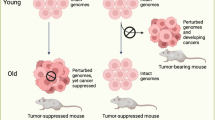
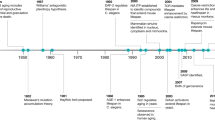
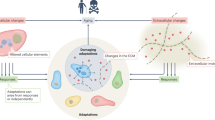
Abstract
Understanding why we age is a long-standing question, and many mechanistic theories of aging have been proposed. Owing to limitations in studying the aging process, including a lack of adequate quantitative measurements, its mechanistic basis remains a subject of debate. Here, I explore theories of aging from the perspective of causal relationships. Many aging-related changes have been observed and touted as drivers of aging, including molecular changes in the genome, telomeres, mitochondria, epigenome and proteins and cellular changes affecting stem cells, the immune system and senescent cell buildup. Determining which changes are drivers and not passengers of aging remains a challenge, however, and I discuss how animal models and human genetic studies have been used empirically to infer causality. Overall, our understanding of the drivers of human aging is still inadequate; yet with a global aging population, elucidating the causes of aging has the potential to revolutionize biomedical research.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Woodcox, A. Aristotle’s theory of aging. In Cahiers des Études Anciennes 65–78 (2018).
de Magalhães, J. P. In An Introduction to Gerontology (ed. Stuart-Hamilton, I.) 21–47 (Cambridge Univ. Press, 2011).
Lopez-Otin, C., Blasco, M. A., Partridge, L., Serrano, M. & Kroemer, G. Hallmarks of aging: an expanding universe. Cell 186, 243–278 (2023).
Weinert, B. T. & Timiras, P. S. Invited review: theories of aging. J. Appl. Physiol. 95, 1706–1716 (2003).
de Magalhães, J. P. et al. Human Ageing Genomic Resources: updates on key databases in ageing research. Nucleic Acids Res. https://doi.org/10.1093/nar/gkad927 (2023).
Gems, D. & de Magalhães, J. P. The hoverfly and the wasp: a critique of the hallmarks of aging as a paradigm. Ageing Res. Rev. 70, 101407 (2021).
de Magalhães, J. P., Lagger, C. & Tacutu, R. In Handbook of the Biology of Aging 151–171 (Elsevier, 2021).
Horvath, S. DNA methylation age of human tissues and cell types. Genome Biol. 14, R115 (2013).
Horvath, S. & Raj, K. DNA methylation-based biomarkers and the epigenetic clock theory of ageing. Nat. Rev. Genet. 19, 371–384 (2018).
Pon, J. R. & Marra, M. A. Driver and passenger mutations in cancer. Annu. Rev. Pathol. 10, 25–50 (2015).
Runge, J., Nowack, P., Kretschmer, M., Flaxman, S. & Sejdinovic, D. Detecting and quantifying causal associations in large nonlinear time series datasets. Sci. Adv. 5, eaau4996 (2019).
Cristofalo, V. J. & Pignolo, R. J. Replicative senescence of human fibroblast-like cells in culture. Physiol. Rev. 73, 617–638 (1993).
Bodnar, A. G. et al. Extension of life-span by introduction of telomerase into normal human cells. Science 279, 349–352 (1998).
Gosden, R. Cheating Time (W.H. Freeman, 1996).
Bartke, A. et al. Genes that prolong life: relationships of growth hormone and growth to aging and life span. J. Gerontol. A Biol. Sci. Med. Sci. 56, B340–B349 (2001).
de Magalhães, J. P. Open-minded scepticism: inferring the causal mechanisms of human ageing from genetic perturbations. Ageing Res. Rev. 4, 1–22 (2005).
Watts, D. J. & Strogatz, S. H. Collective dynamics of ‘small-world’ networks. Nature 393, 440–442 (1998).
Proctor, R. N. The history of the discovery of the cigarette–lung cancer link: evidentiary traditions, corporate denial, global toll. Tob. Control 21, 87–91 (2012).
Keshavarz, M., Xie, K., Schaaf, K., Bano, D. & Ehninger, D. Targeting the ‘hallmarks of aging’ to slow aging and treat age-related disease: fact or fiction? Mol. Psychiatry 28, 242–255 (2023).
Xie, K. et al. Deep phenotyping and lifetime trajectories reveal limited effects of longevity regulators on the aging process in C57BL/6J mice. Nat. Commun. 13, 6830 (2022).
de Magalhães, J. P. The genetics of a long life. Science 377, 1489–1490 (2022).
Weindruch, R. & Walford, R. L. The Retardation of Aging and Disease by Dietary Restriction (C.C. Thomas, 1988).
Prowse, K. R. & Greider, C. W. Developmental and tissue-specific regulation of mouse telomerase and telomere length. Proc. Natl Acad. Sci. USA 92, 4818–4822 (1995).
Martin, G. M., Austad, S. N. & Johnson, T. E. Genetic analysis of ageing: role of oxidative damage and environmental stresses. Nat. Genet. 13, 25–34 (1996).
Partridge, L. & Gems, D. Mechanisms of ageing: public or private? Nat. Rev. Genet. 3, 165–175 (2002).
Xiao, H. et al. A quantitative tissue-specific landscape of protein redox regulation during aging. Cell 180, 968–983 (2020).
Beckman, K. B. & Ames, B. N. The free radical theory of aging matures. Physiol. Rev. 78, 547–581 (1998).
Harman, D. Aging: a theory based on free radical and radiation chemistry. J. Gerontol. 11, 298–300 (1956).
Van Remmen, H. et al. Life-long reduction in MnSOD activity results in increased DNA damage and higher incidence of cancer but does not accelerate aging. Physiol. Genomics 16, 29–37 (2003).
Lapointe, J. & Hekimi, S. When a theory of aging ages badly. Cell. Mol. Life Sci. 67, 1–8 (2010).
de Magalhães, J. P. & Church, G. M. Cells discover fire: employing reactive oxygen species in development and consequences for aging. Exp. Gerontol. 41, 1–10 (2006).
Freitas, A. A. & de Magalhães, J. P. A review and appraisal of the DNA damage theory of ageing. Mutat. Res. 728, 12–22 (2011).
Schumacher, B., Pothof, J., Vijg, J. & Hoeijmakers, J. H. J. The central role of DNA damage in the ageing process. Nature 592, 695–703 (2021).
Vijg, J. From DNA damage to mutations: all roads lead to aging. Ageing Res. Rev. 68, 101316 (2021).
Franco, I., Revechon, G. & Eriksson, M. Challenges of proving a causal role of somatic mutations in the aging process. Aging Cell 21, e13613 (2022).
Narayanan, L., Fritzell, J. A., Baker, S. M., Liskay, R. M. & Glazer, P. M. Elevated levels of mutation in multiple tissues of mice deficient in the DNA mismatch repair gene Pms2. Proc. Natl Acad. Sci. USA 94, 3122–3127 (1997).
Sun, N., Youle, R. J. & Finkel, T. The mitochondrial basis of aging. Mol. Cell 61, 654–666 (2016).
Bratic, A. & Larsson, N. G. The role of mitochondria in aging. J. Clin. Invest. 123, 951–957 (2013).
Trifunovic, A. et al. Premature ageing in mice expressing defective mitochondrial DNA polymerase. Nature 429, 417–423 (2004).
Vermulst, M. et al. Mitochondrial point mutations do not limit the natural lifespan of mice. Nat. Genet. 39, 540–543 (2007).
Demanelis, K. et al. Determinants of telomere length across human tissues. Science 369, eaaz6876 (2020).
Simons, M. J. Questioning causal involvement of telomeres in aging. Ageing Res. Rev. 24, 191–196 (2015).
de Magalhães, J. P. & Toussaint, O. Telomeres and telomerase: a modern fountain of youth? Rejuvenation Res. 7, 126–133 (2004).
Bernardes de Jesus, B. et al. Telomerase gene therapy in adult and old mice delays aging and increases longevity without increasing cancer. EMBO Mol. Med. 4, 691–704 (2012).
de Magalhães, J. P. & Passos, J. F. Stress, cell senescence and organismal ageing. Mech. Ageing Dev. 170, 2–9 (2018).
Hayflick, L. & Moorhead, P. S. The serial cultivation of human diploid cell strains. Exp. Cell Res. 25, 585–621 (1961).
Biran, A. et al. Quantitative identification of senescent cells in aging and disease. Aging Cell 16, 661–671 (2017).
Avelar, R. A. et al. A multidimensional systems biology analysis of cellular senescence in aging and disease. Genome Biol. 21, 91 (2020).
Tuttle, C. S. L. et al. Cellular senescence and chronological age in various human tissues: a systematic review and meta-analysis. Aging Cell 19, e13083 (2020).
Baker, D. J. et al. Naturally occurring p16Ink4a-positive cells shorten healthy lifespan. Nature 530, 184–189 (2016).
Grosse, L. et al. Defined p16High senescent cell types are indispensable for mouse healthspan. Cell Metab. 32, 87–99 (2020).
Arrojo, E. D. R. et al. Age mosaicism across multiple scales in adult tissues. Cell Metab. 30, 343–351 (2019).
Signer, R. A. & Morrison, S. J. Mechanisms that regulate stem cell aging and life span. Cell Stem Cell 12, 152–165 (2013).
Ahlqvist, K. J. et al. Somatic progenitor cell vulnerability to mitochondrial DNA mutagenesis underlies progeroid phenotypes in Polg mutator mice. Cell Metab. 15, 100–109 (2012).
Franceschi, C. & Campisi, J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J. Gerontol. A Biol. Sci. Med. Sci. 69, S4–S9 (2014).
Desdin-Mico, G. et al. T cells with dysfunctional mitochondria induce multimorbidity and premature senescence. Science 368, 1371–1376 (2020).
Soerens, A. G. et al. Functional T cells are capable of supernumerary cell division and longevity. Nature 614, 762–766 (2023).
Martin, G. M. The genetics and epigenetics of altered proliferative homeostasis in ageing and cancer. Mech. Ageing Dev. 128, 9–12 (2007).
Martinez-Miguel, V. E. et al. Increased fidelity of protein synthesis extends lifespan. Cell Metab. 33, 2288–2300 (2021).
Rubinsztein, D. C., Marino, G. & Kroemer, G. Autophagy and aging. Cell 146, 682–695 (2011).
Cassidy, L. D. et al. Temporal inhibition of autophagy reveals segmental reversal of ageing with increased cancer risk. Nat. Commun. 11, 307 (2020).
Bjedov, I. et al. Fine-tuning autophagy maximises lifespan and is associated with changes in mitochondrial gene expression in Drosophila. PLoS Genet. 16, e1009083 (2020).
Pyo, J. O. et al. Overexpression of Atg5 in mice activates autophagy and extends lifespan. Nat. Commun. 4, 2300 (2013).
Benayoun, B. A., Pollina, E. A. & Brunet, A. Epigenetic regulation of ageing: linking environmental inputs to genomic stability. Nat. Rev. Mol. Cell Biol. 16, 593–610 (2015).
Pal, S. & Tyler, J. K. Epigenetics and aging. Sci. Adv. 2, e1600584 (2016).
Ocampo, A. et al. In vivo amelioration of age-associated hallmarks by partial reprogramming. Cell 167, 1719–1733 (2016).
Alle, Q. et al. A single short reprogramming early in life initiates and propagates an epigenetically related mechanism improving fitness and promoting an increased healthy lifespan. Aging Cell 21, e13714 (2022).
Yang, J. H. et al. Loss of epigenetic information as a cause of mammalian aging. Cell 186, 305–326 (2023).
Goh, A. M., Coffill, C. R. & Lane, D. P. The role of mutant p53 in human cancer. J. Pathol. 223, 116–126 (2011).
Melzer, D., Pilling, L. C. & Ferrucci, L. The genetics of human ageing. Nat. Rev. Genet. 21, 88–101 (2020).
Davey Smith, G. & Hemani, G. Mendelian randomization: genetic anchors for causal inference in epidemiological studies. Hum. Mol. Genet. 23, R89–R98 (2014).
Javidnia, S. et al. Mendelian randomization analyses implicate biogenesis of translation machinery in human aging. Genome Res. 32, 258–265 (2022).
Collier, J. J. et al. Developmental consequences of defective ATG7-mediated autophagy in humans. N. Engl. J. Med. 384, 2406–2417 (2021).
Wallace, D. C. Mitochondrial diseases in man and mouse. Science 283, 1482–1488 (1999).
Robinson, P. S. et al. Increased somatic mutation burdens in normal human cells due to defective DNA polymerases. Nat. Genet. 53, 1434–1442 (2021).
Robinson, P. S. et al. Inherited MUTYH mutations cause elevated somatic mutation rates and distinctive mutational signatures in normal human cells. Nat. Commun. 13, 3949 (2022).
Savage, S. A. & Alter, B. P. Dyskeratosis congenita. Hematol. Oncol. Clin. North Am. 23, 215–231 (2009).
Munoz-Lorente, M. A., Cano-Martin, A. C. & Blasco, M. A. Mice with hyper-long telomeres show less metabolic aging and longer lifespans. Nat. Commun. 10, 4723 (2019).
Codd, V. et al. Polygenic basis and biomedical consequences of telomere length variation. Nat. Genet. 53, 1425–1433 (2021).
Kuo, C. L., Pilling, L. C., Kuchel, G. A., Ferrucci, L. & Melzer, D. Telomere length and aging-related outcomes in humans: a Mendelian randomization study in 261,000 older participants. Aging Cell 18, e13017 (2019).
Schneider, C. V. et al. Association of telomere length with risk of disease and mortality. JAMA Intern. Med. 182, 291–300 (2022).
de Magalhães, J. P. Every gene can (and possibly will) be associated with cancer. Trends Genet. 38, 216–217 (2022).
de Magalhães, J. P. Ageing as a software design flaw. Genome Biol. 24, 51 (2023).
de Magalhães, J. P. Longevity pharmacology comes of age. Drug Discov. Today 26, 1559–1562 (2021).
Acknowledgements
I am grateful to current and past members of the Genomics of Ageing and Rejuvenation Lab for valuable discussions and in particular H. Fitzmaurice, I. Viegas and P. Clark for comments on previous drafts. Work in our laboratory is supported by grants from the Wellcome Trust, Longevity Impetus Grants, LongeCity and the Biotechnology and Biological Sciences Research Council. During the preparation of this work, I used AI-assisted tools, Grammarly, QuillBot and ChatGPT, to improve readability and language. After using these tools, I reviewed and edited the text as needed and take full responsibility for the content of the publication. In trying to cover such a broad topic in a limited amount of space, I apologize to colleagues whose relevant works I was unable to cite.
Author information
Authors and Affiliations
Contributions
J.P.d.M. conceived and wrote the article.
Corresponding author
Ethics declarations
Competing interests
J.P.d.M. is the CSO of YouthBio Therapeutics, a company developing rejuvenation gene therapies based on partial reprogramming, an advisor or consultant for the Longevity Vision Fund, 199 Biotechnologies and NOVOS and the founder of Magellan Science, a company providing consulting services in longevity science.
Peer review
Peer review information
Nature Genetics thanks Tamir Chandra and Abraham Aviv for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
de Magalhães, J.P. Distinguishing between driver and passenger mechanisms of aging. Nat Genet 56, 204–211 (2024). https://doi.org/10.1038/s41588-023-01627-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41588-023-01627-0