
Murray, D. T. et al. Structure of FUS protein fibrils and its relevance to self-assembly and phase separation of low-complexity domains. Cell 171, 615–627 (2017).
Cao, Q., Boyer, D. R., Sawaya, M. R., Ge, P. & Eisenberg, D. S. Cryo-EM structures of four polymorphic TDP-43 amyloid cores. Nat. Struct. Mol. Biol. 26, 619–627 (2019).
Zhou, X. et al. Mutations linked to neurological disease enhance self-association of low-complexity protein sequences. Science 377, eabn5582 (2022).
Lin, Y. et al. Redox-mediated regulation of an evolutionarily conserved cross-β structure formed by the TDP43 low complexity domain. Proc. Natl Acad. Sci. USA 117, 28727–28734 (2020).
Dreyfuss, G., Matunis, M. J., Pinol-Roma, S. & Burd, C. G. hnRNP proteins and the biogenesis of mRNA. Annu. Rev. Biochem. 62, 289–321 (1993).
Martin, E. W. et al. Interplay of folded domains and the disordered low-complexity domain in mediating hnRNPA1 phase separation. Nucleic Acids Res. 49, 2931–2945 (2021). This paper discusses how the interplay between low-complexity domains and folded domains drives phase separation.
Zeng, M. et al. Reconstituted postsynaptic density as a molecular platform for understanding synapse formation and plasticity. Cell 174, 1172–1187 (2018).
Milovanovic, D., Wu, Y., Bian, X. & Camilli, P. D. A liquid phase of synapsin and lipid vesicles. Science 361, 604–607 (2018).
Wootton, J. C. Non-globular domains in protein sequences: automated segmentation using complexity measures. Comput. Chem. 18, 269–285 (1994).
Hughes, M. P. et al. Atomic structures of low-complexity protein segments reveal kinked β sheets that assemble networks. Science 359, 698–701 (2018).
Chen, Z. et al. Screening membraneless organelle participants with machine-learning models that integrate multimodal features. Proc. Natl Acad. Sci. USA 119, e2115369119 (2022).
Li, Q. et al. Protein databases related to liquid-liquid phase separation. Int. J. Mol. Sci. 21, 6796 (2020).
Pancsa, R., Vranken, W. & Mészáros, B. Computational resources for identifying and describing proteins driving liquid-liquid phase separation. Brief. Bioinformatics https://doi.org/10.1093/bib/bbaa408 (2021).
Fritsch Anatol, W. et al. Local thermodynamics govern formation and dissolution of Caenorhabditis elegans P granule condensates. Proc. Natl Acad. Sci. USA 118, e2102772118 (2021).
Cascarina, S. M. & Ross, E. D. Proteome-scale relationships between local amino acid composition and protein fates and functions. PLoS Comput. Biol. 14, e1006256 (2018).
Kroschwald, S. et al. Different material states of Pub1 condensates define distinct modes of stress adaptation and recovery. Cell Rep. 23, 3327–3339 (2018).
Cirillo, L. et al. UBAP2L forms distinct cores that act in nucleating stress granules upstream of G3BP1. Curr. Biol. 30, 698–707 (2020).
McSwiggen, D. T., Mir, M., Darzacq, X. & Tjian, R. Evaluating phase separation in live cells: diagnosis, caveats, and functional consequences. Genes Dev. 33, 1619–1634 (2019). This review summarizes current strategies to characterize phase separation in vivo.
Peng, S. et al. Phase separation at the nanoscale quantified by dcFCCS. Proc. Natl Acad. Sci. USA 117, 27124–27131 (2020).
Van Lindt, J. et al. A generic approach to study the kinetics of liquid-liquid phase separation under near-native conditions. Commun. Biol. 4, 77 (2021).
Woodruff, J. B., Hyman, A. A. & Boke, E. Organization and function of non-dynamic biomolecular condensates. Trends Biochem. Sci. 43, 81–94 (2018).
Woodruff, J. B. et al. The centrosome is a selective condensate that nucleates microtubules by concentrating tubulin. Cell 169, 1066–1077 (2017).
Boke, E. et al. Amyloid-like self-assembly of a cellular compartment. Cell 166, 637–650 (2016).
Zhang, G., Wang, Z., Du, Z. & Zhang, H. mTOR regulates phase separation of PGL granules to modulate their autophagic degradation. Cell 174, 1492–1506 (2018).
Mitrea, D. M. et al. Methods for physical characterization of phase-separated bodies and membrane-less organelles. J. Mol. Biol. 430, 4773–4805 (2018). This review summarizes biophysical strategies to characterize phase-separated MLOs.
Dazzi, A. et al. AFM–IR: combining atomic force microscopy and infrared spectroscopy for nanoscale chemical characterization. Appl. Spectrosc. 66, 1365–1384 (2012).
Delarue, M. et al. mTORC1 controls phase separation and the biophysical properties of the cytoplasm by tuning crowding. Cell 174, 338–349 (2018).
Folkmann, A. W., Putnam, A., Lee, C. F. & Seydoux, G. Regulation of biomolecular condensates by interfacial protein clusters. Science 373, 1218–1224 (2021).
Ma, W., Zheng, G., Xie, W. & Mayr, C. In vivo reconstitution finds multivalent RNA-RNA interactions as drivers of mesh-like condensates. eLife 10, e64252 (2021). This paper shows that RNA can modulate the morphology of liquid-like condensates.
Patel, A. et al. A liquid-to-solid phase transition of the ALS protein FUS accelerated by disease mutation. Cell 162, 1066–1077 (2015).
Courchaine, E. M. et al. DMA-tudor interaction modules control the specificity of in vivo condensates. Cell 184, 3612–3625 (2021).
Rai, A. K., Chen, J.-X., Selbach, M. & Pelkmans, L. Kinase-controlled phase transition of membraneless organelles in mitosis. Nature 559, 211–216 (2018).
Liao, Y.-C. et al. RNA granules hitchhike on lysosomes for long-distance transport, using annexin A11 as a molecular tether. Cell 179, 147–164 (2019).
Padrón, A., Iwasaki, S. & Ingolia, N. T. Proximity RNA labeling by APEX-Seq reveals the organization of translation initiation complexes and repressive RNA granules. Mol. Cell 75, 875–887 (2019).
Hubstenberger, A. et al. P-body purification reveals the condensation of repressed mRNA regulons. Mol. Cell 68, 144–157 (2017).
Wheeler, E. C. et al. Pooled CRISPR screens with imaging on microraft arrays reveals stress granule-regulatory factors. Nat. Methods 17, 636–642 (2020).
Roy, R., Das, G., Kuttanda, I. A., Bhatter, N. & Rajyaguru, P. I. Low complexity RGG-motif sequence is required for Processing body (P-body) disassembly. Nat. Commun. 13, 2077 (2022).
Tsang, B., Pritišanac, I., Scherer, S. W., Moses, A. M. & Forman-Kay, J. D. Phase separation as a missing mechanism for interpretation of disease mutations. Cell 183, 1742–1756 (2020).
Parker, R. & Sheth, U. P bodies and the control of mRNA translation and degradation. Mol. Cell 25, 635–646 (2007).
Lafontaine, D. L. J., Riback, J. A., Bascetin, R. & Brangwynne, C. P. The nucleolus as a multiphase liquid condensate. Nat. Rev. Mol. Cell Biol. 22, 165–182 (2021).
Bienz, M. Head-to-tail polymerization in the assembly of biomolecular condensates. Cell 182, 799–811 (2020).
Zamudio, A. V. et al. Mediator condensates localize signaling factors to key cell identity genes. Mol. Cell 76, 753–766 (2019).
Xiao, Q., McAtee, C. K. & Su, X. Phase separation in immune signalling. Nat. Rev. Immunol. 22, 188–199 (2022).
Huang, X. et al. ROS regulated reversible protein phase separation synchronizes plant flowering. Nat. Chem. Biol. 17, 549–557 (2021).
Riback, J. A. et al. Stress-triggered phase separation is an adaptive, evolutionarily tuned response. Cell 168, 1028–1040 (2017).
Weitzel, G., Pilatus, U. & Rensing, L. Similar dose response of heat shock protein synthesis and intracellular pH change in yeast. Exp. Cell. Res. 159, 252–256 (1985).
Lenard, A. J. et al. Phosphorylation regulates CIRBP arginine methylation, transportin-1 binding and liquid-liquid phase separation. Front. Mol. Biosci. https://doi.org/10.3389/fmolb.2021.689687 (2021).
Kato, M., Tu, B. P. & McKnight, S. L. Redox-mediated regulation of low complexity domain self-association. Curr. Opin. Genet. Dev. 67, 111–118 (2021).
Kato, M. et al. Redox state controls phase separation of the yeast ataxin-2 protein via reversible oxidation of its methionine-rich low-complexity domain. Cell 177, 711–721 (2019).
Yang, Y.-S. et al. Yeast ataxin-2 forms an intracellular condensate required for the inhibition of TORC1 signaling during respiratory growth. Cell 177, 697–710 (2019).
Jin, M. et al. Glycolytic enzymes coalesce in G bodies under hypoxic stress. Cell Rep. 20, 895–908 (2017).
Munder, M. C. et al. A pH-driven transition of the cytoplasm from a fluid- to a solid-like state promotes entry into dormancy. eLife 5, e09347 (2016).
Yasuda, S. et al. Stress- and ubiquitylation-dependent phase separation of the proteasome. Nature 578, 296–300 (2020).
Lau, Y., Oamen, H. P. & Caudron, F. Protein phase separation during stress adaptation and cellular memory. Cells 9, 1302 (2020).
Lu, Y. et al. Phase separation of TAZ compartmentalizes the transcription machinery to promote gene expression. Nat. Cell Biol. 22, 453–464 (2020).
Watanabe, K. et al. Cells recognize osmotic stress through liquid–liquid phase separation lubricated with poly(ADP-ribose). Nat. Commun. 12, 1353 (2021).
Perez-Gonzalez, N. A. et al. YAP and TAZ regulate cell volume. J. Cell Biol. 218, 3472–3488 (2019).
Rawat, P. et al. Stress-induced nuclear condensation of NELF drives transcriptional downregulation. Mol. Cell 81, 1013–1026 (2021).
Xie, D. et al. Phase separation of SERRATE drives dicing body assembly and promotes miRNA processing in Arabidopsis. Nat. Cell Biol. 23, 32–39 (2021).
McDonald, N. A., Fetter, R. D. & Shen, K. Assembly of synaptic active zones requires phase separation of scaffold molecules. Nature 588, 454–458 (2020).
Fang, X. et al. Arabidopsis FLL2 promotes liquid–liquid phase separation of polyadenylation complexes. Nature 569, 265–269 (2019).
Shin, Y. et al. Spatiotemporal control of intracellular phase transitions using light-activated optoDroplets. Cell 168, 159–171 (2017).
Schneider, N. et al. Liquid-liquid phase separation of light-inducible transcription factors increases transcription activation in mammalian cells and mice. Sci. Adv. 7, eabd3568 (2021).
Trojanowski, J. et al. Transcription activation is enhanced by multivalent interactions independent of phase separation. Mol. Cell https://doi.org/10.1016/j.molcel.2022.04.017 (2022).
Annunziata, O. et al. Effect of polyethylene glycol on the liquid-liquid phase transition in aqueous protein solutions. Proc. Natl Acad. Sci. USA 99, 14165–14170 (2002).
Hoffmann, C. et al. Fluorescent labeling of tetracysteine-tagged proteins in intact cells. Nat. Protoc. 5, 1666–1677 (2010).
Shulga, N. & Goldfarb David, S. Binding dynamics of structural nucleoporins govern nuclear pore complex permeability and may mediate channel gating. Mol. Cell. Biol. 23, 534–542 (2003).
Kroschwald, S. et al. Promiscuous interactions and protein disaggregases determine the material state of stress-inducible RNP granules. eLife https://doi.org/10.7554/eLife.06807 (2015).
Lin, Y. et al. Toxic PR poly-dipeptides encoded by the C9orf72 repeat expansion target LC domain polymers. Cell 167, 789–802 (2016).
Kroschwald, S., Maharana, S. & Alberti, S. Hexanediol: a chemical probe to investigate the material properties of membrane-less compartments. Matters https://doi.org/10.19185/matters.201702000010 (2017).
Ming, Y. et al. Targeting liquid-liquid phase separation in pancreatic cancer. Transl. Cancer Res. 8, 96–103 (2019).
Acknowledgements
This work was supported by grants from the Ministry of Science and Technology of the People’s Republic of China (2022ZD0213900 and 2022ZD0204900 to Y.L.), the National Key R&D Program (2019YFA0508403 to P.L.) and the National Natural Science Foundation of China (32170684 to Y.L.; 32150023, 32125010 and 31871443 to P.L.).
Author information
Authors and Affiliations
Contributions
X.L. and Y.G. created the figures. Y.G., X.L., P.L. and Y.L. conceived and wrote this Perspective.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemical Biology thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gao, Y., Li, X., Li, P. et al. A brief guideline for studies of phase-separated biomolecular condensates. Nat Chem Biol 18, 1307–1318 (2022). https://doi.org/10.1038/s41589-022-01204-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41589-022-01204-2
This article is cited by
-
Asymmetric oligomerization state and sequence patterning can tune multiphase condensate miscibility
Nature Chemistry (2024)
-
MolPhase, an advanced prediction algorithm for protein phase separation
The EMBO Journal (2024)
-
A chaperone-like function of FUS ensures TAZ condensate dynamics and transcriptional activation
Nature Cell Biology (2024)
-
LATS2 condensates organize signalosomes for Hippo pathway signal transduction
Nature Chemical Biology (2024)
-
Liquid–Liquid Phase Separation within Dense-Core Vesicles in Sympathetic Adrenal Chromaffin Cells
Neuroscience Bulletin (2024)