Abstract
Potato (Solanum tuberosum) and tomato (Solanum lycopersicon) crops suffer severe losses to late blight caused by the oomycete pathogen Phytophthora infestans. Solanum americanum, a relative of potato and tomato, is globally distributed and most accessions are highly blight resistant. We generated high-quality reference genomes of four S. americanum accessions, resequenced 52 accessions, and defined a pan-NLRome of S. americanum immune receptor genes. We further screened for variation in recognition of 315P. infestans RXLR effectors in 52 S. americanum accessions. Using these genomic and phenotypic data, we cloned three NLR-encoding genes, Rpi-amr4, R02860 and R04373, that recognize cognate P. infestans RXLR effectors PITG_22825 (AVRamr4), PITG_02860 and PITG_04373. These genomic resources and methodologies will support efforts to engineer potatoes with durable late blight resistance and can be applied to diseases of other crops.
Similar content being viewed by others
Main
Potato is one of the most consumed nongrain crops worldwide. However, pests and diseases reduce global yields by ~17% (ref. 1). Potato late blight, which is caused by the oomycete pathogen Phytophthora infestans2, triggered the Irish famine in the 1840s and is still the most damaging disease for global potato production1.
Plant immunity depends on pathogen recognition by both cell-surface pattern recognition receptors (PRRs) and intracellular immune receptors. Many R genes against P. infestans (Rpi genes) were cloned from wild relatives of potato species, such as R2, R3a, R8, Rpi-blb1, Rpi-blb2 and Rpi-vnt1 from Solanum demissum, Solanum bulbocastanum and Solanum venturii3,4,5,6,7,8,9,10. However, most cloned Rpi genes have been overcome by the fast-evolving pathogen.
P. infestans effectors carry a signal peptide and an RXLR-EER motif (where X represents any amino acid). In the P. infestans reference genome (strain T30-4), 563 RXLR effectors were predicted, enabling screens for recognition of these effectors (‘effectoromics’) in various plants11,12.
Reference genome sequences of potato, tomato, eggplant and pepper have been determined13,14,15,16. Phased, chromosome-level genome assemblies of heterozygous diploid and tetraploid potatoes are also available17,18,19. Pan-genome studies of crop plants including potato have also emerged that shed light on the extensive genetic variation in these species20,21,22,23. Sequence capture methods have been developed to sequence plant NLR (RenSeq) and PRR (RLP/KSeq) gene repertoires that reduce the genomic complexity and sequencing costs24,25,26. These methods have led to many important applications, such as AgRenSeq, and defining the pan-NLRome of Arabidopsis27,28.
Diploid Solanum americanum is highly resistant to late blight. Previously, our group cloned Rpi-amr1 and Rpi-amr3 from several resistant S. americanum accessions along with their cognate effectors AVRamr1 and AVRamr3 (refs. 25,29,30,31).
Here, we sequenced and assembled four high-quality genomes of S. americanum, resequenced 52 accessions, and defined the pan-NLRome of S. americanum. We also screened 315 P. infestans RXLR effectors in 52 S. americanum accessions. These genomic resources and functional data led to the rapid identification of three new NLR-encoding genes, Rpi-amr4, R02860 and R04373, that are responsible for PITG_22825 (AVRamr4), PITG_02860 and PITG_04373 recognition, respectively. This study unveils an effector-triggered immunity (ETI) interaction landscape between S. americanum and P. infestans that will enable us to clone more Rpi genes from the gene pool of wild Solanum species and deepen our knowledge of late blight resistance in wild relatives of potato. Potato genome design driven by potato genomics that takes advantage of novel plant breeding technologies32 will help to develop better potato varieties with durable late blight resistance.
Results
Genome assembly and gene model prediction of S. americanum
S. americanum is a globally distributed Solanaceae species that is resistant to many pathogens, including P. infestans and Ralstonia solanacearum25,29,33. Four S. americanum accessions SP1102, SP2271, SP2273 and SP2275 were selected for sequencing based on their variation in resistance to late blight (Supplementary Fig. 1a,b). We generated PacBio high-fidelity, Oxford Nanopore and Illumina paired-end reads and assembled the genomes of SP1102, SP2271, SP2273 and SP2275 into contigs (Supplementary Note 1). We also generated Hi-C data for SP1102, SP2271 and SP2273, and anchored the contigs into 12 pseudomolecules (Supplementary Note 1 and Supplementary Figs. 2 and 3). The completeness of these assemblies was estimated to be ~98.4% (single-copy and duplicated) by BUSCO, which indicates the high quality of genome assembly (Supplementary Fig. 4a). To annotate gene models, we applied EVidenceModeler or GeMoMa pipelines to integrate the ab initio prediction, homology-based annotation and transcriptome evidence for SP1102/SP2271 or SP2273/SP2275. In summary, we predicted an average of 34,193 gene models with an average of 98.1% BUSCO evaluation (single-copy and duplicated) for each S. americanum genome (Supplementary Fig. 4b and Table 1).
Genome evolution of S. americanum
To investigate the evolution of S. americanum genomes, we clustered the representative protein sequences from 15 genomes, comprising the genomes from four S. americanum accessions, four potato accessions, three tomato accessions, four additional Solanaceae species and an outgroup species of Arabidopsis thaliana, into 33,115 orthogroups, from which we further identified 1,363 single-copy orthogroups. The species tree topology suggests that S. americanum is a sister species to the common ancestor of potato and tomato and diverged ~14.1 million years ago (Ma; 95% highest posterior density interval, 11.7–17.2 Ma; Fig. 1a), which is consistent with a former report based on plastid sequences34.
a, Phylogenetic relationship of S. americanum and neighboring species. The red number indicates the bootstrap of each node. The black number denotes the estimated divergence time (million years ago). The scale bar represents the number of amino acid substitutions per site. b, Genome synteny of S. americanum, potato and eggplant. Ribbons between chromosomes show syntenic regions. Large chromosome rearrangements (>1 Mb in size) are marked in orange.
Chromosome rearrangement (CR) is an important evolutionary process35. The reference-grade genome assemblies enabled us to explore S. americanum chromosome evolution. We observed 45 large CRs (>1 Mb in size), comprising 26 inversion and 19 inter-chromosome translocation events, between the S. americanum and potato genomes (Fig. 1b and Supplementary Fig. 5). In contrast, 67 large CRs (30 inversions and 37 inter-chromosome translocations) were found between S. americanum and eggplant (Fig. 1b). Notably, CRs were not evenly distributed across the genome. No CR was identified on chromosome 2 between S. americanum and potato, while 11 CRs occurred on chromosome 11.
Structural variation between S. americanum genomes
Structural variations (SVs), including insertions, deletions, duplications, inversions and translocations, cause and maintain phenotypic diversity36. The chromosome assemblies of three S. americanum genomes enabled the analysis of large SVs (>1 Mb in size). Using SP1102 as the reference, we identified 56 large SVs in SP2271 (Supplementary Fig. 6a), impacting ~256 Mb of the reference genome. However, only 14 large SVs were identified in SP2273, covering ~54 Mb of the reference genome (Supplementary Fig. 6b). Most of the SVs reside in single contigs and are supported by the Hi-C interaction map, suggesting the high reliability of SV identification (Supplementary Fig. 6c and Supplementary Table 1). The large differences in SV numbers among S. americanum genomes shed light on their complex evolutionary history. We further characterized the small SVs (40 bp–1 Mb in size) among S. americanum genomes and found that SVs might contribute to the differential expression of 1,084 genes between SP1102 and SP2271 leaves (Supplementary Note 2 and Supplementary Figs. 7 and 8).
Defining the S. americanum pan-NLR repertoire
To understand NLR gene diversity, a phylogenetic tree was generated using the NB-ARC domain of the NLR proteins from SP1102 (Fig. 2a) and the position of these NLR genes in the SP1102 genome was visualized in the physical map (Fig. 2b). We found that 71% of SP1102 NLR genes were in clusters and the rest were singletons (Fig. 2c). Due to the complexity of NLR gene clusters, most automatic annotation pipelines produce incorrect gene models37. To generate better models of the NLR genes, we manually annotated 528, 579 and 524 NLR genes from SP1102, SP2271 and SP2273 genomes by incorporating NLR-annotator results and cDNA sequence data (Fig. 2d). Next, presence/absence (P/A) polymorphisms of NLR genes among S. americanum accessions were compared (Fig. 2a). Further, a pan-NLRome was built, which suggests that the accessions in our research are representative of the S. americanum NLR repertoire (Supplementary Note 3 and Supplementary Fig. 9).
a, The NB-ARC domains of S. americanum SP1102 were predicted by NLR-annotator and used to generate a maximum-likelihood tree using IQ-TREE with the JTT + F + R9 model. Known NLR proteins from Solanaceae species were included (highlighted in red). The NLRs are classified into different groups based on a previous report29. The RNL, TNL and NRC superclade are shown. CED-4 from Caenorhabditis elegans was used as the outgroup. The expression profile is shown by a heatmap (white to red) based on the cDNA RenSeq data of SP1102. The P/A polymorphism of NLRs from the three other S. americanum genomes and SMRT RenSeq assemblies of 16 additional accessions are shown by the heatmap (white to blue). The accession order from top to bottom is SP3409, SP3408, SP3406, SP3400, SP3399, SP3370, SP2360, SP2308, SP2307, SP2300, SP2298, SP2272, SP1123, SP1101, SP1034, SP1032, SP2275, SP2273 and SP2271. The absent NLRs are shown by black blocks. The scale bar represents the number of amino acid substitutions per site. b, The physical map of NLR genes in the SP1102 genome. CNLs are shown by yellow blocks, TNLs are shown by red blocks and RNLs are shown by pink blocks. Some functionally characterized NLR clusters are noted on this map. Some previously reported NLR clusters (NRC1, NRG1, R3, ADR1, NRC3, R1, RB, Rpi-vnt1, NRC2, Rpi-chc1, Rpi-amr1, NRC6, NRC4b, NRC5, NRC4a, Rpi-amr3, Gpa2) are shown in the physical map. (c) The proportion of NLR singletons and NLRs in clusters. d, Number of manually curated NLR genes (red circle), and the number of NLR genes predicted by NLR-annotator (yellow circle). Manual curation of NLR genes from SP2275 was not performed. e, Phylogeny of the NRC helper NLR family. The NRC homologs from potato, tomato and N. benthamiana are marked in orange, red and green, respectively. The NRC proteins from S. americanum are in black. The number indicates the bootstrap of each node. The scale bar represents the number of amino acid substitutions per site. f, The log10 transformed TPM values for NLR genes are classified into five groups, and some homologs of known R genes are noted. The NLR IDs are shown in Supplementary Table 2.
We also inspected the expression level of SP1102 NLR genes by re-analyzing RenSeq cDNA data25. The transcripts per million (TPM) values of NLR genes were visualized with a heatmap (Fig. 2a). Many well-known NLR genes were relatively highly expressed, such as the sensor NLR Rpi-amr3, and helper NLRs ADR1, NRG1, NRC4a, NRC2 and NRC3 (Fig. 2f and Supplementary Table 2). Many Solanaceae coiled-coil NLRs (CC-NLRs, or CNLs) require helper NLR NRCs that are phylogenetically related and we found that about 50% of the S. americanum NLRs lie within the NRC superclade38 (Fig. 2a). To investigate the NRC family, we generated a phylogenetic tree for the NRC genes. We found NRC1, NRC2, NRC3, NRC4a and NRC6 homologs, and two NRC5a homologs in the S. americanum genome (Fig. 2b, e). Interestingly, NRC4b genes (seven homologs) have expanded in the S. americanum genome compared to Nicotiana benthamiana (two homologs) (Fig. 2e). Previously, we reported that Rpi-amr3 and Rpi-amr1 require NbNRC2/NbNRC3/NbNRC4 and NbNRC2/NbNRC3 in N. benthamiana, respectively29,30. However, NRC1 is missing in N. benthamiana39. To test whether NRC1 from S. americanum can support Rpi-amr1/Rpi-amr3 function, we cloned the SaNRC1 from SP1102 and showed that SaNRC1 enables Rpi-amr3 but not Rpi-amr1 function in N. benthamiana nrc2/mc3/mc4 knockout plants (Supplementary Fig. 10). This result indicates that distinct members in different plant species might enable NRC functions.
The S. americanum genome and pan-NLRome also enabled us to investigate the diversity of NLR genes. We found that sequence diversity in NLR regions was significantly higher than in non-NLR regions (Supplementary Fig. 11a), consistent with a previous report37. In addition, we found extensive sequence diversity and copy number variation within NLR clusters. For example, the Rpi-amr3 locus varied greatly among the S. americanum genomes (Supplementary Fig. 11b), showing that high-quality genomes are required for reliable NLRome annotation.
In summary, we generated a pan-NLRome of 20 S. americanum accessions and manually annotated the NLR genes from three reference genomes. This resource is important for the investigation of NLR gene evolution and facilitates functional studies of ETI in S. americanum and other Solanaceae species.
The ETI landscape of the S. americanum and P. infestans interaction
There are 563 predicted RXLR effectors in the T30-4 P. infestans reference genome. In this study, we showed that there are ~550 NLR genes in S. americanum reference genomes (Fig. 2d). To reveal one-to-one effector-receptor interactions and clone more immune receptors, we screened ~315 RXLR effectors on 52 S. americanum accessions (Fig. 3). Based on cDNA PenSeq data, all these RXLR effectors are expressed during colonization of a susceptible potato leaf31. We found that five effectors triggered hypersensitive response (HR) on most S. americanum accessions (≥ 50), including effectors from the AVRblb2 family, while 185 effectors did not trigger HR in any S. americanum accessions, 71 effectors were recognized by fewer than five S. americanum accessions and 54 effectors showed differential recognition by different S. americanum accessions. AVRamr1 (36/52) and AVRamr3 (43/52) were also widely recognized by different S. americanum accessions (Fig. 3). The four reference accessions SP2271, SP2275, SP1102 and SP2273 could recognize 25, 18, 30 and 30 RXLR effectors, respectively, of which 5, 3, 7 and 9 effectors were specifically recognized in each accession (Supplementary Fig. 12). Notably, accession SP2271 was susceptible to P. infestans in the detached leaf assay (DLA), but susceptibility was age-dependent (Supplementary Fig. 1b), and this accession is resistant to late blight in the field. As expected, SP2271 did not recognize AVRamr1 and AVRamr3. We found premature stop codons in both Rpi-amr1 and Rpi-amr3 homologs from SP2271. Intriguingly, 25 RXLR effectors triggered HR in SP2271. These RXLR effector recognitions might contribute to the age-dependent resistance and field resistance of SP2271 to late blight. Taken together, these results reveal the ETI landscape of S. americanum against the late blight pathogen.
A total of 315 RXLR effectors were transiently expressed in 52 S. americanum accessions. The HR index (2, strong HR; 1, weak HR; 0, no HR, NA, not available) was used for the heatmap. These effectors were screened on N. benthamiana30, and their recognitions are included in this heatmap. Empty PVX vector was used as negative control, and co-expression of Rpi-amr3–HisFlag and AVRamr3–GFP was used as positive control. The S. americanum accessions were ordered based on the phylogenetic tree; SP3400 is not included in this tree. The scale bar represents the number of amino acid substitutions per site. The S. americanum accessions were classified into four groups (gray or yellow shading). The four reference accessions are marked by red arrows. The effectors were ordered based on the total HR index. For each effector, the numbers of responsive S. americanum accessions is visualized by a bar chart on the top of the heatmap. For each S. americanum accession, the numbers of recognized effectors is visualized by a bar chart on the right of the heatmap. Some RXLR effectors previously characterized or mentioned in this study are indicated by gray arrows.
Resequencing of S. americanum accessions
To investigate genetic diversity in the S. americanum accessions in our collection, we performed PCR-free, 150 bp paired-end sequencing for 52 S. americanum accessions at 10× coverage. We constructed a phylogenetic tree using all genic SNPs, and eggplant, potato, and tomato were used as outgroups (Fig. 3 and Supplementary Fig. 13a). Structural and inbreeding coefficient values were also analyzed (Supplementary Fig. 13b, c). The 52 accessions can be assigned into four groups (Supplementary Fig. 13a). All six accessions in group 1 lacked Rpi-amr1 and Rpi-amr3 based on the effectoromics screening (Fig. 3 and Supplementary Table 3). SP2275 and SP1102 were in group 2 and SP2273 was in group 3. No reference genome was available for group 4, but we generated SMRT-RenSeq assemblies for several of these accessions. Surprisingly, four accessions (SP2303, SP2310, SP3393 and SP3051) were not closely related to other groups and are highly heterozygous (Supplementary Fig. 13c), suggesting that they may be polyploid species like Solanum nigrum. Two other accessions, SP3052 and SP3376, were also not closely related to the four S. americanum groups and might belong to another Solanaceae species. These resequencing data could be used for genome-wide association studies (GWAS) and molecular marker development.
Cloning of Rpi-amr4 by GWAS and linkage analysis
During effectoromics screening, we found an effector, PITG_22825, that triggered HR in 28 of 52 S. americanum accessions (Fig. 3 and Supplementary Table 3), including SP1102 and SP2271 but not SP2298 (Fig. 4a). PITG_22825 is an RXLR effector with a signal peptide and RQLR and EER motifs followed by the effector domain (Fig. 4a). This effector had not received attention before our cDNA PenSeq study31. To map the gene conferring its recognition, a GWAS analysis was performed, and a strong signal was identified in an NLR singleton located on chromosome 01 of SP1102 (Figs. 2b and 4b). This gene encodes a CNL that belongs to the CNL-13 Rpi-amr3 phylogenetic clade (Fig. 2a), although the Rpi-amr3 gene cluster locates on chromosome 11 (Fig. 2b). This indicates that the candidate gene on chromosome 1 might have translocated from the Rpi-amr3 locus on chromosome 11, which probably explains another weaker GWAS signal in the Rpi-amr3 cluster of chromosome 11 (Fig. 4b). Based on cDNA RenSeq data from SP1102, the corresponding NLR gene carries an extra exon compared to Rpi-amr3 (Fig. 4b). To verify this GWAS signal, we performed a bulked segregant analysis and resistance gene enrichment sequencing (BSA-RenSeq) in a segregating F2 population of SP2271 x SP2298 (Supplementary Fig. 14). The PITG_22825 responsive gene from SP2271 was mapped to the same position on chromosome 1 in both the SP2271 and SP1102 reference genomes.
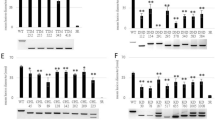
a, PITG_22825 is an RXLR effector. 35S::PITG_22825 triggers cell death on S. americanum SP2271 and SP1102 leaves, but not SP2298 leaves. b, Manhattan plot of the GWAS of PITG_22825 recognition. The SNPs associated with PITG_22825 recognition is located in an NLR singleton sp1102chr01_nlr39; (red arrow). c, HR assay of candidate genes. Rpi-amr4-1102 and Rpi-amr4-2271 were expressed alone or co-expressed with either 35S::PITG_22825 or 35S::AVRamr3–GFP constructs in N. benthamiana leaves. Rpi-amr4-2271 is auto-active in N. benthamiana, but when co-expressed with PITG_22825, the HR was stronger. Rpi-amr4-1102 specifically recognizes PITG_22825. Rpi-amr3 was used as control. OD600 = 0.5. Four leaves from two plants were used for each experiment and three biological replicates were performed with the same results. HF, HisFlag. d, Phylogeny of Rpi-amr4 homologs in different S. americanum accessions. Rpi-amr3 was used as an outgroup. PITG_22825-mediated HR is shown by red (HR) or blue (no HR) circles. Percent identity of the amino acid sequence relative to Rpi-amr4-1102 is shown. a,c,d–f, The scale bars represent the number of amino acid substitutions per site. e, Rpi-amr4-knockout lines lose the capacity for PITG_22825 recognition. Two sgRNAs (black arrows) were designed on Rpi-amr4-2271. The genotype of the two knockout lines is shown. Both lines failed to recognize PITG_22825, but HR could be complemented when co-expressing PITG_22825 with Rpi-amr4-1102. Wild-type (WT) SP2271 plants were used as control plants. Rpi-amr3 and AVRamr3 were used as positive control. OD600 = 0.5. f, DLA with 35S::Rpi-amr4-1102. 35S::Rpi-amr4-1102 (green), Rpi-amr3 (positive control, red) and Rpi-amr3a (a non-functional Rpi-amr3 paralog, negative control, blue) were transiently expressed in N. benthamiana, OD600 = 0.5. Zoospores from P. infestans strain T30-4 were used to inoculate the leaves 1 day post-infiltration (dpi). Lesion sizes were measured at 6 dpi. Four biological replicates were performed, and all data points (74 data points per treatment) were visualized as a box-and-whisker plot. Center line, median; box limits, upper and lower quartiles. The whiskers (top and bottom) comprise values within 1.5 times the interquartile range (IQR). The outliers are indicated by black dots. Statistical differences were analyzed by one-way ANOVA with Tukey’s HSD test (P < 0.001) and were indicated by the lower-case letters. Representative leaves are shown.
To test gene function, the ORFs of the candidate genes from SP2271 and SP1102 were PCR amplified and cloned into an over-expression binary vector with the 35S promoter and Ocs terminator and the resulting constructs were then transformed into Agrobacterium tumefaciens. The candidate genes were expressed in N. benthamiana alone or co-expressed with PITG_22825 or AVRamr3-GFP as the negative control. Rpi-amr3-HisFlag was used as a positive control. We found that the SP2271 allele (Rpi-amr4-2271 hereafter) was auto-active in N. benthamiana, but when it was co-expressed with PITG_22825 the HR was faster and stronger compared to the control (Fig. 4c). In contrast, the SP1102 allele (Rpi-amr4-1102 hereafter) was not auto-active in N. benthamiana. HR was triggered when Rpi-amr4-1102 was co-expressed with PITG_22825, but not AVRamr3–GFP (Fig. 4c). There are only three amino-acid differences between the proteins encoded by Rpi-amr4-1102 and Rpi-amr4-2271, and these differences might cause the auto-activity of the SP2271 allele (Supplementary Fig. 15). We also found that Rpi-amr4 was conserved in the PITG_22825 responsive accessions (Fig. 4d). To verify the function of Rpi-amr4, we generated Rpi-amr4-knockout SP2271 lines by CRISPR-Cas9. In total, 16 CRISPR-Cas9 knockout lines were generated (Supplementary Table 4) and 2 lines are shown in Fig. 4e. Wild-type SP2271 could recognize PITG_22825, but the Rpi-amr4-knockout lines could not. The HR phenotype could be complemented when Rpi-amr4-1102 was co-expressed with PITG_22825 in the knockout lines (Fig. 4e). Therefore, we conclude that Rpi-amr4 encodes the PITG_22825-recognizing immune receptor and that PITG_22825 is Avramr4.
To test whether Rpi-amr4 confers late blight resistance, we transiently expressed Rpi-amr4-1102 in N. benthamiana leaves and inoculated the leaves with P. infestans zoospores. Rpi-amr3 was used as a positive control and non-functional Rpi-amr3a from SP1102 was used as a negative control (Fig. 4f). This assay showed that Rpi-amr4-1102 confers resistance against P. infestans isolate T30-4. However, the resistance was weaker than that with Rpi-amr3 (Fig. 4f). We also generated stable Rpi-amr4-1102 transgenic N. benthamiana lines. As expected, the T0 transgenic plants gained the capacity for PITG_22825 recognition, and were resistant to two P. infestans isolates T30-4 and 88069. We also verified this finding in the T1 Rpi-amr4 transgenic lines (Supplementary Fig. 16a,b).
In summary, we successfully cloned a new Rpi gene Rpi-amr4 from S. americanum and defined its cognate effector gene Avramr4 (PITG_22825). Rpi-amr4 confers late blight resistance and may serve as a resource for producing late blight-resistant potatoes.
Cloning of R02860 and R04373
Although Rpi-amr4 could be identified using a GWAS approach, the number of effectors recognized by a few S. americanum accessions was small and did not enable a clear GWAS signal. We therefore deployed BSA-RenSeq to clone two more immune receptors.
PITG_02860 (Fig. 5a) targets the host protein NRL1 and attenuates plant immunity and increases pathogen virulence, but the cognate receptor was unknown40. We found that PITG_02860 triggered HR in 5 of 52 S. americanum accessions, including SP2271. We tested an F2 population of SP2271 (PITG_02860 responsive, R) × SP2272 (PITG_02860 non-responsive, NR), and found that recognition of PITG_02860 segregated according to a 3:1 ratio (104 R and 34 NR; χ2 (1, Ν = 138) = 0.00966, P = 0.92169) (Fig. 5b). The RenSeq pipeline was performed on the F2 population, and most filtered SNPs were located within a 1-Mb region on the top of chromosome 4 of SP2271 (Fig. 5c). SCAR markers were designed based on the resequencing data and used for genotyping. The candidate gene was mapped to a 295 kb region between markers S42 and S36. Seven NLRs genes reside within the mapping interval and all belong to the R3 family (Figs. 2a,b and 5c). To test these candidate genes, the ORFs from four candidate genes (nlr13, nlr14, nlr16, nlr17) were cloned into a binary vector under the control of the 35S promoter and Ocs terminator and transformed into Agrobacterium for transient expression. The candidate genes were expressed alone or with PITG_02860 or AVRamr3 in N. benthamiana and Nicotiana tabacum. NLR16 and NLR17 were auto-active in N. benthamiana, but we found that co-expression of NLR16 and PITG_02860 activated HR in N. tabacum (Fig. 5d). To verify this finding, we generated nlr16 knockout SP2271 lines by using the CRISPR–Cas9 system. As expected, the knockout lines lost recognition of PITG_02860 (Supplementary Fig. 17). Therefore, we conclude that NLR16 (R02860 hereafter) is the immune receptor for PITG_02860.
a, PITG_02860 is an RXLR effector from P. infestans. An illustration and predicted structure are shown. b, An F2 population was generated from a cross between SP2271 (responds to PITG_02860, R) and SP2272 (no response to PITG_02860, NR). The R bulk (104 progenies) and NR bulk (34 progenies) were used for BSA-RenSeq. c, A total of 218 linked SNPs (red dots) on the top of chromosome 4 of SP2271 were identified. The gray bar represents the chromosome. The physical positions (in Mb) are shown by number. Five molecular markers (S30, S42, S35, S36 and S31) were used for the map-based cloning. The number of recombination events per total tested gametes is shown. d, HR assay of the candidate PITG_02860 receptor. The candidate genes were expressed alone, or co-expressed with PVX::PITG_02860 in N. tabacum leaves. Rpi-amr3 and AVRamr3 were used as controls. NLR16 turned out to be the PITG_02860 receptor (R02860 hereafter). OD600 = 0.5. Four-week-old N. tabacum plants were used, and the photos were taken at 4 dpi. Three biological replicates were performed with the same results. e, PITG_04373 is an RXLR effector from P. infestans. An illustration and predicted structure are shown. f, Both backcross (BC1) and F2 populations were generated from SP2271 and SP2300. The BC1 population of 192 responsive plants and 182 non-responsive progenies were bulked for BSA-RenSeq. The F2 populations were used for fine mapping. g, Informative SNPs (red dots) on the top of chromosome 10 of SP2271 were identified. Five molecular markers (S11, S13, S5, S7 and S16) were used for fine mapping. The number of recombination gametes per total tested gametes is shown. Nine genes from SP2300 SMRT-RenSeq assemblies that mapped to the mapping interval of the SP2271 genome were regarded as candidate genes. All candidates belong to the Rpi-chc1 family except C444. h, HR assay of the PITG_04373 receptor candidates. The candidate genes were expressed alone or co-expressed with 35S::PITG_04373 in N. benthamiana leaves, Rpi-amr3 and AVRamr3 were used as controls. C168 turned out to be the PITG_04373 receptor (R04373 hereafter). OD600 = 0.5. Four-week-old N. benthamiana plants were used and photos were taken at 4 dpi. Three biological replicates were performed with the same results.
PITG_04373 (Fig. 5e) triggered HR in only 3 of 50 S. americanum accessions including in SP2300, which carries both functional Rpi-amr1 and Rpi-amr3 (Fig. 3). To clone the corresponding immune receptor gene, we first phenotyped a BC1 backcross population of SP2271 (NR) × SP2300 (R). The BC1 population segregated for PITG_04373 responsiveness with a 1:1 ratio (198 R and 182 NR; χ2 (1, N = 380) = 0.67368, P = 0.41177) (Fig. 5f). The DNA from responsive or nonresponsive progenies was bulked for BSA-RenSeq and most linked SNPs mapped to SP2271 chromosome 10 (Fig. 5g). SCAR markers were designed and an F2 population of SP2271 × SP2300 was phenotyped and genotyped. The PITG_04373 responsiveness was mapped to a 1.447-Mb interval with eight genes based on the SP2271 genome (Fig. 5g). Most candidates belong to the Rpi-chc1 family, except an R1 homolog (Fig. 5g). In the absence of a reference genome for SP2300, we used the SMRT-RenSeq assembly as the reference NLRome29. The SMRT-RenSeq contigs mapped to this region of the SP2271 genome, and candidate genes from SP2300 were cloned into a vector with the 35S promoter and Ocs terminator for transient assays. Five candidate genes were tested (C18.1, C18.2, C127, C168 and C829). We found that the candidate immune receptor C168 (R04373 hereafter) can specifically recognize PITG_04373 after transient expression in N. benthamiana (Fig. 5h). Therefore, we conclude R04373 is the immune receptor of PITG_04373.
SP2300 also carries functional Rpi-amr1 and Rpi-amr3 homologs. To test the function of R04373 in SP2300, we generated Rpi-amr1-2300/Rpi-amr3-2300/R04373 triple knockout lines (Supplementary Fig. 18a). Forty transgenic SP2300 knockout lines were generated and phenotyped and 13 of these 40 knockout lines lost recognition of the three effectors (PpAVRamr1, AVRamr3 and PITG_04373). Two of these lines, SLJ25603#3 and SLJ25603#17, were genotyped and the knockout events were confirmed (Supplementary Fig. 18b–e). We also co-expressed R04373 with PITG_04373; however, the HR phenotype was not restored in these knockout lines (Supplementary Fig. 18e) and we hypothesized that the truncated R04373 fragment might produce interfering RNAs. Interestingly, the triple knockout lines showed slightly elevated susceptibility to P. infestans (Supplementary Fig. 18f, g) compared to wild-type SP2300, but were more resistant than SP2271, suggesting that there are additional Rpi genes in SP2300.
To test the late blight resistance conferred by R02860 and R04373, we transiently expressed R02860, R04373, Rpi-amr4 and their combinations in N. benthamiana and measured P. infestans growth. However, although we observed a slight significant decrease in lesion size after transient expression of R02860 and R04373, the pathogen could still infect the plants. We also co-expressed Rpi-amr4 with R02860 or R04373 without enhancing the resistance of Rpi-amr4 (Supplementary Fig. 19). These results indicate that although R02860 and R04373 can recognize the RXLR effectors PITG_02860 and PITG_04373 from P. infestans, the resistance they confer can be overcome by P. infestans.
In summary, by using BSA-RenSeq, SMRT-RenSeq and map-based cloning strategies, we successfully cloned two new immune receptors, R02860 and R04373, and defined their recognized RXLR effectors, PITG_02860 and PITG_04373.
Discussion
Solanum is the largest genus of the Solanaceae family, comprising more than 1,500 species, including many important crop plants such as potato, tomato and eggplant for which extensive genome sequence data are available. The S. nigrum complex is composed of many species with different ploidy levels, including S. nigrum (6×), Solanum scabrum (6×), Solanum villosum (4×) and S. americanum (2×). Some are regarded as weeds, but others are consumed as food and medicine in various countries41. Importantly, these species carry valuable genetic variation for resistance to diseases, including, but not limited to, potato late blight and bacterial wilt25,29,31. In this study, we sequenced and assembled four S. americanum genomes, and generated multi-omics datasets. These data enabled us to build an S. americanum pan-NLRome to study the evolution and function of the NLR genes in S. americanum.
Potato late blight triggered the Irish famine in the 1840s and remains a global challenge that greatly constrains potato production. To understand ETI of S. americanum to P. infestans, we used ‘effectoromics’ to dissect the ETI interactions between them. We generated a matrix of 315 RXLR effectors × 52 S. americanum accessions. Interestingly, AVRamr1 (36/52) and AVRamr3 (43/52) recognition is widely distributed in S. americanum accessions, indicating that Rpi-amr1 and Rpi-amr3 play important roles in the late blight resistance of S. americanum. This finding is consistent with the conclusions of a pan-genome ETI study of the interaction between Arabidopsis and Pseudomonas syringae42. Some effectors induce cell death in all S. americanum accessions, such as effectors in the AVRblb2 family (Fig. 3). This observation is consistent with previous findings that AVRblb2 (PexRD39/PexRD40) induces cell death in all tested wild potato species, but not in N. benthamiana43,44; this non-specific cell death might be a result of the virulence activities of AVRblb2. Some resistant accessions lack AVRamr1 and AVRamr3 recognition and thus are valuable sources of novel Rpi genes.
Three new immune receptors Rpi-amr4, R02860 and R04373 were cloned in this study (Figs. 4 and 5). We showed that Rpi-amr4 elevates P. infestans resistance in N. benthamiana. PITG_02860 was reported to promote host susceptibility by targeting the host protein NRL1 (ref.40), and the virulence functions and host targets of AVRamr4 and PITG_04373 remain to be discovered. P. infestans is a fast-evolving pathogen and may be able to overcome single Rpi genes in the field within a few years. Resistance based on the recognition of a single effector can be easily overcome by mutations or silencing, as shown for Avrvnt1 (refs.45,46). R gene stacking is a better way to deploy cloned R genes in the field47,48. Therefore, Rpi-amr4 can be stacked with other Rpi genes to provide stronger and more durable potato late blight resistance.
The two other immune receptors R02860 and R04373, recognizing PITG_02860 and PITG_47373 were cloned from SP2271 and SP2300, respectively. The resistance conferred by these genes might be too weak to be applied in the field (Supplementary Fig. 19). Many effectors are suppressors of host immunity, notably AVRcap1, which can attenuate the function of the helper NLRs NRC2 and NRC3 (ref.49), and this attenuation may explain why some plant immune receptors that recognize effectors nevertheless do not confer strong disease resistance. To understand the complex nature of plant–pathogen interaction, our work provides an assay to identify the suppressor of R02860 and R04373 in the future.
In summary, our study provides valuable genomic and genetics tools that should accelerate the path to understanding and achieving durable resistance against potato late blight and other plant diseases and shows that S. americanum is an excellent model plant to study molecular plant–microorganism interactions and plant immunity.
Methods
Sequencing and assembly of S. americanum genomes
Four representative S. americanum accessions, SP1102, SP2271, SP2273 and SP2275, were selected for sequencing. The Pacific Bioscience Sequel II platform in the circular consensus sequencing (CCS) mode was applied to sequence the genomes of SP1102 and SP2271 and generated 30.5 Gb and 28.5 Gb of high-fidelity (HiFi) reads, respectively. The PromethION and GridION platforms of Oxford Nanopore Technologies were applied to sequence the genomes of SP2273 and SP2275, and generated ~81.1 Gb and ~114.5 Gb of data, respectively. To estimate the genome heterozygosity and polish the raw assembled genomes, we also prepared libraries for Illumina paired-end short-reads sequencing following the standard protocol and generated an average of 99.2 Gb of clean data for each S. americanum accession using the Illumina Hiseq 2500 platform. Hi-C libraries of three S. americanum accessions, SP1102, SP2271 and SP2273, were created from young seedlings based on the restriction enzyme MboI. The Illumina Hiseq 2500 platform was applied to generate 86.5, 81.8 and 54.8 Gb of paired-end reads for SP1102, SP2271 and SP2273, respectively.
The genome size and heterozygosity were estimated using a k-mer-based approach by KAT50 and GenomeScope51. The estimated genome size was calculated as the total number of k-mers divided by the estimated sequencing coverage. The total number of k-mers could be calculated from sequencing data, and sequencing coverage could be assessed based on k-mer distribution frequency. In this study, we applied KAT to calculate k-mer frequency with k = 19 and the Perl script estimate_genome_size.pl (https://github.com/josephryan/estimate_genome_size.pl) to estimate the genome size of S. americanum. Hifiasm52 was applied to assemble assemble SP1102 and SP2271 de novo using default parameters. To assemble the genomes of SP2273 and SP2275, we first corrected ONT reads using Canu53 with parameters ‘correct corOutCoverage=500 corMinCoverage=2 minReadLength=2000 genomeSize=1g -nanopore-raw’. The corrected reads were then assembled into raw contigs by SMARTdenovo54 with the following command line arguments ‘perl smartdenovo.pl -c 1 -t 24 -k 17’. The raw assemblies were then iteratively polished using Illumina short reads. Reads were aligned to the raw assemblies using BWA55, and resulting bam files were passed to Pilon56 for polishing. Pseudo-chromosomes were built by using the juicer57 and 3d-DNA58 pipeline with parameters ‘-m haploid -i 15000 -r 0’. The quality of the assemblies was assessed by BUSCO (Benchmarking Universal Single-Copy Orthologs)59 with the solanales_odb10 database.
Protein-coding genes prediction
For SP1102 and SP2271, to help with gene model prediction, the transcriptomes of S. americanum whole seedlings, roots, stems, leaves, flowers and fruits were sequenced by using the Illumina HiSeq 2500 platform with three replications for each tissue and 4 Gb of clean data for each sample. The reads were aligned to the genome by HISAT60, transcripts were assembled using StringTie61, Cufflinks62 and Trinity63 and the assemblies were then imported into PASA64 for protein-coding gene prediction. Ab initio and homologous protein search strategies were also performed by using SNAP65, AUGUSTUS66, GlimmerHMM67 and exonerate68. All predicted evidence was integrated using EVM64. To predict gene models in SP2273 and SP2275, we used the ITAG4.0 (ref.14) and SolTub_3.0 (ref.13) datasets for homology-based gene prediction in the GeMoMa program69. RNA-seq data, obtained from SP2273 were also incorporated for splice site prediction.
Phylogenetic analysis of S. americanum
The representative protein sequences of Arabidopsis thaliana, Petunia inflata, Capsicum annuum, Solanum melongena, Solanum tuberosum Group Phureja (DM1-3 516 R44), S. tuberosum Group Tuberosum (RH89-039-16), Solanum commersonii, Solanum chacoense, Solanum pennellii, Solanum pimpinellifolium, Solanum lycopersicum and four S. americanum accessions (SP1102, SP2271, SP2273 and SP2275) were extracted and input into OrthoFinder70 to cluster orthogroups using the MCL algorithm. The protein sequences of 1,363 single-copy orthogroups were extracted to infer the phylogenetic relationship following the supermatrix method. Sequences from 15 genomes were aligned using MAFFT71 with parameter ‘--auto’ and trimmed using trimAl with parameters ‘-phylip -gt 0.8’. IQ-TREE72 was applied to infer the phylogenetic relationships with parameters ‘--alrt 1000 -B 1000’. We used BASEML and MCMCTREE from the PAML software package73 to estimate the divergence time. The coding sequence (CDS) sequences of 1,363 single-copy orthogroups were extracted for a rough estimation of the substitution rate using BASEML with model = 7. MCMCTREE with parameters ‘model = 7, burnin = 5,000,000, sampfreq = 300, nsample = 20,000’ was applied to estimate the divergence time. The divergence times of potato–tomato (7–10 Ma)34 and potato–Arabidopsis (111–131 Ma; http://www.timetree.org/) were used for calibration. Two rounds of estimation were performed with similar results.
Genomic alignments of S. americanum and neighboring species
Genomic alignment between SP1102 and eggplant/potato was performed using MUMMER74 with parameters ‘ --maxmatch -c 100 -b 500 -l 50 ‘. The alignment was further filtered with parameters ‘ -1 -i 80 -l 100’. The delta format was then converted to PAF format using the paftools.js script75 and passed to D-Genies76 for dot plot visualization.
Syntenic analysis of S. americanum and neighboring species
We applied the Python-based program MCscan (v1.1.8) (ref. 77) to perform the syntenic analysis. The representative protein sequences and corresponding gene model annotations in BED format of potato (DMv6.1), eggplant (HQ-1315) and four S. americanum genomes were extracted to search for homologous with parameters ‘-m jcvi.compara.catalog ortholog --cscore = .99’. Syntenic regions were identified with ‘-m jcvi.compara.synteny screen --minspan=30’ and visualized with ‘-m jcvi.graphics.karyotype’ parameters.
Identification of SVs
To identify large SVs (>1 Mb in length), we aligned the chromosome-grade assembly (SP2271 and SP2273) to the SP1102 reference genome by using MUMMER with parameters: ‘--batch 1 -t 20 -l 100 -c 500’ and further filtered the alignment with parameters: ‘-i 90 -l 100’. SyRI v1.4 was adopted to identify SVs based on the alignment delta files; only large SVs were kept for further analysis. We adopted the Hi-C interaction map and SV location to validate the large SVs. Of the 70 SVs identified in SP2271 and SP2273, 68 SVs reside in single contig, suggesting high reliability. Of these, 40 SVs could be verified by a Hi-C interaction map.
The assembly-based approach was applied to identify SVs (> 40 bp in length) among S. americanum genomes following the pipeline of SVIM-asm78. The contig assemblies of SP2271, SP2273 and SP2275 were aligned to the SP1102 reference using minimap2 (ref.76) with the following parameters ‘--paf-no-hit -a -x asm5 --cs -r2k’. SVs, which consist of insertions, deletions, duplications and inversions were identified using SVIM-asm with ‘haploid’ mode. The SVs were further annotated by SnpEff79.
Calculation of sequence diversity across the S. americanum genome
We used the SV information generated by SVIM-asm and a sliding-window (window = 500 kb, step = 50 kb) method to calculate sequence diversity across the S. americanum genome. The diversity of a window was calculated as follows: diversity = (sum of SV length in a window) / window length. The final diversity value of each window was generated from the average of SP2271, SP2273 and SP2275. Higher diversity values refer to higher variation levels of a window. If a window overlapped an NLR gene, this window was counted as an NLR-region and a total of 2,304 NLR-regions were extracted from the SP1102 genome. To compare the variations between NLR-region and non-NLR-regions, we randomly selected 2,304 non-NLR-regions and compared their diversity values with those for NLR-regions by Wilcoxon rank-sum test. Ten rounds of random selection and comparisons were done between non-NRL-regions and NLR-regions.
Annotation of NLR gene models
The NLR genes from the four S. americanum reference genomes were predicted by NLR-annotator80. To obtain a better gene model for these NLR genes, all the NLR genes from SP2271, SP1102 and SP2273 genomes were manually curated. In brief, the outputs of NLR-annotator were imported into Geneious (v10.2.6) (ref. 81) as annotations of the reference genomes and the predicted NLR fragments with 2 kb flanking sequences from both sides were extracted. Augustus66 was then used to predict the gene model based on the trained dataset of tomato. The gene models were curated based on functionally validated NLR genes from public databases and cDNA RenSeq data were also used to assist the manual annotation.
Phylogenetic analysis of NLRs in S. americanum genomes
To infer the phylogeny of NLRs, the protein sequences for the NB-ARC domain found using NLR-annotator were aligned using MAFFT71 and IQ-TREE was used to build a phylogenetic tree. The JTT + F + R9 substitution model was selected by ModelFinder82 and used to infer the maximum-likelihood tree. Ultrafast bootstrap (UFBoot)83 was set to 1,000. CED-4 from C. elegans was selected as an outgroup.
To analyze the NLR presence and absence in S. americanum genomes, we collected 13 previously reported SMRT RenSeq assemblies37 and generated 3 new assemblies from accessions SP2298, SP3370 and SP2308. NLR-annotator was used to annotate the NLR genes in the SMRT-RenSeq dataset. We used GMAP84 to predict the NLR homologs among the S. americanum genomes and SMRT-RenSeq assemblies. The CDS sequences of manually curated NLRs in the SP1102 genome were extracted and mapped to the three S. americanum genomes and 16 SMRT RenSeq assemblies using GMAP with parameters ‘-f 2 -n 1 --min-trimmed-coverage=0.70 --min-identity=0.70’. NLRs that failed to align were marked as absent. In the v4 RenSeq library85, more baits were included compared to the v3 RenSeq library; thus, if a certain NLR was absent in all v3 RenSeq assemblies but present in v4 assemblies, the absence might be a false-positive and was marked as NA. To calculate the expression level of NLR genes in SP1102, RNA was isolated from young leaves of SP1102 and cDNA RenSeq was done as described previously86. We mapped the reads from SP1102 cDNA RenSeq to its genome using STAR (2.6.0c) (ref. 87), the BAM files were imported into Geneious (v10.2.6) (ref. 81) and the TPM values for NLR genes were calculated using the ‘Calculate Expression Levels’ function. The NLR phylogeny, TPM and PAV results were passed to the online software iTOL88 for final visualization.
Analysis of the S. americanum pan-NLRome
The NLR protein sequences from 4 genome assemblies as well as 16 SMRT-RenSeq assemblies were classified into orthogroups by OrthoFinder using the MCL algorithm. The orthogroups matrix was then processed with PanGP (v.1.0.1) (ref. 89) using the random algorithm. The sample size and sample repeat parameters were set to 500 and 30, respectively. These parameters indicate that at each given accession number (n), n accessions will be randomly selected for pan- and core-NLR analysis. A 500 times random selection was performed with 30 replicates. The estimated size of pan- and core-NLRomes were illustrated with a box plot and fitted with exponential models. The orthogroups were classified into three categories according to their frequency of occurrence: core (orthogroups present in all 18–20 accessions); dispensable (orthogroups that were missed in more than 3 accessions and present in at least 2 accessions); and unique (orthogroups present in only 1 accession). For each accession, the numbers of NLRs in different categories were summarized and illustrated with a stacked bar chart.
Resequencing of 52 S. americanum accessions
The genomic DNA of 4-week-old young leaves from 52 S. americanum accessions was sampled and isolated using a Qiagen DNeasy plant kit (Qiagen, 69104). A whole-genome PCR-free, 2 × 150 bp paired-end Illumina library was generated and sequenced by Novogene (Beijing, China), generating ~10 Gb of data for each S. americanum accession. The raw reads were trimmed using trimmomatic v0.36 (ref. 90). The clean reads of each accession were mapped to the SP1102 reference genome with minimap2 (v2.16) (ref. 75), and converted to BAM format with samtools (v1.9). SNP calling was carried out with samtools and bcftools (v1.9).
To infer the phylogenetic relationships of S. americanum accessions, we selected the genomes of potato (DM 1-3 516 R44 v6.1), tomato (Heinz 1706 v4.0) and eggplant (v3) as an outgroup. Wgsim (https://github.com/lh3/wgsim) was used to simulate whole-genome sequencing reads from the potato, tomato and eggplant genomes with parameters: ‘-e 0 -d 350 -N 500000000 -1 150 -2 150 -r 0 -R 0 -X 0’. The simulated reads mapping and SNP calling were performed using the same approaches. Bedtools (v2.17) was used to extract SNPs in coding regions. The SNP-based phylogenetic tree was inferred by IQ-TREE with UFBoot set to 1,000 and the TVMe+R2 best-fit model, which was automatically selected by ModelFinder. The phylogenetic tree was visualized with FigTree (v1.4.4).
GWAS analysis
For the GWAS analysis, all the SNPs residing in NLR gene regions, as well as the 3 kb upstream and 1 kb downstream regions, were extracted with bedtools (v2.17). The SNPs were filtered and processed using Plink (v1.90) with parameters ‘--make-bed --allow-extra-chr --allow-no-sex --mind 1 --maf 0.05 -geno 0.05 --recode --out’. The responsiveness scores of each effector were used as the phenotype and passed to Plink for association analysis with parameters ‘--allow-extra-chr --allow-no-sex --assoc --bfile --pheno’. The Manhattan plot was created using the R package qqman (v0.1.8).
Effectoromics screening
An RXLR effector library of 311 RXLR effectors was used in the effectoromics screening. The signal peptides were removed, and the effector domains were cloned into overexpression vectors (pMDC32 or pICSL86977) or PVX vectors. S. americanum plants were grown in a containment glasshouse. Four- or 5-week-old plants were used for the agroinfiltration. For the overexpression vectors, cell death was scored at 4 dpi; for the PVX vectors, cell death was scored at 7 dpi. OD600 = 0.5. The cell death phenotype was scored (2, strong HR; 1, weak HR; 0, no HR). Two leaves each from two plants were used for each experiment.
Constructs for transient overexpression
To verify the candidate genes, the ORF of each candidate genes was amplified by Phusion high-fidelity DNA polymerase (NEB, M0530S) or KAPA HiFi Uracil+ DNA polymerase (Roche, 07959052001) and then cloned into the pICLS86922 overexpression vector with the 35S promoter and Ocs terminator using BsaI (NEB, #R3733) or a USER cloning vector (pICSLUS0004OD) with the 35S promoter and Ocs terminator using USER enzyme (NEB, #M5508). The verified constructs were transformed into Agrobacterium for transient expression in plants.
Gene knockout with the CRISPR–Cas9 system
For the knockout constructs, guide RNAs were designed in Geneious (v10.2.6) using the ‘Find CRISPR Site’ function with parameters: ‘Maximum mismatches allowed against off-targets = 3; Maximum mismatches allowed to be indels = 0; pair CRISPR Sites: Maximum overlap of paired sites = 100; Maximum allowed space between paired sites = 300’. The reference genome or SMRT-RenSeq assembly was used for scoring of off-target activity. The selected guide RNAs are shown in Supplementary Table 3. Two guide RNAs for each candidate gene were amplified with the sgRNA scaffold by Q5 high-fidelity DNA polymerase (NEB, M0491S) and the pICSL70001 vector was used as the template. The fragments were then fused with an Arabidopsis U6-26 promoter (pICL90002) and cloned into level 1 vectors at different positions (position 3, pICH47751; position 4, pICH47761; position 5, pICH47772; position 6, pICH47772). For the final level 2 constructs, Cas9 with introns (position 1, pICSL11197), NPTII (position 2, pICSL11055), an end linker (pICH41922) and the guide RNAs were assembled into pICSL4723_OD. The final constructs were then transformed into S. americanum accessions from gene knockout. After transformation, the T0 lines were moved into a containment glasshouse for phenotyping and genotyping. Agroinfiltration of the corresponding effector was used for the phenotyping. Genomic DNA from the individual T0 lines was isolated, and specific primers were designed for the Cas9 gene and the target genes. Amplicons from the target genes were sub-cloned into the pGEM-Teasy TA cloning vector (Promega, A1360) or pICSL86977 for sequencing. The sequencing data were analyzed in Geneious (v10.2.6).
Plant growth and transformation
The N. benthamiana and N. tabacum cv. Petit Gerard plants were sowed and grown in a controlled environment room (CER) at 22 °C and 45–65% humidity with a 16-h photoperiod. Four-week-old plants were used for the HR assay.
For the S. americanum transformation, sterilized seeds (SP2271 and SP2300) were sown in MS medium (2% sucrose). Leaf disks were cut from 4 to 6-week-old in-vitro plants. Overnight Agrobacterium (AGL1) culture (100 µl) and 200 µM acetosyringone were added to 20 ml of LSR broth and the leaf discs were gently dipped into the solution using sterile forceps for 20 min. The leaf discs were then removed from the Agrobacterium suspension, blotted dry, and incubated under low light conditions at 18–24 °C for 3 days. The dried leaf discs were plated on LSR1 + 200 µM Acetosyringone solid medium. Co-cultivated explants were transferred to LSR1 medium in petri dishes with selection antibiotics (about seven leaf discs per plate). Explants were subcultured onto fresh LSR1 medium approximately every 14 days. Once the calli were sufficiently developed they were transferred onto LSR2 medium. Subculturing continued every 14 days when shoots started to appear. The shoots were removed with a sharp scalpel and planted into MS2R solid medium with selection antibiotics. Transgenic plants harboring appropriate antibiotic or herbicide resistance genes should root normally by the fourth week and can then be weaned out of tissue culture into sterile peat blocks before being transferred to the glasshouse. Media used had the following components: LSR broth (1× MS medium, 3% sucrose, pH 5.7); LSR1 medium (1× MS medium, 3% sucrose, 2.0 mg L-1 zeatin riboside, 0.2 mg L-1 NAA, 0.02 mg L-1 GA3, 0.6% agarose, pH 5.7); LSR2 medium (1× MS medium, 3% sucrose, 2.0 mg L-1 zeatin riboside, 0.02 mg L-1 GA3, 0.6% agarose, pH 5.7); MS2R (1× MS medium, 2% Sucrose, 100 mg L-1 myo-inositol, 2.0 mg L-1 glycine, 0.2% Gelrite, pH 5.7).
Disease assay
P. infestans isolates T30-4 and 88069 were used for the disease test and were maintained on rye sucrose agar (RSA) medium in an 18 °C incubator. To induce zoospores, ice-cold water was added to the plates after 10–14 days. The plates were then incubated at 4 °C for 1–2 h and a hemocytometer was used to count the number of zoospores. The zoospore suspension was used for the DLA (100–500 zoospores per droplet).
BSA-RenSeq and map-based cloning
Three mapping populations were used in this study: (1) F2 populations of SP2271 × SP2272 and (2) BC1 and (3) F2 populations of SP2271 × SP2300. The populations were phenotyped by agroinfiltration of RXLR effectors. A cork borer was used for sampling, and the leaf discs from the responsive and non-responsive progenies were pooled. The Genomic DNA was isolated using the Qiagen DNeasy plant kit (Qiagen, 69104). RenSeq libraries were then prepared, as described previously24. The libraries were sequenced (Illumina 2 × 250-bp reads) by Novogene (Beijing, China). The SNP filtering and calling steps were described previously26.
To design molecular markers, the 10× PCR-free resequencing reads were mapped to the SP2271 S. americanum reference genome. Then SCAR markers that linked with the BSA-RenSeq signals were designed; amplicons should only be present in the non-responsive allele. The SCAR markers were first tested on the parental lines and the verified markers were then used on genomic DNA from individual non-responsive plants. GoTaq G2 DNA polymerase (Promega, 0000066542) was used for genotyping.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The raw sequencing data and genome assemblies of SP1102, SP2271, SP2273 and SP2275 genomes have been deposited at the National Center for Biotechnology Information (NCBI) Sequence Read Archive (SRA) with BioProject accession number PRJNA845062; The raw SMRT RenSeq data were deposited in ENA under project number PRJEB38240. The whole-genome resequencing data were deposited in ENA under project number PRJEB57057. The BSA-RenSeq data were deposited in ENA under project numbers PRJEB57070 and PRJEB57074. The assembled genomes, gene structure annotations, SMRT-RenSeq assemblies and manually annotated NLR genes as well as variation information are available at Figshare (https://figshare.com/projects/The_Solanum_americanum_pangenome_and_effectoromics_reveals_new_resistance_genes_against_potato_late_blight/145449). The SaNRC1-1102, SaNRC2-1102, SaNRC3-1102, Rpi-amr4-1102, Rpi-amr4-2271, R02860 (Rpi-amr16) and R04373 (Rpi-amr17) sequences were deposited at NCBI GenBank under accession number OP918030–OP918036. Source data are provided with this paper.
Code availability
Custom scripts and codes used in this study are available at Zenodo (https://doi.org/10.5281/zenodo.7928678)91.
Change history
25 September 2023
A Correction to this paper has been published: https://doi.org/10.1038/s41588-023-01550-4
References
Savary, S. et al. The global burden of pathogens and pests on major food crops. Nat. Ecol. Evol. 3, 430–439 (2019).
Fry, W. Phytophthora infestans: the plant (and R gene) destroyer. Mol. Plant Pathol. 9, 385–402 (2008).
Lokossou, A. A. et al. Exploiting knowledge of R/Avr genes to rapidly clone a new LZ-NBS-LRR family of late blight resistance genes from potato linkage group IV. Mol. Plant Microbe Interact. 22, 630–641 (2009).
Huang, S. et al. Comparative genomics enabled the isolation of the R3a late blight resistance gene in potato. Plant J. 42, 251–261 (2005).
Vossen, J. H. et al. The Solanum demissum R8 late blight resistance gene is an Sw-5 homologue that has been deployed worldwide in late blight resistant varieties. Theor. Appl Genet 129, 1785–1796 (2016).
Song, J. et al. Gene RB cloned from Solanum bulbocastanum confers broad spectrum resistance to potato late blight. Proc. Natl Acad. Sci. USA 100, 9128–9133 (2003).
Vossen, E. et al. An ancient R gene from the wild potato species Solanum bulbocastanum confers broad‐spectrum resistance to Phytophthora infestans in cultivated potato and tomato. Plant J. 36, 867–882 (2003).
van der Vossen, E. A. G. et al. The Rpi-blb2 gene from Solanum bulbocastanum is an Mi‐1 gene homolog conferring broad-spectrum late blight resistance in potato. Plant J. 44, 208–222 (2005).
Foster, S. J. et al. Rpi-vnt1.1, a Tm-22 homolog from Solanum venturii, confers resistance to potato late blight. Mol. Plant Microbe Interact. 22, 589–600 (2009).
Pel, M. A. et al. Mapping and cloning of late blight resistance genes from Solanum venturii using an interspecific candidate gene approach. Mol. Plant Microbe Interact. 22, 601–615 (2009).
Haas, B. J. et al. Genome sequence and analysis of the Irish potato famine pathogen Phytophthora infestans. Nature 461, 393–398 (2009).
Vleeshouwers, V. G. A. A. et al. Understanding and exploiting late blight resistance in the age of effectors. Annu. Rev. Phytopathol. 49, 507–531 (2011).
Xu, X. et al. Genome sequence and analysis of the tuber crop potato. Nature 475, 189–195 (2011).
Tomato Genome Consortium. The tomato genome sequence provides insights into fleshy fruit evolution. Nature 485, 635–641 (2012).
Wei, Q. et al. A high-quality chromosome-level genome assembly reveals genetics for important traits in eggplant. Hortic. Res. 7, 153 (2020).
Kim, S. et al. Genome sequence of the hot pepper provides insights into the evolution of pungency in Capsicum species. Nat. Genet. 46, 270–278 (2014).
Zhou, Q. et al. Haplotype-resolved genome analyses of a heterozygous diploid potato. Nat. Genet. 52, 1018–1023 (2020).
Sun, H. et al. Chromosome-scale and haplotype-resolved genome assembly of a tetraploid potato cultivar. Nat. Genet. 54, 342–348 (2022).
Hoopes, G. et al. Phased, chromosome-scale genome assemblies of tetraploid potato reveal a complex genome, transcriptome, and predicted proteome landscape underpinning genetic diversity. Mol. Plant https://doi.org/10.1016/j.molp.2022.01.003 (2022).
Zhao, Q. et al. Pan-genome analysis highlights the extent of genomic variation in cultivated and wild rice. Nat. Genet. 50, 278–284 (2018).
Liu, Y. et al. Pan-genome of wild and cultivated soybeans. Cell 182, 162–176 (2020).
Gao, L. et al. The tomato pan-genome uncovers new genes and a rare allele regulating fruit flavor. Nat. Genet. 51, 1044–1051 (2019).
Tang, D. et al. Genome evolution and diversity of wild and cultivated potatoes. Nature 606, 535–541 (2022).
Jupe, F. et al. Resistance gene enrichment sequencing (RenSeq) enables reannotation of the NB-LRR gene family from sequenced plant genomes and rapid mapping of resistance loci in segregating populations. Plant J. 76, 530–544 (2013).
Witek, K. et al. Accelerated cloning of a potato late blight–resistance gene using RenSeq and SMRT sequencing. Nat. Biotechnol. 34, 656–660 (2016).
Lin, X. et al. RLP/K enrichment sequencing; a novel method to identify receptor-like protein (RLP) and receptor-like kinase (RLK) genes. New Phytol. 227, 1264–1276 (2020).
Arora, S. et al. Resistance gene cloning from a wild crop relative by sequence capture and association genetics. Nat. Biotechnol. 37, 139–143 (2019).
Van de Weyer, A.-L. et al. A species-wide inventory of NLR genes and alleles in Arabidopsis thaliana. Cell 178, 1260–1272 (2019).
Witek, K. et al. A complex resistance locus in Solanum americanum recognizes a conserved Phytophthora effector. Nat. Plants 7, 198–208 (2021).
Lin, X. et al. A potato late blight resistance gene protects against multiple Phytophthora species by recognizing a broadly conserved RXLR-WY effector. Mol. Plant 15, 1457–1469 (2022).
Lin, X. et al. Identification of Avramr1 from Phytophthora infestans using long read and cDNA pathogen‐enrichment sequencing (PenSeq). Mol. Plant Pathol. 21, 1502–1512 (2020).
Zhang, C. et al. Genome design of hybrid potato. Cell 184, 3873–3883 (2021).
Moon, H. et al. Identification of RipAZ1 as an avirulence determinant of Ralstonia solanacearum in Solanum americanum. Mol. Plant Pathol. 22, 317–333 (2021).
Särkinen, T., Bohs, L., Olmstead, R. G. & Knapp, S. A phylogenetic framework for evolutionary study of the nightshades (Solanaceae): a dated 1000-tip tree. BMC Evol. Biol. 13, 214 (2013).
Rieseberg, L. Chromosomal rearrangements and speciation. Trends Ecol. Evol. 16, 351–358 (2001).
Yuan, Y., Bayer, P. E., Batley, J. & Edwards, D. Current status of structural variation studies in plants. Plant Biotechnol. J. 19, 2153–2163 (2021).
Barragan, A. C. & Weigel, D. Plant NLR diversity: the known unknowns of pan-NLRomes. Plant Cell 33, 814–831 (2021).
Wu, C.-H. et al. NLR network mediates immunity to diverse plant pathogens. Proc. Natl Acad. Sci. USA 114, 8113–8118 (2017).
Adachi, H. et al. An atypical NLR protein modulates the NRC immune receptor network in Nicotiana benthamiana. PLoS Genet. 19, e1010500 (2023).
Yang, L. et al. Potato NPH3/RPT2-like protein StNRL1, targeted by a Phytophthora infestans RXLR effector, is a susceptibility factor. Plant Physiol. 171, 645–657 (2016).
Sangija, F., Martin, H. & Matemu, A. African nightshades (Solanum nigrum complex): the potential contribution to human nutrition and livelihoods in sub-Saharan Africa. Compr. Rev. Food Sci. Food Saf. 20, 3284–3318 (2021).
Laflamme, B. et al. The pan-genome effector-triggered immunity landscape of a host–pathogen interaction. Science 367, 763–768 (2020).
Rietman, H. Putting the Phytophthora infestans genome sequence at work: multiple novel avirulence and potato resistance gene candidates revealed. PhD thesis (Wageningen University, 2011).
Oh, S.-K. et al. In planta expression screens of Phytophthora infestans RXLR effectors reveal diverse phenotypes, including activation of the Solanum bulbocastanum disease resistance protein Rpi-blb2. Plant Cell 21, 2928–2947 (2009).
Vleeshouwers, V. G. A. A. & Oliver, R. P. Effectors as tools in disease resistance breeding against biotrophic, hemibiotrophic, and necrotrophic plant pathogens. Mol. Plant Microbe Interact. 27, 196–206 (2014).
Pais, M. et al. Gene expression polymorphism underpins evasion of host immunity in an asexual lineage of the Irish potato famine pathogen. BMC Evol. Biol. 18, 93 (2018).
Zhu, S., Li, Y., Vossen, J. H., Visser, R. G. F. & Jacobsen, E. Functional stacking of three resistance genes against Phytophthora infestans in potato. Transgenic Res. 21, 89–99 (2012).
Ghislain, M. et al. Stacking three late blight resistance genes from wild species directly into African highland potato varieties confers complete field resistance to local blight races. Plant Biotechnol. J. 17, 1119–1129 (2019).
Derevnina, L. et al. Plant pathogens convergently evolved to counteract redundant nodes of an NLR immune receptor network. PLoS Biol. 19, e3001136 (2021).
Mapleson, D., Garcia Accinelli, G., Kettleborough, G., Wright, J. & Clavijo, B. J. KAT: a K-mer analysis toolkit to quality control NGS datasets and genome assemblies. Bioinformatics 33, 574–576 (2017).
Ranallo-Benavidez, T. R., Jaron, K. S. & Schatz, M. C. GenomeScope 2.0 and Smudgeplot for reference-free profiling of polyploid genomes. Nat. Commun. 11, 1432 (2020).
Cheng, H., Concepcion, G. T., Feng, X., Zhang, H. & Li, H. Haplotype-resolved de novo assembly using phased assembly graphs with hifiasm. Nat. Meth.18, 170–175 (2021).
Koren, S. et al. Canu: scalable and accurate long-read assembly via adaptive k-mer weighting and repeat separation. Genome Res. 27, 722–736 (2017).
Liu, H., Wu, S., Li, A. & Ruan, J. SMARTdenovo: a de novo assembler using long noisy reads. GigaByte https://doi.org/10.46471/gigabyte.15 (2021).
Li, H. & Durbin, R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25, 1754–1760 (2009).
Walker, B. J. et al. Pilon: an integrated tool for comprehensive microbial variant detection and genome assembly improvement. PLoS ONE 9, e112963 (2014).
Durand, N. C. et al. Juicer provides a one-click system for analyzing loop-resolution Hi-C experiments. Cell Syst. 3, 95–98 (2016).
Dudchenko, O. et al. De novo assembly of the Aedes aegypti genome using Hi-C yields chromosome-length scaffolds. Science 356, 92–95 (2017).
Simão, F. A., Waterhouse, R. M. & Ioannidis, P. BUSCO: assessing genome assembly and annotation completeness with single-copy orthologs. Bioinformatics 31, 3210–3212 (2015).
Kim, D., Langmead, B. & Salzberg, S. L. HISAT: a fast spliced aligner with low memory requirements. Nat. Meth. 12, 357–360 (2015).
Pertea, M. et al. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 33, 290–295 (2015).
Roberts, A. et al. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat. Protoc. 7, 562–578 (2012).
Grabherr, M. G. et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 29, 644–652 (2011).
Haas, B. J. et al. Automated eukaryotic gene structure annotation using EVidenceModeler and the program to assemble spliced alignments. Genome Biol. 9, R7 (2008).
Korf, I. Gene finding in novel genomes. BMC Bioinforma. 5, 59 (2004).
Stanke, M. & Morgenstern, B. AUGUSTUS: a web server for gene prediction in eukaryotes that allows user-defined constraints. Nucleic Acids Res. 33, W465–W467 (2005).
Majoros, W. H., Pertea, M. & Salzberg, S. L. TigrScan and GlimmerHMM: two open source ab initio eukaryotic gene-finders. Bioinformatics https://doi.org/10.1093/bioinformatics/bth315 (2004).
Slater, G. S. C. & Birney, E. Automated generation of heuristics for biological sequence comparison. BMC Bioinformatics. 6, 31 (2005).
Keilwagen, J., Hartung, F., Paulini, M., Twardziok, S. O. & Grau, J. Combining RNA-seq data and homology-based gene prediction for plants, animals and fungi. BMC Bioinformatics. 19, 189 (2018).
Emms, D. M. & Kelly, S. OrthoFinder: solving fundamental biases in whole genome comparisons dramatically improves orthogroup inference accuracy. Genome Biol. 16, 157 (2015).
Katoh, K. & Standley, D. M. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol. Biol. Evol. 30, 772–780 (2013).
Minh, B. Q. et al. IQ-TREE 2: new models and efficient methods for phylogenetic inference in the genomic era. Mol. Biol. Evol. 37, 1530–1534 (2020).
Yang, Z. PAML 4: phylogenetic analysis by maximum likelihood. Mol. Biol. Evol. 24, 1586–1591 (2007).
Marçais, G. et al. MUMmer4: a fast and versatile genome alignment system. PLoS Comput. Biol. 14, e1005944 (2018).
Li, H. Minimap2: pairwise alignment for nucleotide sequences. Bioinformatics 18, 3094–3100 (2018).
Cabanettes, F. & Klopp, C. D-GENIES: dot plot large genomes in an interactive, efficient and simple way. PeerJ 6, e4958 (2018).
Wang, Y. et al. MCScanX: a toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 40, e49 (2012).
Heller, D. & Vingron, M. SVIM-asm: structural variant detection from haploid and diploid genome assemblies. Bioinformatics https://doi.org/10.1093/bioinformatics/btaa1034 (2020).
Cingolani, P. et al. A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff: SNPs in the genome of Drosophila melanogaster strain w1118; iso-2; iso-3. Fly 6, 80–92 (2012).
Steuernagel, B. et al. The NLR-Annotator tool enables annotation of the intracellular immune receptor repertoire. Plant Physiol. 183, 468–482 (2020).
Kearse, M. et al. Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 28, 1647–1649 (2012).
Kalyaanamoorthy, S., Minh, B. Q., Wong, T. K. F., von Haeseler, A. & Jermiin, L. S. ModelFinder: fast model selection for accurate phylogenetic estimates. Nat. Meth. 14, 587–589 (2017).
Hoang, D. T., Chernomor, O., von Haeseler, A., Minh, B. Q. & Vinh, L. S. UFBoot2: improving the ultrafast bootstrap approximation. Mol. Biol. Evol. 35, 518–522 (2018).
Wu, T. D. & Watanabe, C. K. GMAP: a genomic mapping and alignment program for mRNA and EST sequences. Bioinformatics 21, 1859–1875 (2005).
Seong, K., Seo, E., Witek, K., Li, M. & Staskawicz, B. Evolution of NLR resistance genes with noncanonical N-terminal domains in wild tomato species. New Phytol. 227, 1530–1543 (2020).
Andolfo, G. et al. Defining the full tomato NB-LRR resistance gene repertoire using genomic and cDNA RenSeq. BMC Plant Biol. 14, 120 (2014).
Dobin, A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 (2013).
Letunic, I. & Bork, P. Interactive Tree Of Life (iTOL) v5: an online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 49, W293–W296 (2021).
Zhao, Y. et al. PanGP: a tool for quickly analyzing bacterial pan-genome profile. Bioinformatics 30, 1297–1299 (2014).
Bolger, A. M., Lohse, M. & Usadel, B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30, 2114–2120 (2014).
Jia Y. Codes of Solanum americanum genome-assisted discovery of immune receptors that detect potato late blight pathogen effectors. Zenodo https://doi.org/10.5281/zenodo.7928678 (2023).
Acknowledgements
This research was financed by BBSRC grants BB/P021646/1 (J.D.G.J.), BB/S018832/1 (J.D.G.J.), BB/W017423/1 (J.D.G.J.), the Gatsby Charitable Foundation (J.D.G.J.), National Key Research and Development Program of China (2019YFA0906200, S.H.), Guangdong Major Project of Basic and Applied Basic Research (2021B0301030004, S.H.), Agricultural Science and Technology Innovation Program (CAAS-ZDRW202101, S.H.), Shenzhen Outstanding Talents Training Fund (S.H.), National Research Foundation of Korea (2018R1A5A1023599 and 2023R1A2C3002366, K.H.S.) and New Breeding Technologies Development Program (PJ015799 and PJ016538, S.J.P.). We thank TSL transformation team (A. Wawryk-Khamdavong), SynBio team (M. Youles and L. Egan), media services (N. Stammars), bioinformatics team (D. MacLean and C. Jégousse) and horticultural team (S. Perkins, J. Smith, L. Phillips, C. Taylor, T. Wells, D. Alger and S. Able) for their support. We thank P. Robinson (JIC) for the scientific photography. We thank S. Marillonnet (Icon Genetics GmbH, Halle/Saale, Germany) for sharing the Cas9 construct (pAGM47523). We thank Experimental Garden and Genebank of Radboud University, Nijmegen, the Netherlands, IPK Gatersleben, Germany and S. Knapp (Natural History Museum, London, UK) for access to S. americanum genetic diversity. We thank V. G. A. A. Vleeshouwers at Wageningen University and Research, P. Birch, I. Hein and B. Harrower at James Hutton Institute for making available clones of some effectors. We thank B. B.H. Wulff, S. Arora and K. Gaurav (JIC) for helpful discussions.
Author information
Authors and Affiliations
Contributions
X.L. Y.J., K.H.S., S.H. and J.D.G.J. conceived and designed the project. X.L. and Y.J. wrote the first draft with input from all the authors. X.L., Y.J., K.H.S., S.H. and J.D.G.J. reviewed and edited the manuscript. Y.J., M.P., X.L., R.K.S. and J.H. performed the bioinformatics analyses. X.L. and M.M. performed the effectoromics screening. X.L., R.H., M.S., A.C.O.A., S.F. and A.N. contributed to cloning and characterization of Rpi-amr4, R02860 and R04373. M. Smoker and J.T. performed the plant transformation. K.W., Y.L., C.Z., S.J.P., K.H.S., S.H. and J.D.G.J. contributed resources.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Genetics thanks Doil Choi, Xiu-Fang Xin, and Jack H. Vossen for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Notes 1–3 and Figs. 1–19.
Supplementary Tables
Supplementary_Tables
Source data
Source Data Fig. 1
Phylogenetic relationship of S. americanum and neighboring species; genome synteny of S. americanum, potato and eggplant.
Source Data Fig. 4
The lesion size measurement.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lin, X., Jia, Y., Heal, R. et al. Solanum americanum genome-assisted discovery of immune receptors that detect potato late blight pathogen effectors. Nat Genet 55, 1579–1588 (2023). https://doi.org/10.1038/s41588-023-01486-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41588-023-01486-9
This article is cited by
-
Chromosome-scale assembly and gene editing of Solanum americanum genome reveals the basis for thermotolerance and fruit anthocyanin composition
Theoretical and Applied Genetics (2024)