Abstract
Myeloproliferative neoplasms (MPNs) are chronic cancers characterized by overproduction of mature blood cells. Their causative somatic mutations, for example, JAK2V617F, are common in the population, yet only a minority of carriers develop MPN. Here we show that the inherited polygenic loci that underlie common hematological traits influence JAK2V617F clonal expansion. We identify polygenic risk scores (PGSs) for monocyte count and plateletcrit as new risk factors for JAK2V617F positivity. PGSs for several hematological traits influenced the risk of different MPN subtypes, with low PGSs for two platelet traits also showing protective effects in JAK2V617F carriers, making them two to three times less likely to have essential thrombocythemia than carriers with high PGSs. We observed that extreme hematological PGSs may contribute to an MPN diagnosis in the absence of somatic driver mutations. Our study showcases how polygenic backgrounds underlying common hematological traits influence both clonal selection on somatic mutations and the subsequent phenotype of cancer.
Similar content being viewed by others
Main
Myeloproliferative neoplasms (MPNs) are rare chronic hematological cancers characterized by the overproduction of mature blood cells leading to elevated blood cell parameters. They are typically driven by somatically mutated JAK2-mediated, calreticulin (CALR)-mediated or MPL-mediated clonal expansion1. JAK2 mutations are found in both polycythemia vera (PV) and essential thrombocythemia (ET), which are distinct but overlapping MPNs characterized by increased numbers of red blood cells and platelets, respectively. Mutant JAK2 is commonly detectable in 0.1–3% of the healthy population as clonal hematopoiesis (CH)2,3,4,5,6,7, with the vast majority of carriers not meeting or going on to develop disease-defining characteristics of MPN. Little is understood about why only a minority of individuals with mutated JAK2 develop more severe hematological manifestations of MPN and the factors that influence blood count heterogeneity in MPNs.
The 46/1 haplotype near JAK2 is a known germline risk factor for MPNs in the population8. Genome-wide association studies (GWAS) have identified additional disease-associated germline risk loci, estimating the liability-scale heritability of MPNs based on common single-nucleotide polymorphisms (SNPs) to be ~6.5% (refs. 9,10,11). However, these germline risk loci insufficiently explain the phenotypic heterogeneity observed within MPNs and in JAK2-mutated healthy carriers.
Blood cell traits vary widely in the healthy population. The genetic architecture underlying these traits is highly polygenic, with more than 11,000 independently associated genetic variants discovered so far12,13,14. These genome-wide associated variants, when combined in polygenic scores (PGSs), explain a large proportion of phenotypic variance among healthy individuals (from 2.5% for basophil count to 27.3% for mean platelet volume) and are associated with multiple common diseases and rare hematological disorders14. We hypothesized that a genetic burden of germline variants associated with extreme hematological traits could influence phenotypic heterogeneity in association with mutated JAK2, by influencing the clonal dynamics of mutant JAK2 and/or modifying its downstream consequences. In this study, we integrate information on somatic driver mutations, germline genetic variants associated with MPNs, and CH and hematological trait PGSs to study how inherited polygenic variation underlying blood cell traits influences clonal selection on mutated JAK2 and MPN disease phenotypes (Supplementary Fig. 1).
Results
Inherited polygenic contribution to JAK2 V617F positivity
One in 30 healthy individuals reportedly harbors JAK2V617F in their blood, as determined using sensitive assays6. The majority of such individuals have low levels of JAK2V617F and do not meet clinical criteria for MPN due to the absence of elevated blood cell parameters. We wished to understand whether inherited polygenic loci that underlie blood cell traits influence the strength of clonal selection on JAK2V617F.
We studied the germline characteristics of individuals in UK Biobank (UKBB) with and without JAK2V617F. From 162,534 genetically unrelated individuals of European ancestry within the UKBB whole-exome sequencing cohort (‘200k UKBB-WES cohort’; Methods), we identified 540 individuals with one or more mutant reads for JAK2V617F (0.3%, median variant allele frequency (VAF) = 0.056, range = 0.019–1; Supplementary Fig. 2; ‘UKBB-JAK2V617F cohort’). The lower rate of JAK2V617F in the UKBB-WES cohort compared to other population studies6,7 could be explained by its low sequencing coverage (21.5× depth), as also reported previously15 (Supplementary Fig. 3). As expected, there was some overlap among individuals with JAK2V617F and those with a diagnosis of MPN. Of the 423 individuals labeled with a diagnosis of MPN (156 with ET, 161 with PV and 106 with myelofibrosis (MF)), 72 were positive for JAK2V617F (Supplementary Table 1).
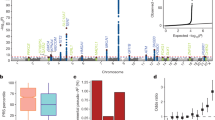
We built PGSs for 29 blood cell traits covering a wide range of hematopoietic parameters (Supplementary Table 2). Blood cell trait-specific PGSs were then weighted (by effect size) by the sum of all common (minor allele frequency (MAF) > 0.01) variants that were independently associated with a blood cell trait at genome-wide significance (P < 5 × 10−8) in UKBB (Methods)14. To assess the association between hematological PGSs and small (VAF < 0.1, n = 397) or large (VAF ≥ 0.1, n = 143) JAK2V617F clones, we used multinomial logistic regression including PGSs for each hematological trait (units of s.d.), together with previously reported germline sites associated with MPN9 and CH16 (PGSMPN and PGSCH) as covariates. To account for the recognized predisposition risk for MPN driven by the JAK2 46/1 haplotype8, we computed two PGSMPN scores, separating rs1327494 (tagging the JAK2 46/1 haplotype; PGSMPN-46/1) from nontagging JAK2 variants (PGSMPN-other). We found a negative association between the PGSs for both mean reticulocyte volume (PGSMRV) and immature reticulocyte fraction (PGSIRF) and small JAK2V617F clones (P = 6.2 × 10−4 and 0.0018, false discovery rate (FDR) < 0.05; Supplementary Table 3). We also found significant positive associations with small JAK2V617F clones for the PGSs of plateletcrit (PGSPCT) and monocyte count (PGSMONO) (P = 9.5 × 10−4 and 0.0036, FDR < 0.05). Germline predisposition to high MONO and PCT values was also positively associated with large JAK2V617F clones at modest significance (P = 0.033 and 0.0022, FDR-adjusted P = 0.31 and 0.064; Fig. 1a). Repeating the analysis above excluding MPN cases still demonstrated a significant association between PGSPCT or PGSMONO and small JAK2V617F clones (P < 0.013, Bonferroni corrected; Supplementary Table 4), suggesting that the inherited effects on JAK2V617F were not driven by the subset of MPN cases. These associations were independent of the known germline risk loci associated with MPN and CH (Supplementary Table 3). Validating these associations in the full UKBB-WES dataset (n = 799 and 326 for small and large clones, respectively, and n = 338,919 for controls), we again replicated the associations between PGSPCT and small JAK2V617F clones and between PGSMONO and large JAK2V617F clones at FDR < 0.05 (PCT: odds ratio (OR) = 1.15 (change in odds per increase of 1 s.d. in PGS), 95% confidence interval (CI) = 1.07–1.24, P = 1.4 × 10−4; MONO: OR = 1.20, 95% CI = 1.07–1.34, P = 0.0014; Supplementary Table 5).
Data are presented as ORs (solid dots) with 95% CIs (error bars). a, PGSs with significant associations with small clone size of JAK2V617F (FDR < 0.05) compared to the CH and MPN PGSs (Supplementary Table 3). OR was defined as the change in odds per increase of 1 s.d. in PGS. b, Causal effects estimated by four MR methods for the exposure traits whose PGSs were found to have significant predisposition risk for JAK2V617F positivity (Supplementary Table 7). OR was defined as the change in odds per increase of 1 s.d. in exposure. The MR results shown were based on GWAS summary statistics for JAK2V617F positivity in the full UKBB (Supplementary Fig. 4). Results based on the main discovery set (200k UKBB-WES cohort) are shown in Supplementary Table 6. The MR result for MRV was not available due to a lack of corresponding GWAS summary data in INTERVAL.
To understand the causal relationship among these associations, we undertook Mendelian randomization (MR) analyses with GWAS estimates for the exposure (blood traits) and the outcome (JAK2V617F positivity; Supplementary Fig. 4) obtained from two independent sources. We used genetic instruments for hematological traits identified from UKBB, with effect size estimates from INTERVAL17 (n = 30,305), an external independent cohort. MRV was excluded due to a lack of data in INTERVAL. Both PCT and MONO showed significant causality on the presence of a JAK2V617F clone based on inverse variance-weighted (IVW)18 MR and demonstrated consistent effect estimates using two other MR methods (simple median and weighted median), suggesting that higher MONO and higher PCT values cause a detectable JAK2V617F clone (Supplementary Table 6).
Extending this analysis to the full UKBB-WES cohort (JAK2V617F, n = 1,125; controls, n = 338,919) validated these causal associations with greater estimation accuracy (PCT: ORIVW = 1.52, 95% CI = 1.29–1.78, P = 3.0 × 10−7; MONO: ORIVW = 1.3, 95% CI = 1.15–1.49, P = 4.6 × 10−5; Fig. 1b and Supplementary Table 7). The IVW method of MR (Methods) assumes that the germline loci that drive MONO and PCT have no direct causal effect on driving a JAK2V617F clone (that is, there are no direct causal effects of the genetic instruments on the outcome). We found no evidence of pleiotropy using the MR-Egger19 test; the estimated intercept was not significantly different from zero with P = 0.84 and P = 0.90 for PCT and MONO, respectively. The causal relationship was also significant for PCT and MONO (P < 0.05; Supplementary Table 7 and Supplementary Fig. 5). Additionally, the estimates were not biased by any potential pleiotropic outlier variants and were highly consistent with outlier-corrected causal estimates (Supplementary Table 7 and Methods). Lastly, to ensure the results were not confounded by the possibility that the genetic loci used as instruments for MR directly promoted the outcome (that is, JAK2V617F positivity), we repeated the analysis excluding genetic instruments associated with JAK2V617F positivity (Passociation < 10−6), as well as those that correlated with JAK2V617F variants (that is, those variants and JAK2V617F variants are in linkage disequilibrium (LD) r2 > 0.01) or were in proximity to JAK2V617F variants (in the 10-Mb region centered on each variant), and found no major changes (Supplementary Table 8). Importantly, any reverse causal effect we detected for MONO and PCT was subtle and with pleiotropic effects (PEgger > 0.05 and PEgger-intercept < 0.05; Supplementary Table 9 and Supplementary Fig. 6).
Overall, the association results combined with MR suggest that higher PCT and MONO are causal for the presence of a JAK2V617F clone. This would also explain why individuals with germline predisposition to high PCT and MONO are also more likely to harbor a JAK2V617F clone. Given that acquisition of somatic mutations in blood is largely stochastic in healthy populations20, our data suggest that genetically predicted PCT and MONO influence clonal selection on nascent JAK2V617F cells to promote mutation acquisition.
Germline contribution to blood cell count variation in MPNs
Having shown that polygenic germline loci can predispose to JAK2 clone positivity through their influence on blood cell trait levels, we next studied the contribution of these inherited sites to clinical phenotypes of MPN. We first considered the four blood cell traits that are used to define MPN subcategories clinically21 as follows: hemoglobin concentration (HGB) (g dl–1 divided by 10), hematocrit (HCT) (%), platelet count (PLT) (×109 divided by 1,000) and white blood cell count (WBC) (×109 divided by 100). We used SNP arrays to measure genome-wide polymorphism in an MPN cohort of 761 patients (PV, n = 112; ET, n = 581; MF, n = 68), in whom diagnostic blood cell counts were available and mutation status for a panel of cancer-associated genes (Fig. 2a) had previously been characterized22.
a, Table summarizing the MPN patient cohort (total n = 761) across demographic and genetic features (somatically mutated genes present in >20 patients). 46/1 Hap, JAK2 46/1 germline haplotype; Chr9, chromosomal gain or loss of heterozygosity on chromosome 9. b, Plot showing the distribution of germline and somatic changes in the MPN patient cohort. Somatic mutations, chromosomal aberrations and germline risk loci present in at least ten patients are shown. Chr1p, chromosomal gain or loss of heterozygosity on chromosome 1p. c,e, Regression coefficients of significant (P < 0.0011, Bonferroni corrected) demographic, somatic or germline variables for hematological traits in the MPN patient cohort (c) and JAK2 and PGS (P < 0.0063, Bonferroni corrected) in the UKBB-MPN cohort (e). The regression coefficient (standard error (s.e.)) was estimated using linear regression analysis. Variables shown were identified from a set of variables selected by a stepwise model selection procedure (Supplementary Tables 10 and 11 and Methods). d,f, Plots showing the estimated hematological trait variation (%; bars) with 1 s.e. (error bars) explained by the variables in c and e. The percentage variation shown for a variable was estimated conditional on the remaining variables in a selected model with the s.e. estimated via bootstrapping (Methods). Blood trait phenotype data were available for n = 380–577 patients in the MPN patient cohort and n = 268–281 individuals in the UKBB-MPN cohort (varied across traits). For a specific trait, a variable has a blank result if it did not pass the significance threshold (P = 0.0011 for the MPN patient cohort and 0.0063 for the UKBB-MPN cohort, Bonferroni corrected; Methods). JAK2 includes V617F and exon 12 mutation in the MPN patient cohort and V617F only in the UKBB-MPN cohort; CALR, exon 9 frameshift (+1 bp) mutation.
We built PGSs for the four blood cell traits in both patients with MPN and a cohort of healthy blood donors from the INTERVAL study (n = 30,305; Methods). For each trait, we built a linear regression model with predictors that included the corresponding PGS (for example, PGSHGB), demographic variables (age and sex), JAK2 46/1 haplotype status (due to its influence on hematological traits23,24) and somatic mutation status for genes frequently (>20 patients) mutated in the MPN patient cohort (Fig. 2b). Following stepwise regression, we identified three somatic mutations (JAK2 mutation, CALR mutation and chromosome 9 aberration resulting in homozygous JAK2 mutation), PGS and sex as significant explanatory variables for at least one of the four traits (P < 0.001, Bonferroni corrected; Fig. 2c). We then estimated the phenotypic variation in blood cell traits explained by each variable conditional on the others (Fig. 2d and Methods). As a benchmark, we estimated PGS-explained hematological trait variation in controls from the INTERVAL cohort based on similar linear regression models with covariates such as age, sex and ten principal components (PCs) controlling for population stratification. Blood cell trait phenotypes were inverse-normal transformed, and only genetically unrelated individuals were included (Supplementary Fig. 7 and Methods).
The estimated PGS-explained phenotypic variance in blood cell traits in INTERVAL was as follows: 6.8% (95% CI = 6.2–7.4%) for HGB; 6.7% (95% CI = 6.1–7.2%) for HCT; 10% (95% CI = 9.7–11%) for WBC; and 25% (95% CI = 24–26%) for PLT, highly consistent with previously published results14. In the MPN patient cohort, when taking into account the effects of somatic mutations, there remained significant but smaller PGS effects on HGB, HCT and WBC (P < 0.001, Bonferroni corrected; n = 380 to 577; Fig. 2c and Methods), explaining only 2.0% (95% CI = 0.62–4.2%), 3.0% (95% CI = 0.79–7.0%) and 2.8% (95% CI = 1.0–5.3%) of trait variance, respectively (Fig. 2d), while PGS had no significant effect on PLT (Fig. 2c; P > 0.05).
To validate these findings, we analyzed the UKBB-WES cohort including patients with MPN (‘UKBB-MPN cohort’; patients, n = 423; healthy controls, n = 161,872; Supplementary Table 1) with hematological PGS and JAK2V617F somatic mutation as the main explanatory variables (Fig. 2e). Around 60% of MPNs were positive for JAK2V617F. However, only 44 of 423 individuals (10.4%) with labels of ET, PV or MF in UKBB had two or more mutant reads for JAK2V617F at a median sequencing depth of 21× and only 72 of 423 individuals (17.2%) had one or more mutant reads for JAK2V617F (Supplementary Table 1), suggesting that, despite the low depth of exome sequencing coverage, this cohort of individuals may represent a mixture of those with true somatic mutation-driven MPN and those with high blood counts driven by other causes. Both PGS and JAK2V617F in the UKBB-MPN cohort captured a substantial proportion of the phenotypic variation for all four traits (PGS, 9.8–14.3%; JAK2V617F, 2.8–12.0%; Methods and Fig. 2f). Interestingly, the contribution of PGS to blood cell traits was notably higher in the UKBB-MPN cohort than in the MPN patient cohort (Fig. 2d,f). This may reflect an ascertainment bias in estimation of the genetic weights of the PGSs from the UKBB. However, it is also possible that some individuals with labels of MPN included in the UKBB-MPN cohort had elevated blood counts driven by a high PGS. This could also contribute to the low prevalence of JAK2V617F in the UKBB-MPN cohort compared to the more strictly defined clinical MPN patient cohort.
Germline polygenic impact on MPN subtype at diagnosis
We next explored whether polygenic loci underlying hematological traits also affect initial disease classification, severity and subsequent disease evolution. MPNs can be classified into chronic phase conditions (ET and PV) and advanced phase MF. To assess whether PGSs for hematological traits influence MPN classification at diagnosis, we used multinomial logistic regression to explore the associations with standardized PGSs, including age, sex and ten PCs as covariates. We performed this analysis in genetically unrelated individuals across PGSs for the 29 different blood cell traits, including the 4 tested previously (Supplementary Table 2; ET, n = 581; PV, n = 112; control, n = 30,305).
We found that PGSs for multiple MPN-relevant blood traits showed significant associations with ET at FDR < 0.05. High PGSPLT, PGSPCT and PGSWBC were associated with increased risk while high PGSHCT, PGSHGB and PGSPDW (platelet distribution width) were associated with decreased risk of having an ET diagnosis (Supplementary Fig. 8a and Supplementary Table 12). Increased risk of PV diagnosis was modestly associated with an increased PGS for several red blood cell traits (PGSHGB, PGSHCT and PGSRBC), PGSPCT and PGSs for white blood cell traits (eosinophil count (EO) and MONO). PGSMRV showed a risk-decreasing effect for PV (P < 0.05; Supplementary Fig. 8a and Supplementary Table 12). We repeated this analysis in the UKBB-MPN cohort and healthy controls (ET, n = 156; PV, n = 161; control, n = ~161,000; Supplementary Table 1), taking into account the VAF of JAK2V617F (Supplementary Fig. 2) because it can influence blood count parameters (Fig. 2d,f). We found that the PGSs for two platelet traits (PGSPCT and PGSPLT) were significant risk factors for an ET diagnosis while those for four red blood cell traits (PGSHGB, PGSHCT, PGSRBC and PGSMCHC (mean corpuscular hemoglobin concentration)) were significant risk factors for PV diagnosis at FDR < 0.05 (Supplementary Fig. 8b and Supplementary Table 13). Thus, we replicated the significant polygenic germline risk effects of PGSPLT and PGSPCT for ET and PGSHGB, PGSHCT and PGSRBC for PV in both the MPN patient and UKBB-MPN cohorts. These results provide evidence of a strong polygenic germline predisposition for one hematological malignancy over the other, in this case ET versus PV, irrespective of somatic driver mutation status and driven by inherited variants implicated in basic hematopoietic processes.
We next asked whether an individual such as a JAK2V617F carrier might be protected from developing an MPN by inheriting a low PGS for relevant blood cell traits. Using enrichment tests in the full UKBB-WES cohort across the PGSs of six hematological traits that were identified to be either putative causal factors for JAK2V617F clones or associated factors for MPN diagnosis (MONO, PCT, PLT, HGB, HCT and RBC; Methods), we found that healthy JAK2V617F carriers were enriched in the low-PGS group for the two platelet traits and monocytes, with an enrichment OR around 2 (PGSPCT: OR = 2.8, 95% CI = 1.51–5.42, P = 3.8 × 10−4; PGSPLT: OR = 2.35, 95% CI = 1.29–4.43, P = 0.0027; PGSMONO: OR = 1.99, 95% CI = 1.11–3.67, P = 0.015), indicating a protective effect that makes low-PGS individuals around two times less likely to have ET than those in the high-PGS group. This is interpreted as a relative risk, which is very close to OR given the low incidence of ET (~1.6 per 100,000; Supplementary Table 14). Importantly, this indicates that an individual’s PGS for several hematological traits also influences the risk of developing subsequent disease from JAK2V617F CH. The association of low PGS with healthy JAK2V617F carriers was also confirmed in a logistic regression analysis (PGSPCT: OR = 2.32, 95% CI = 1.27–4.25, P = 0.0065; PGSPLT: OR = 2.48, 95% CI = 1.36–4.54, P = 0.0032; PGSMONO: OR = 2.08, 95% CI = 1.15–3.77, P = 0.016) with covariates included (Methods).
Of note, only 10–17% of the UKBB-PV cohort had a JAK2V617F mutation (n = 1 or ≥2 reads, respectively; Supplementary Table 1), although mutated JAK2 is expected to be found in >99% of PV cases. This raises the possibility that polygenic germline predisposition to high red blood cell indices may also contribute to other causes of clinical polycythemia not driven by JAK2V617F mutation25. However, we cannot exclude the possibility that some JAK2 mutations were missed due to the length of time between UKBB blood sampling and diagnosis (Supplementary Fig. 9) and the low sequencing coverage of JAK2, although these factors would equally affect the PV subgroups positive and negative for JAK2V617F in the UKBB cohort (Supplementary Fig. 3).
Combined germline impact on MPN classification at diagnosis
Because several SNPs and germline loci have been found to be associated with both MPN and CH9,15,26,27,28,29, we assessed whether germline predisposition to an ET versus PV diagnosis through PGSs for blood cell traits was independent of previously reported germline risk loci. To this end, we estimated the independent germline effects of each of the five hematological traits significant to MPN (for example, PGSPCT), taking into account previously reported genetic loci associated with MPN9 and CH16 (PGSMPN-46/1, PGSMPN-other and PGSCH). Across both MPN patient cohort and UKBB-MPN cohort, the strongest (P < 0.05 in both cohorts) germline risk factors for a diagnosis of ET were PGSPLT and PGSPCT, followed by MPN-specific risk loci (that is, PGSMPN-other). In PV, PGSHGB and PGSMPN-other were the strongest risk factors (Fig. 3a, Supplementary Table 15 and Methods). Our data confirmed strong risk effects for these five hematological PGSs independent of all currently known genetic loci predisposing to risk of MPN and CH. As a sensitivity analysis, we confirmed these associations after excluding variants associated with MPN and CH and their proxies (LD r2 > 0.6) from the hematological PGSs (Methods). Interestingly, the 46/1 haplotype, which was most strongly associated with PV in the MPN patient cohort, was not significant for PV in the less well-defined UKBB-PV cohort (Fig. 3a), in which the majority of individuals were JAK2V617F negative, suggesting that this locus is not a risk factor for developing high red blood cell indices independently of mutant JAK2, such as in secondary or apparent polycythemia.
a, The estimated OR for ET and PV across five significantly associated hematological trait PGSs, PGSCH, PGSMPN-46/1 and PGSMPN-other in the MPN patient cohort (red; ET, n = 581; PV, n = 112; control, n = 30,305) and the UKBB-MPN cohort (gray; ET, n = 156; PV, n = 161; control, n = 161,872). ORs were estimated in a multinomial logistic regression model. Data are presented as the OR (change in odds per increase of 1 s.d. in PGS; solid dots) with 95% CI (error bars). An asterisk indicates significant germline PGSs (P < 0.05 in both cohorts). The corresponding ORs and P values are shown in Supplementary Table 15. Age, sex and ten PCs were fit as covariates for both datasets. The VAF of JAK2V617F and the sample batch were two additional covariates included for the UKBB-MPN cohort. b, Pearson correlation coefficients across the PGSs for blood cell traits, CH and MPN in the MPN patient cohort. c, ΔR2 between the full and reduced models (y axis) after removing each PGS component (x axis). ‘HGB+MPN+CH’ and ‘PCT+MPN+CH’ represent the results when simultaneously excluding the three PGS items.
Next, we fit the same variables as above in a linear regression model and quantified the contribution of each of the germline components (that is, PGSMPN-46/1, PGSMPN-other, PGSCH and PGS of a blood cell trait) to ET or PV as the relative difference in the variance explained (R2) on the liability scale between a full model and a reduced model excluding one or more variables (\(\Delta {R}^{2}\left( \% \right)=\frac{{R}_{{\rm{{full}}}}^{2}-{R}_{{{\rm{reduced}}}}^{2}}{{R}_{{{\rm{full}}}}^{2}}( \% )\); Methods). We selected one representative hematological PGS for each analysis because the PGS correlations were high within trait categories (for example, Pearson’s r = 0.72 between PGSPLT and PGSPCT) and very small between trait categories (for example, r = −0.08 between PGSPLT and PGSHCT; Fig. 3b). Thus, we selected PGSPCT for ET and PGSHGB for PV, both of which showed the strongest disease predisposition risk. Consistent with the pattern of estimated risk (Fig. 3a), the germline contribution (relative to the full model-explained variation) of PCT polygenic loci to ET (26.6%) was largely comparable to the germline components of CH (29.2%) and MPN (33.4% for PGSMPN-other and 35.3% for PGSMPN-46/1). For PV, germline loci associated with MPN (15.8% for PGSMPN-other and 38.5% for PGSMPN-46/1) were more dominant than polygenic loci for HGB (1.6% for PGSHGB; Fig. 3c). For both ET and PV, more than 50% of the variance explained by the full model was accounted for by using combined germline components, highlighting the strong heritable predisposition to specific MPN subtypes. We also explored germline associations with MF presentation and MF transformation, but we did not see any significant associations that replicated across both the MPN patient and UKBB-MPN cohorts.
Influence of hematological PGSs on somatic mutation subtypes of MPN
Finally, we explored whether the influence of PGSs for blood cell trait variation on MPN classification was altered by the different somatic driver mutations underlying MPN. We stratified patients with ET according to their somatic driver mutation into patients heterozygous for JAK2 mutation (JAK2het; that is, JAK2 mutation without chromosome 9 aberrations; n = 308), patients with CALR and/or MPL mutations (n = 169), and patients negative for JAK2, MPL and CALR mutations (triple-negative (TN) patients; n = 81). We then compared diagnoses for patients in either the top or bottom quintile of the PGS distribution to those for patients in the middle quintiles (Methods). Our results showed consistent associations of both PGSPLT and PGSPCT with ET (n = 581) regardless of the driver mutation (Fig. 4a). Indeed, the top quintiles for PGSPLT and PGSPCT were associated with higher odds of an ET diagnosis, even in individuals without somatic driver mutations (ET TN, n = 81), suggesting that such individuals may simply represent those with extreme PGSs for PLT and PCT. We replicated these results in the UKBB-MPN cohort (Fig. 4a).
a,b, ORs of the top and bottom PGS quintiles relative to ORs of the middle three quintiles in the MPN patient cohort (red and yellow) and the UKBB-MPN cohort (blue and gray) for ET (a) and PV (b). Data are presented as the OR (change in odds per increase of 1 s.d. in PGS; solid dots) with the 95% CI (error bars). Mutation-stratified groups are shown wherever these data were available. n is the sample size of ET (or PV) cases in a specific group. Notably, PV negative for JAK2 mutation should be a rare diagnosis in PV (normally <5%), but several such individuals were present in the UKBB-WES cohort (n = 121 of 161; Supplementary Table 1). It may be that many of these individuals do not have an underlying clonal disorder such as PV but present with secondary erythrocytosis, due, for example, to smoking, alcohol or lung disease, where a germline predisposition to high HGB or HCT may contribute to blood count phenotypes that mimic PV (Supplementary Fig. 11). There was limited statistical power to demonstrate any significance for the PV subgroup positive for JAK2 mutation given the small sample size (UKBB-MPN cohort, n = 40). Furthermore, we cannot exclude the possibility that a mutant JAK2 clone was missed during sequencing in some of these individuals due to the low sequencing depth in UKBB or the timing of the blood sample relative to diagnosis.
For patients with PV in the MPN patient cohort, we did not see significant differentiation between the top and bottom quintiles for blood cell trait PGSs, possibly because the mutant JAK2 clone dominates the PV phenotype in this patient cohort (PV JAK2+ including JAK2het and JAK2hom in Fig. 4b; n = 112), resulting in the PGSs not having an observed impact. A similar pattern was also present in individuals with PV positive for JAK2 mutation in the UKBB-MPN cohort, although the number of such individuals was small (n = 40) as the overall UKBB-PV cohort had much higher than expected rates of PV negative for JAK2 mutation. Despite similar observations for patients in both the UKBB-MPN and MPN patient cohorts, we cannot rule out the possibility that we had limited statistical power to discover any significance for blood cell trait PGSs. This is especially true for the UKBB-MPN cohort, which had only 40 individuals with PV carrying JAK2V617F, as we noted that the higher PGS quintiles (for example, the fourth and fifth quintiles) for PGSHGB, PGSHCT and PGSRBC tended to have more patients with PV than the lower quintiles, and similarly so for PGSPLT and PGSPCT for patients with ET (Supplementary Fig. 10). In the UKBB-MPN cohort, we consistently observed that the top PGS quintile of PGSHGB, PGSHCT and PGSRBC presented risk effects for a PV label without JAK2V617F (n = 121; Fig. 4b). We did not see any significant associations between the PGSs for blood cell traits and progression of disease to MF that replicated in both cohorts, consistent with the notion that disease transformation is driven more by the acquisition of additional somatic driver mutations.
Discussion
Germline variants have been shown to contribute to CH26,28,29,30,31,32,33, as well as blood cancers such as MPNs8,9,10. However, the influence of polygenic inherited variation controlling normal hematopoiesis on the consequences of somatic driver mutations in blood and the development of blood cancers has not previously been explored.
Recent data suggest that acquisition of driver mutations in blood is much more frequent than previously appreciated20,34. In the presence of a high stochastic acquisition rate of driver mutations, variation in the presence of a detectable mutant JAK2 clone could reflect differential selection landscapes among individuals. Indeed, JAK2 somatic mutations generally occur long before the onset of MPNs35,36, and heterogeneity in the strength of clonal selection on mutant JAK2 is evident in both healthy individuals37 and those with MPN35. The factors driving such differences in selection remain unclear. We find that high PGSMONO and high PGSPCT are associated with detectable clone positivity for JAK2V617F. Our forward MR analyses suggest that the mode of action is via a route where the elevated blood counts themselves positively select on mutant JAK2. Indeed, clonal expansion of nascent mutated hematopoietic stem cells in MPNs and CH has been suggested to be influenced by inflammation38,39,40,41. However, we caution that a sensitivity analysis distinguishing causality from potential genetic correlation should be considered in future studies42, although no significant genetic correlation was detected in our data. We note that the causal relationship inferred by MR does not necessarily suggest a direct association between baseline MONO or PCT levels themselves and positivity for JAK2V617F, as blood cell parameters can suffer from measurement error and/or high levels of variability due to unknown or unmeasured environmental factors. These factors can counteract genetic effects, the latter of which provide better proxies for stable long-term exposure of a trait on an outcome.
In patients with MPN, we showed that germline variants affecting platelets (PGSPCT and PGSPLT) and HGB (PGSHGB and PGSHCT) significantly increased the risk of developing ET or PV independently of somatically mutated clones. This may be because some individuals, through their polygenic germline risk, have higher or lower baseline levels of blood count traits, which causes them to meet the diagnostic criteria for a particular MPN disease subtype21 sooner. However, it is also conceivable that somatic driver mutations result in differential downstream consequences in the presence of a high germline predisposition to one versus another hematological trait. Importantly, our data highlight how extreme PGSs for hematological traits may contribute to individuals meeting the diagnostic criteria for MPN in the absence of somatic mutations, potentially resulting in erroneous labels of MPN or ‘triple-negative’ MPN. This emphasizes the clinical need to consider whether somatic mutation-negative individuals with elevated blood cell counts suspicious of MPN, currently around 10% of patients, truly have underlying clonal neoplastic disease.
We explored the reasons for low rates of JAK2V617F positivity in the UKBB-MPN cohort. We found that nearly half the individuals with PV were diagnosed after a blood draw in UKBB, potentially explaining the low rate of positivity for JAK2 mutation (Supplementary Fig. 9). Furthermore, the overall depth of sequencing for JAK2 was low, as previously reported15, compared to other CH-related genes. However, these factors would have similarly affected the PV groups positive and negative for JAK2V617F (Supplementary Figs. 3 and 9) and therefore should not unduly bias the results for germline polygenic risk in the context of somatic mutations in the UKBB-MPN cohort, where a significant differentiation in polygenic effects between the top and bottom quintiles of PGSs was observed in PV cases that were not carriers of JAK2V617F compared to those that were carriers (Fig. 4b). This suggests that polygenic germline predisposition contributes to a PV diagnostic label in JAK2V617F-negative individuals in UKBB and should be considered as a potential contributory factor in JAK2-unmutated individuals with high HGB or HCT.
In summary, by analyzing two large MPN disease cohorts and UKBB, we provide new insights into the interaction between germline polygenic variation involved in basic hematopoiesis and clonal selection on somatic driver mutations in blood and describe how this interaction can influence the phenotype of subsequent blood cancer. Our results highlight an independent and causal new component of the overall susceptibility to clonal disease and provide a new framework for considering an individual’s genetic background in the context of their clinical presentation.
Methods
Samples and consent
UKBB analyses were undertaken under application numbers 56844 and 13745. Samples from patients with MPN were obtained following written informed consent and ethics approval as described previously22. Briefly, samples from patients with MPN were collected from outpatient clinics at Addenbrooke’s Hospital, Guy’s and St Thomas’ Hospital in the UK, under the clauses of the ‘Causes of Clonal Haematological Disorders Project’, which had regional ethical approval from the Eastern Multiregion Ethics Committee (MREC 02/5/22 and 07/MRE05/44) and local research and ethical approval at participating UK hospitals. Additional MPN samples were obtained from the University of Florence Careggi Hospital, Italy, with local ethics approval. Whole blood-derived samples were additionally analyzed from the Primary Thrombocythaemia-1 (PT1) trials, a multicenter international trial in ET. Analyses for this study were conducted under Cambridge Blood and Stem Cell Biobank ethics, 18/EE/0199 expiry 14 July 2024.
Genotyping, quality control and imputation
We genotyped peripheral blood-derived DNA extracted from whole blood or granulocyte samples from a cohort of 1,358 patients with MPN22 (‘MPN patient cohort’) using the Affymetrix UKBB array (m = ~731,000 loci). For the raw genotype data, we removed samples with dish quality control (QC) <0.82 (a default threshold of a measure of the extent to which the distribution of signal values separates from background values), sample call rate <97% (an estimate of the overall quality for a sample), plate pass rate <98% and average QC plate pass rate for the remaining samples for a given plate <99%. We used the program apt-genotype-axiom to make genotyping calls. We excluded samples with an outlying heterozygosity rate of greater than ±3 s.d. and high identity-by-descent sharing of >0.9 to obtain 1,207 samples. Genotype QC for the control cohort INTERVAL (n = ~44,000) was as previously published12. We performed PC analysis in a combined dataset of MPN cases, INTERVAL samples and the 1000 Genomes Project (1000G) with major global populations. We removed individuals more than 5 s.d. from the mean of the British ancestry group (GBR-1000G) to obtain 1,010 patients with MPN and 30,949 control individuals from INTERVAL. For genotype imputation, we included autosomal variants with an SNP call rate of >99%, MAF of >0.01, and Hardy–Weinberg equilibrium P > 10−6 in controls (m = ~523,000) and P > 10−10 in patients with MPN (m = ~576,000). We imputed genotype data to the HRC v1.1 reference panel using the Michigan imputation server (minimac4)43. We extracted the SNPs with an imputation accuracy R2 value of >0.6, MAF of >0.01, and Hardy–Weinberg equilibrium P > 10−6 for controls and P > 10−10 for cases (m = ~7.6 million) in unrelated individuals with genetic relatedness of <0.05 for both patients with MPN (n = 980) and controls in INTERVAL (n = 30,630). A genetic relationship matrix (GRM) was built on genotyped variants after LD pruning (LD r2 < 0.2; m = ~215,000) in the combined case–control dataset. For UKBB, genotype imputation, variant QC, and sample filtering of ancestry outliers and genetic relatedness of individuals were as previously described44. The corresponding approved application number was 13745.
Inclusion criteria for MPN and JAK2 V617F positivity
The MPN disease cohort had detailed somatic mutation status for 69 myeloid cancer-associated genes, clinical phenotypes of four blood cell traits (HGB and HCT levels and platelet and white blood cell counts) at diagnosis, plus age, sex and information on disease transformation. UKBB-WES44 data from 200,450 individuals (200k UKBB-WES as our main discovery dataset) were used to identify known MPN cases through annotations for ET (ICD-10 D47.3, D75.2), PV (ICD-10 D45), MF (ICD-10 D47.4, D75.81), chronic myeloid leukemia (CML; ICD-10 C921, C922, C931), and chronic myeloproliferative disease (CMD; ICD-10 D47.1). For both the MPN patient cohort and the UKBB-MPN cohort (n = 761 and 423, respectively, with genotype data available), we included only patients with MPN who were explicitly diagnosed as having ET, PV or MF. Patients with conflicting records/unclassified/other MPN (for example, CML) were excluded.
Note that the MPN cases in the UKBB-MPN cohort were not restricted to participants who had existing diagnoses at the time of blood sampling. Any participants whose inpatient records could be matched to the ICD-10 codes for MPN were included as cases in this study. To calculate the time interval from the blood draw to MPN diagnosis, we matched MPN cases to information regarding the date a blood sample was collected and when a particular diagnosis was first recorded in the hospital data (episode start date), downloaded from the UKBB data portal. When an MPN case could be matched to multiple episode start dates, the earliest date was selected as the diagnosis date. We then calculated the time interval between the two dates for ET and PV.
UKBB-WES data were also used to identify a set of individuals (n = 540, of whom 72 had a corresponding diagnosis of ET, PV or MF, 6 had a diagnosis of CMD, 3 had a diagnosis of CML, 63 had more than one record of MPN subtypes and 396 were healthy) with mutant reads (either 1 or ≥2; n = 359 and n = 181, respectively) corresponding to the JAK2V617F mutation (‘UKBB-JAK2V617F cohort’); these patients comprised those with and without a diagnosis of MPN. The mpileup function of Samtools 1.9 was used with the FASTA file of the GRCh38 assembly and the parameter ‘-r chr9:5073767-5073775’ to calculate the number of mutant reads and coverage of each base around the V617 hotspot. We excluded reads with base quality of <13 using the base quality filter in the Samtools mpileup tool. JAK2V617F clone size was measured using VAF in the UKBB-WES cohort. Descriptions of the 200k UKBB-WES dataset can be found in Kar et al.15.
Germline associations of hematological traits with JAK2 V617F positivity
To detect germline associations with JAK2V617F positivity, we classified the UKBB-JAK2V617F cohort as individuals with either small or large clones (VAF cut-off = 0.1) and applied multinomial logistic regression analysis (small, n = 397; large, n = 143 (regardless of whether the individual had MPN) versus n = ~162,000 UKBB controls). The corresponding approved application number was 56844. PGSMPN-46/1, PGSMPN-other and PGSCH along with age, sex, WES batch and ten PCs (controlling for population stratification) were fitted as covariates. The significance threshold was FDR < 0.05. The ORs (95% CIs) of PGSMPN-46/1, PGSMPN-other and PGSCH were estimated in the same model without fitting hematological PGSs. Hematological PGSs were sourced from a published GWAS conducted in UKBB (n = 408,112)18. SNPs associated with MPN and CH were discovered by Bao et al.9 (m = 25 with association P < 10−6) and Kessler et al.16 (m = 57 with P < 5 × 10−8), respectively. We adopted the suggestive significance threshold (P < 10−6) to extract MPN-associated SNPs to obtain variants that are potentially functionally important to MPN9. Only SNPs that overlapped with our common SNPs (MAF > 0.01) were incorporated in PGSMPN and PGSCH (UKBB-MPN cohort, m = 25; UKBB controls, m = 51). The PGS in the regression analysis was standardized with units of s.d., and an OR estimate indicates the change in odds per increase of 1 s.d. in the PGS.
We applied MR methods to identify causal associations of blood cell traits with JAK2V617F positivity in the UKBB-JAK2V617F cohort. For JAK2V617F positivity (≥1 mutant reads) as the outcome, we focused on the traits that showed significant JAK2V617F associations as exposures. Summary statistics of JAK2V617F positivity were obtained by conducting a case–control GWAS in unrelated individuals in the 200k UKBB-WES cohort (controls, n = 161,994; cases, n = 540) and also in the full UKBB-WES cohort (controls, n = 338,919; cases, n = 1,125) using SAIGE (v0.38)45. A GRM was built on variants for which imputation, QC and LD pruning with an r2 threshold of 0.2 had been performed (m = 341,000; MAF > 0.01). Age, sex, WES batch and ten PCs were fit as covariates. This GWAS had sample overlap with the UKBB where the GWAS of hematological traits was done. Thus, to maximize power and minimize bias due to overfitting, we selected independent genetic instruments of blood cell traits from UKBB14 but re-estimated weights and s.e. from an external source (INTERVAL; LD r2 < 0.05; P < 5 × 10−8 in UKBB and <0.05 in INTERVAL). For these traits, we estimated genetic causal effects using four main MR methods implemented in mr_allmethods() in the MendelianRandomization package46, namely, simple median-based MR47, weighted median-based MR, IVW MR46 and MR-Egger19. We preliminarily excluded variants that had a large difference in allele frequency (>0.2) between the GWAS summary data and the reference sample (the 10,000 random unrelated individuals in the UKBB-EUR set). We then selected independent common variants (MAF > 0.01; LD r2 < 0.05) that were genome-wide significant in UKBB-EUR (P < 5 × 10−8) and nominally significant in INTERVAL (P < 0.05; GWAS summary data sourced from Vuckovic et al.14) as genetic instruments (n = 373–703 depending on the trait). Only blood cell traits that had GWAS summary data available in INTERVAL were included in the MR analysis. A result was identified as significant if it had PIVW < 0.0042 (= 0.05/(3 ⨯ 4), Bonferroni corrected), consistent directions of the estimated causal effects across the four main methods and an MR-Egger intercept not significantly different from 0 (Pintercept > 0.05). We tested whether there was significant distortion in the causal estimate before and after removing outliers (if any) using a published method called MR pleiotropy residual sum and outlier (MR-PRESSO)48. MR-PRESSO detected one outlier horizontal pleiotropic variant for MONO and nine for PCT, but the distortion test before and after removing outliers was not significant for either MONO or PCT (Pdistortion = 0.91 and 0.48, respectively). MR-PRESSO and MR-Egger regression complement each other in providing a less biased causal estimate with better precision: the MR-PRESSO outlier test assumes that more than half of the instruments are valid (with no horizontal pleiotropy) and have balanced pleiotropy, while MR-Egger, which corrects for the global average pleiotropy effect among all variants, is best suited when the percentage of horizontal pleiotropic variants is large (>50%)48. We also examined whether there was reverse causality using independent genome-wide-significant variants for JAK2V617F positivity as genetic instruments (P < 10−6 (a less stringent threshold with more instruments) and LD (r2 < 0.05; m = 11). The corresponding genetic effects of hematological traits were extracted from the GWAS in INTERVAL.
PGS computation for blood cell traits
We obtained published summary statistics for participants of European ancestry (n = ~408,000) in UKBB for 29 blood cell traits14. We refined the GWAS associations for each trait using GCTA-COJO49 (v1.93.3 beta) with Passociation < 5 × 10−8 in a 10-Mb window. GCTA-COJO selects SNPs based on conditional Passociation values through a genome-wide stepwise selection procedure and estimates the joint effects of all selected SNPs after model optimization. We randomly sampled 10,000 unrelated individuals (relatedness <0.05) of European ancestry from the UKBB as the LD reference for COJO analysis. We then computed PGSs in the MPN patient cohort (n = 761) and controls in INTERVAL (n = 30,305) using the selected SNPs based on the joint effects estimated using COJO (m = 179–1,093 depending on the trait). PGSs for the 29 blood cell traits in the UKBB were obtained from published data14. Only common SNPs (MAF > 0.01) that passed the after-imputation QC and were in common among datasets (GWAS summary data, reference data and individual-level data; m = ~7.5 million) contributed to COJO and the PGSs. PGSMPN and PGSCH in the MPN patient cohort and INTERVAL were based on the SNPs discovered by Bao et al.9 (19 variants with Passociation < 10−6 and MAF > 0.01) and Kessler et al.26 (49 variants with association P < 5 × 10−8 and MAF > 0.01).
Hematological trait variation explained by germline and somatic genetic features in patients with MPN
We studied four hematological traits available in our MPN patient cohort: HGB (g dl–1 divided by 10), HCT (%), WBC (×109 divided by 100) and PLT (×109 divided by 1,000). To estimate the phenotypic variation explained by a variable, we built a linear regression model with the hematological PGS, a set of driver genes, age and sex. We focused on the eight genes for which mutations were carried by more than 20 patients with MPN. We used this model as an initial model and selected the model that explained the greatest amount of phenotypic variation with the fewest independent explanatory variables. Model selection was carried out using the function stepAIC() in the package MASS (v7.3-54)50. Based on the selected model, we estimated the phenotypic variation explained by a variable conditional on remaining variables using the function Anova (type = 2) in the car package (v3.0-10)51. The s.e. was the square root of the sampling variance of the estimated proportion of explained variation computed following bootstrapping (boot() in the R package boot (v1.3-28) with the default 1,000 replications)52,53. A variable was considered as significant at P < 0.0011 = 0.05/(4 ⨯ (8 + 3)) (PGS, age and sex that are relevant to blood cell counts and eight germline and somatic genetic changes) across four traits. We performed the analyses for ET and PV separately. For a comparison with healthy individuals, we estimated PGS-explained phenotypic variation using the same approach in the control dataset (INTERVAL) with age, sex and ten PCs as covariates. The PGSs were standardized and the phenotypes for the four traits in both the MPN patient cohort and INTERVAL controls were inverse-normal transformed. The raw phenotypes in the MPN patient cohort were preliminarily adjusted for subcohorts wherever necessary. The raw data of phenotypes in INTERVAL controls were processed and described as previously published14. We replicated the analysis in the UKBB-MPN cohort (n between 268 and 281 due to varied data availability across traits) and UKBB controls (n = ~140,000 to ~146,000), based on a model with PGS and JAK2V617F mutation status as explanatory variables (age, sex and batch as covariates). The Passociation cut-off was 0.0063 = 0.05/(4 ⨯ 2). The PGSs based on common SNPs (MAF > 0.01) and phenotype data for the UKBB participants were as previously published14. Raw phenotypes were processed from a published study12, including an adjustment for sex and environmental factors and inverse-normal transformation.
Germline associations of blood cell traits with MPN
In the MPN patient cohort, we studied the germline associations with three aspects of MPN diagnosis: subtype classification (ET versus PV), initial severity (ET + PV versus primary MF) and transformation (primary MF versus transformed MF). For subtype classification, we applied a multinomial logistic regression model to the patients with ET or PV and controls with age, sex, ten PCs and 29 hematological PGSs (standardized). The same model was applied to the association analysis for initial severity and transformation. The significance threshold of germline associations was FDR-adjusted P < 0.05. For replication, the same model was applied to the UKBB-MPN cohort for subtype classification (ET versus PV) and severity (ET + PV versus MF), with the VAF of JAK2V617F and sample batch fit as two additional covariates. Note that it is unknown whether patients with MF in the UKBB-MPN cohort had primary MF or their disease had transformed from ET or PV.
For significant associations that were replicated in both datasets, we first quantified the germline contribution of a blood cell trait (for example, PGSPCT) to ET and PV by fitting the same multivariate logistic regression model plus PGSMPN-46/1, PGSMPN-other and PGSCH (and VAF of JAK2V617F in the UKBB-MPN cohort) as covariates. The germline effects of PGSMPN-46/1, PGSMPN-other and PGSCH were estimated from the model without fitting a hematological PGS. We also repeated the analysis excluding the hematological variants that overlapped or were in LD (r2 > 0.6) with MPN and CH risk loci.
We performed an enrichment analysis to test whether there was a polygenic germline protective effect on MPN in JAK2V617F carriers in the full UKBB-MPN cohort. For this, we defined high-PGS and low-PGS groups by the median of the PGS distribution for a hematological trait within the JAK2V617F carriers and tested whether healthy individuals were enriched in the low-PGS group for ET and PV separately. We considered PGSs for the traits significantly associated with MPN classification (HCT, HGB, RBC, PLT and PCT) and those that were causally associated with JAK2V617F positivity (PCT and MONO). We estimated the PGS effect based on a logistic regression model within JAK2V617F carriers, where healthy carriers were coded as cases and carriers with ET or PV were coded as controls. PGS was fitted as a factor with the low-PGS group coded as 1. PGSMPN-46/1, PGSMPN-other, PGSCH, age, sex, WES batch and ten PCs were fit as covariates.
Contributions of germline components to MPN classification
We quantified the contribution of each of the germline components (PGSMPN-46/1, PGSMPN-other, PGSCH and PGS of a blood cell trait) to ET or PV by comparing the full model for a disease (for example, ET) with a reduced model excluding one or more variables. We quantified the difference in variance explained (R2) between the two models, defined as \(\Delta {R}^{2}\left( \% \right)=\frac{{R}_{{\rm{{full}}}}^{2}-{R}_{{{\rm{reduced}}}}^{2}}{{R}_{{{\rm{full}}}}^{2}}( \% )\), where the R2 component (for example, \({R}_{{{\rm{full}}}}^{2}\)) was the variance explained on the liability scale transformed from the observed scale under-reported disease prevalence. Instead of modeling disease status on an observation scale (unaffected or affected), the liability threshold model describes disease liability on an unobserved continuous scale, assuming the sum of environmental and additive genetic components from an independent normal distribution54. Individuals are affected if liability exceeds a truncation threshold value. We adopted a published transformation method where the variance explained R2 on a liability scale was a function of R2 on an observed scale (\({R}_{{{\rm{full}}}}^{2}\) and \({R}_{{{\rm{reduced}}}}^{2}\)), population disease prevalence (that is, 9 per 100,000 for ET and 5.4 per 100,000 for PV55), proportion of cases in the ascertained sample (581/(581 + 30,305) for ET and 112/(112 + 30,305) for PV in the MPN patient cohort) and height of the standard normal probability density function at the truncation threshold (equation (2) in Zhang et al.56).
Germline impact in MPN influenced by somatic driver mutation
For significant germline associations that were replicated in both datasets, we performed mutation-stratified analyses focusing on JAK2, CALR and MPL. The stratification resulted in four ET groups and three PV groups, which included the following: a JAK2het group (JAK2+C9−MPL−CALR−; n = 308), a CALR+ and/or MPL+ group (JAK2−C9−MPL+CALR−, JAK2−C9−MPL−CALR+ and JAK2−C9−MPL+CALR+; n = 169) and a TN group (JAK2−C9−MPL−CALR−; n = 81) in the MPN patient cohort and one ET-all group in the UKBB-MPN cohort as a replicate for ET and a PV-all group (JAK2+ including 50 JAK2het and 62 JAK2hom) in the MPN patient cohort and JAK+ PV and JAK2− PV groups in the UKBB-MPN cohort for PV. For an MPN subtype (for example, ET), we combined the patients in a specific stratified group (for example, ET JAK2het; n = 308) from the MPN patient cohort together with controls from INTERVAL and then divided the combined individuals into groups according to PGS quintiles and estimated the PGS effect on the disease in the top or bottom quintile versus the middle quintiles via logistic regression analysis with age, sex and ten PCs as covariates.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
GWAS summary statistics for the 29 blood cell traits were sourced from a published study (Vuckovic et al.14) and are available from the GWAS Catalog (https://www.ebi.ac.uk/gwas/) with accession numbers GCST90002379–GCST90002407. Individual-level phenotype/genotype data for the UKBB can be requested by application at https://www.ukbiobank.ac.uk. Individual-level genotype data for the 1000 Genomes Project are available at https://www.internationalgenome.org/. INTERVAL data can be requested by application to the study leaders (https://www.intervalstudy.org.uk/), and MPN patient cohort data can be requested from the authors.
Code availability
The case–control GWAS for JAK2V617F positivity in the UKBB-WES cohort was conducted using SAIGE version 0.38 (https://github.com/weizhouUMICH/SAIGE). GRM computation for the case–control GWAS for JAK2V617F positivity and conditional analysis for the GWAS associations for 29 blood cell traits were carried out using GCTA-GRM (https://yanglab.westlake.edu.cn/software/gcta/#MakingaGRM) and GCTA-COJO (https://yanglab.westlake.edu.cn/software/gcta/#COJO), respectively, in GCTA version 1.93.3beta2 (https://yanglab.westlake.edu.cn/software/gcta/#Download). PGS computation in the MPN patient cohort and INTERVAL, LD-based variant pruning and LD r2 computation were conducted in PLINK version 1.9 (https://www.cog-genomics.org/plink/). MR analysis for JAK2V617F positivity was performed using the R package MendelianRandomization version 0.5.1 (https://cran.r-project.org/src/contrib/Archive/MendelianRandomization/). Hematological trait variation explained by germline and somatic genetic features in patients with MPN was estimated in R package MASS version 7.3-57 (https://cran.r-project.org/src/contrib/Archive/MASS/), car version 3.0-10 (https://cran.r-project.org/src/contrib/Archive/car/) and boot version 1.3-28 fv.
References
Nangalia, J. & Green, A. R. Myeloproliferative neoplasms: from origins to outcomes. Blood 130, 2475–2483 (2017).
Genovese, G. et al. Clonal hematopoiesis and blood-cancer risk inferred from blood DNA sequence. N. Engl. J. Med. 371, 2477–2487 (2014).
Jaiswal, S. et al. Age-related clonal hematopoiesis associated with adverse outcomes. N. Engl. J. Med. 371, 2488–2498 (2014).
McKerrell, T. et al. Leukemia-associated somatic mutations drive distinct patterns of age-related clonal hemopoiesis. Cell Rep. 10, 1239–1245 (2015).
Nielsen, C., Bojesen, S. E., Nordestgaard, B. G., Kofoed, K. F. & Birgens, H. S. JAK2V617F somatic mutation in the general population: myeloproliferative neoplasm development and progression rate. Haematologica 99, 1448–1455 (2014).
Cordua, S. et al. Prevalence and phenotypes of JAK2V617F and calreticulin mutations in a Danish general population. Blood 134, 469–479 (2019).
Xu, X. et al. JAK2V617F: prevalence in a large Chinese hospital population. Blood 109, 339–342 (2006).
Jones, A. V. et al. JAK2 haplotype is a major risk factor for the development of myeloproliferative neoplasms. Nat. Genet. 41, 446–449 (2009).
Bao, E. L. et al. Inherited myeloproliferative neoplasm risk affects haematopoietic stem cells. Nature 586, 769–775 (2020).
Hinds, D. A. et al. Germ line variants predispose to both JAK2V617F clonal hematopoiesis and myeloproliferative neoplasms. Blood 128, 1121–1128 (2016).
Tapper, W. et al. Genetic variation at MECOM, TERT, JAK2 and HBS1L-MYB predisposes to myeloproliferative neoplasms. Nat. Commun. 6, 6691 (2015).
Astle, W. J. et al. The allelic landscape of human blood cell trait variation and links to common complex disease. Cell 167, 1415–1429 (2016).
Chen, M.-H. et al. Trans-ethnic and ancestry-specific blood-cell genetics in 746,667 individuals from 5 global populations. Cell 182, 1198–1213 (2020).
Vuckovic, D. et al. The polygenic and monogenic basis of blood traits and diseases. Cell 182, 1214–1231 (2020).
Kar, S. P. et al. Genome-wide analyses of 200,453 individuals yield new insights into the causes and consequences of clonal hematopoiesis. Nat. Genet. 54, 1155–1166 (2022).
Kessler, M. D. et al. Common and rare variant associations with clonal haematopoiesis phenotypes. Nature 612, 301–309 (2022).
Moore, C. et al. The INTERVAL trial to determine whether intervals between blood donations can be safely and acceptably decreased to optimise blood supply: study protocol for a randomised controlled trial. Trials 15, 363 (2014).
Burgess, S., Butterworth, A. & Thompson, S. G. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet. Epidemiol. 37, 658–665 (2013).
Bowden, J., Davey Smith, G. & Burgess, S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int. J. Epidemiol. 44, 512–525 (2015).
Mitchell, E. et al. Clonal dynamics of haematopoiesis across the human lifespan. Nature 606, 343–350 (2022).
Arber, D. A. et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 127, 2391–2405 (2016).
Grinfeld, J. et al. Classification and personalized prognosis in myeloproliferative neoplasms. N. Engl. J. Med. 379, 1416–1430 (2018).
Pardanani, A., Fridley, B. L., Lasho, T. L., Gilliland, D. G. & Tefferi, A. Host genetic variation contributes to phenotypic diversity in myeloproliferative disorders. Blood 111, 2785–2789 (2008).
Smalberg, J. H. et al. The JAK2 46/1 haplotype in Budd–Chiari syndrome and portal vein thrombosis. Blood 117, 3968–3973 (2011).
McMullin, M. F. The classification and diagnosis of erythrocytosis. Int. J. Lab. Hematol. 30, 447–459 (2008).
Kessler, M. D. et al. Common and rare variant associations with clonal hematopoiesis phenotypes. Nature 612, 301–309 (2022).
Silver, A. J., Bick, A. G. & Savona, M. R. Germline risk of clonal haematopoiesis. Nat. Rev. Genet. 22, 603–617 (2021).
Bick, A. G. et al. Inherited causes of clonal haematopoiesis in 97,691 whole genomes. Nature 586, 763–768 (2020).
Uddin, M. M. et al. Germline genomic and phenomic landscape of clonal hematopoiesis in 323,112 individuals. Preprint at medRxiv https://doi.org/10.1101/2022.07.29.22278015 (2022).
Thompson, D. J. et al. Genetic predisposition to mosaic Y chromosome loss in blood. Nature 575, 652–657 (2019).
Zhou, W. et al. Mosaic loss of chromosome Y is associated with common variation near TCL1A. Nat. Genet. 48, 563–568 (2016).
Loh, P.-R. et al. Insights into clonal haematopoiesis from 8,342 mosaic chromosomal alterations. Nature 559, 350–355 (2018).
Loh, P.-R., Genovese, G. & McCarroll, S. A. Monogenic and polygenic inheritance become instruments for clonal selection. Nature 584, 136–141 (2020).
Poon, G. Y. P., Watson, C. J., Fisher, D. S. & Blundell, J. R. Synonymous mutations reveal genome-wide levels of positive selection in healthy tissues. Nat. Genet. 53, 1597–1605 (2021).
Williams, N. et al. Life histories of myeloproliferative neoplasms inferred from phylogenies. Nature 602, 162–168 (2022).
Van Egeren, D. et al. Reconstructing the lineage histories and differentiation trajectories of individual cancer cells in myeloproliferative neoplasms. Cell Stem Cell 28, 514–523 (2021).
Fabre, M. A. et al. The longitudinal dynamics and natural history of clonal haematopoiesis. Nature 606, 335–342 (2022).
Andersen, M. et al. Mathematical modelling as a proof of concept for MPNs as a human inflammation model for cancer development. PLoS ONE 12, e0183620 (2017).
Lai, H. Y. et al. Defective negative regulation of Toll-like receptor signaling leads to excessive TNF-α in myeloproliferative neoplasm. Blood Adv. 3, 122–131 (2019).
Koschmieder, S. et al. Myeloproliferative neoplasms and inflammation: whether to target the malignant clone or the inflammatory process or both. Leukemia 30, 1018–1024 (2016).
Avagyan, S. et al. Resistance to inflammation underlies enhanced fitness in clonal hematopoiesis. Science 374, 768–772 (2021).
O’Connor, L. J. & Price, A. L. Distinguishing genetic correlation from causation across 52 diseases and complex traits. Nat. Genet. 50, 1728–1734 (2018).
Das, S. et al. Next-generation genotype imputation service and methods. Nat. Genet. 48, 1284–1287 (2016).
Szustakowski, J. D. et al. Advancing human genetics research and drug discovery through exome sequencing of the UK Biobank. Nat. Genet. 53, 942–948 (2021).
Zhou, W. et al. Efficiently controlling for case–control imbalance and sample relatedness in large-scale genetic association studies. Nat. Genet. 50, 1335–1341 (2018).
Yavorska, O. O. & Burgess, S. MendelianRandomization: an R package for performing Mendelian randomization analyses using summarized data. Int. J. Epidemiol. 46, 1734–1739 (2017).
Bowden, J., Davey Smith, G., Haycock, P. C. & Burgess, S. Consistent estimation in Mendelian randomization with some invalid instruments using a weighted median estimator. Genet. Epidemiol. 40, 304–314 (2016).
Verbanck, M., Chen, C.-Y., Neale, B. & Do, R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat. Genet. 50, 693–698 (2018).
Yang, J., Lee, S. H., Goddard, M. E. & Visscher, P. M. GCTA: a tool for genome-wide complex trait analysis. Am. J. Hum. Genet. 88, 76–82 (2011).
Venables, W. N. & Ripley, B. D. Modern Applied Statistics with S 4th edn (Springer, 2002); https://www.stats.ox.ac.uk/pub/MASS4/
Fox, J. & Weisberg, S. An R Companion to Applied Regression 3rd edn (Sage Publications, 2019).
Canty, A. & Ripley, B. boot: bootstrap functions (originally by Angelo Canty for S). cran.r-project.org/web/packages/boot/index.html (2022).
Davison, A. C. & Hinkley, D. V. Bootstrap Methods and Their Applications (Cambridge Univ. Press, 1997).
Falconer, D. S. The inheritance of liability to certain diseases, estimated from the incidence among relatives. Ann. Hum. Genet. 29, 51–76 (1965).
McMullin, M. F. & Anderson, L. A. Aetiology of myeloproliferative neoplasms. Cancers 12, 1810 (2020).
Zhang, Q. et al. Risk prediction of late-onset Alzheimer’s disease implies an oligogenic architecture. Nat. Commun. 11, 4799 (2020).
Acknowledgements
J.N. is a Cancer Research UK (CRUK) advanced clinician scientist fellow. Work in the Nangalia laboratory is supported by CRUK, Wellcome core funding, and the Alborada Trust and Rosetrees Trust. D.V. is a member of the health protection research unit in chemical and radiation threats and hazards, a partnership between Public Health England and Imperial College London, which is funded by the National Institute for Health Research (NIHR). J.D. holds a British Heart Foundation professorship and an NIHR senior investigator award. Samples from patients with MPN were provided by the Cambridge Blood and Stem Cell Biobank, which is supported by the Cambridge NIHR Biomedical Research Centre, the Wellcome Trust–MRC Stem Cell Institute and the Cambridge Experimental Cancer Medicine Centre, UK. We thank Q. Zhang, N. Williams and D. Leongamornlert at the Wellcome Sanger Institute (Cambridge, UK) and N. Pirastu at the Fondazione Human Technopole (Milan, Italy) for their thoughtful discussions and constructive advice.
Author information
Authors and Affiliations
Contributions
J.G. performed all data analyses and prepared figures under the supervision of N.S. and J.N. K.W. supported data preparation. P.M.Q., M.G. and G.S.V. analyzed somatic mutations in the UKBB. E.J.B. provided MPN samples. J.D., E.D.A. and D.R. shared cohort data from INTERVAL. P.G., C.N.H., A.L.G., A.R.G. and J.N. provided MPN data and samples. D.V., J.N. and N.S. supervised the work. J.G., D.V., N.S. and J.N. wrote the paper. All authors reviewed the paper. The study was conducted in accordance with the journal’s guidelines on inclusion and ethics in global research.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Genetics thanks Mitchell Machiela and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Guo, J., Walter, K., Quiros, P.M. et al. Inherited polygenic effects on common hematological traits influence clonal selection on JAK2V617F and the development of myeloproliferative neoplasms. Nat Genet 56, 273–280 (2024). https://doi.org/10.1038/s41588-023-01638-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41588-023-01638-x
This article is cited by
-
Germline determinants of aberrant signaling pathways in cancer
npj Precision Oncology (2024)