Abstract
Cellular differentiation requires extensive alterations in chromatin structure and function, which is elicited by the coordinated action of chromatin and transcription factors. By contrast with transcription factors, the roles of chromatin factors in differentiation have not been systematically characterized. Here, we combine bulk ex vivo and single-cell in vivo CRISPR screens to characterize the role of chromatin factor families in hematopoiesis. We uncover marked lineage specificities for 142 chromatin factors, revealing functional diversity among related chromatin factors (i.e. barrier-to-autointegration factor subcomplexes) as well as shared roles for unrelated repressive complexes that restrain excessive myeloid differentiation. Using epigenetic profiling, we identify functional interactions between lineage-determining transcription factors and several chromatin factors that explain their lineage dependencies. Studying chromatin factor functions in leukemia, we show that leukemia cells engage homeostatic chromatin factor functions to block differentiation, generating specific chromatin factor–transcription factor interactions that might be therapeutically targeted. Together, our work elucidates the lineage-determining properties of chromatin factors across normal and malignant hematopoiesis.
Similar content being viewed by others
Main
Cell fate decisions are governed by the coordinated activities of transcription factors and chromatin factors, which together form gene regulatory complexes (GRCs), to orchestrate tissue-specific gene expression and cellular phenotypes1. The widescale description of transcription factor binding and its relationship to chromatin accessibility and gene expression, obtained from epigenomic and transcriptomic analyses across multiple developmental processes, have provided us with a highly developed understanding of the instructional role for transcription factors in governing cell fates2,3. Conversely, although the role of individual chromatin factors, particularly those mutated across malignancies4, are being elucidated, we still lack a global understanding of chromatin factor functions in cellular differentiation. Specifically, whether chromatin factors have specific or redundant roles during lineage differentiation, the identity of specific transcription factor–chromatin factor interactions and the molecular mechanisms that govern these interactions are unresolved questions.
We have chosen to address these fundamental questions in the exemplar process of hematopoiesis, where multiple mature cells with diverse, specific functions derive from a single self-renewing cell type, the hematopoietic stem cell (HSC)5. The study of hematopoiesis benefits from a comprehensive cellular blueprint3, which describes normal differentiation and malignant transformation, and a well-annotated molecular blueprint2,3,4, including detailed single-cell transcriptional landscapes and comprehensive maps of transcription factor activity derived from epigenomic and in vivo loss-of-function (LOF) studies across hematopoietic lineages6,7,8,9,10,11. In addition, the importance of both chromatin factors and transcription factors in hematopoietic differentiation has been further emphasized by recent studies, which have documented mutations that alter the function of transcription factors and chromatin factors to be highly recurrent and almost uniform in hematological malignancies such as acute myeloid leukemia (AML)12.
Given our increasing ability to manipulate the dynamic epigenome13, understanding chromatin regulation in normal and malignant hematopoiesis is key for harnessing regenerative therapeutics to restore damaged bone marrow function and in the treatment of hematological malignancies. However, many unanswered fundamental questions remain, including: What are the key interacting components (chromatin factors and transcription factors) of the GRCs that orchestrate lineage differentiation? Does their composition change during differentiation? Do chromatin factors have specificity for lineage determination and, if so, is this dependent on transcription factor recruitment to target loci? What cross talk occurs within the components of individual GRCs or between different GRCs? Which of these mechanisms are corrupted in leukemia and does this drive the induction or maintenance of the disease? In this study, we sought to answer many of these fundamental questions, combining comprehensive CRISPR-mediated knockout of multiple chromatin factors ex vivo and in vivo, with functional, epigenetic and transcriptional studies at both bulk and single-cell resolution.
Results
Functional screens of chromatin factors in hematopoiesis
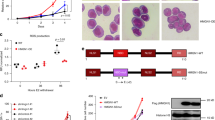
To interrogate the roles of chromatin factors in regulating normal hematopoiesis, we developed four screening platforms coupling cytokine-instructed differentiation of primary hematopoietic progenitors collected from mice, with fluorescence-activated cell sorting (FACS) readouts to study key lineage transitions (Fig. 1a,b): (1) self-renewal versus differentiation; (2) branching between myeloid and mega-erythroid lineages; (3) myeloid differentiation of multipotent progenitors; and (4) myeloid differentiation of myeloid-primed granulocyte-macrophage progenitors (GMPs). Cross-referencing the single-cell RNA sequencing (scRNA-seq)-derived expression profiles of each readout population to existing hematopoietic expression, we demonstrated fidelity with their in vivo counterparts (Extended Data Fig. 1a,b). Next, we generated a CRISPR library targeting 680 genes, including the vast majority of chromatin factors expressed by myeloid and mega-erythroid lineages (Supplementary Tables 1 and 2). We then delivered our library to both Cas9 (green fluorescent protein (GFP)+) and non-Cas9 (GFP−) progenitors ex vivo, cocultured them throughout our in vitro differentiation conditions, sorted ‘readout’ populations based on surface markers and quantified their single-guide RNA (sgRNA) distributions. Next, we calculated a lineage score for each chromatin factor by analyzing the differences in sgRNA content between populations, using the non-Cas9 distributions as a background. This analysis identified 142 chromatin factors with significant lineage scores in any of the four lineage transitions (Fig. 1c,d and Extended Data Fig. 1e). Finally, replicate screens for the strongest 200 chromatin factors demonstrated high correlation between replicates, indicating reproducible methodology (Extended Data Fig. 1d).
a, Schema of the experimental approach used, describing the murine progenitor populations used, their isolation and transduction with the CRISPR library, subsequent differentiation, flow sorting of specific differentiation readouts and read-count based analysis of function. CF, chromatin factor; TF, transcription factor. b, Differentiation systems and FACS-based readouts: (1) self-renewal versus differentiation; (2) lineage priming: mega-erythroid versus myeloid; (3) myeloid differentiation: mature myeloid versus non-myeloid; and (4) terminal myeloid maturation versus immature. c, Averaged lineage scores of ‘differentiation versus self-renewal’ (y axis) versus ‘lineage priming’ (x axis) for 554 genes. Significant hits (n = 93 genes with 50% or more significant guide RNAs (gRNAs) in either comparison) are shown with a red cross. NTC sgRNAs are shown with a blue cross. Data for non-Cas9 cells are shown in the background using a yellow-blue density. d, Lineage scores for chromatin factors grouped on the basis of complex membership. The dot color represents the lineage score, the dot size the percentage of significant guides (Supplementary Table 3). HDAC, histone deacetylase; PRMT, protein arginine methyltransferase. e, Exemplar immunophenotypic validations for chromatin factors in the lineage priming (top) and myeloid differentiation (bottom) systems. SSC-A, side scatter area.
Examination of the lineage scores revealed a high degree of phenocopy among factors belonging to the same complex, but also antagonistic behavior for specific chromatin factor families (Fig. 1c,d and Extended Data Fig. 1e). For instance, several members of the cohesin and mediator complexes (Stag2, Med20) were required to elicit differentiation toward myeloid or mega-erythroid fates, confirming dynamic chromatin looping as a general requirement for differentiation14,15. However, genes associated with the RNA elongation machinery (Phf5a, Ash1l) operated to preserve progenitor multipotency. As reported in other systems, H3K4 methyltransferases and chromatin remodelers showed high functional diversity16. Myeloid/lymphoid or mixed-lineage leukemia protein 4 (MLL4) complex genes (Kmt2d, Kdm6a) regulated progenitor identities and early myeloid priming, while histone-lysine N-methyltransferase Set1-like (SET1) complex components were required for differentiation and erythroid priming. BRG1- or BRM-associated factors (BAF) members16 behaved predominantly as pro-myeloid regulators, but nucleosome remodeling deacetylase (NuRD) and imitation switch (ISWI) factors17,18,19 facilitated mega-erythroid fates. Finally, and in contrast, certain repressive complexes demonstrated functional homogeneity, where heterochromatin (Setdb1, Cbx3), histone deacetylases (Hdac1 and Hdac3) and coREST20,21 members all functioned as myeloid repressors. Validating these results, the screen-based phenotypes of ten individual chromatin factor knockouts were confirmed by analyzing their ex vivo differentiation patterns (Fig. 1e and Extended Data Fig. 2a–d).
Collectively, these findings highlight substantial functional diversity within chromatin complexes, suggesting that specific chromatin factor subcomplexes work at different branches and stages along the differentiation trajectories.
Chromatin factor roles during in vivo hematopoiesis
Next, we used Perturb-seq to explore the functional diversity of 80 factors in their proper physiological context, in vivo hematopoiesis, at single-cell resolution. This list comprises 60 chromatin factors with strong dependencies combined with 20 lineage-specific transcription factors, with known functional effects, as control perturbations.
Experimentally, multipotent progenitors (Lin−Sca1+c-Kit+ (LSKs)) were transduced with targeted libraries before transplantation into sublethally irradiated mice. Thereafter, 2 weeks after transplant, we isolated their progeny (Lin− and Lin+c-Kit+ cells) and used Perturb-seq to jointly measure their transcriptomes and chromatin factor perturbations (Fig. 2a and Supplementary Fig. 5). This approach reconstituted the main hematopoietic lineages: progenitor (HSC) myeloid (GMP) (granulocyte progenitor, granulocytes and monocytes), mega-erythroid, basophil and lymphoid (B cell), with most cells spanning the myeloid/erythroid branches (Fig. 2b and Extended Data Fig. 3a–c).
a, Schematic drawing of the in vivo Perturb-seq: LSK progenitors were sorted from Cas9-GFP mice infected with the chromatin factor knockout library and transplanted into irradiated recipient mice; bone marrow was collected 14 days after transplant, sorted for an immature phenotype (lineage− and Lin+c-Kit+) and the Perturb-seq and downstream analyses were performed. b, Uniform manifold approximation and projection (UMAP) of the single-cell transcriptomes. Clusters are annotated using external reference maps56. The analysis integrates seven different biological replicates. CLP, common lymphoid progenitor; Eo/Ba, esosinophil-basophil progenitor; Ery, erythroblast; G1, G1 phase; Gran, granulocyte; IMP, immature myeloid progenitor; MEP, mega-erythroid progenitor; MKP, megakaryocyte progenitor; Mono, monocyte; S, S phase. c, UMAP showing the distribution of unperturbed cells (NTC sgRNAs) and specific perturbations. d, Scatterplot showing comparisons between experimental batches. Each dot represents the abundance of two NTC sgRNAs in a given population. Pearson correlation between NTC and sgRNAs per experimental batch = 0.962 with P = 1.20 × 10−67. e, Enrichment and depletion of chromatin factor knockouts across hematopoietic populations (Supplementary Table 4). Dot color and size relate to the log2 odds ratio (OR) and the percentage of significant enrichments. f, Effect of specific chromatin factor knockouts on myeloid versus erythroid priming. Positive values (red) show enhanced myeloid priming. Negative values (blue) indicate reduced myeloid priming. g, Trajectory analysis of specific chromatin factor knockouts along myeloid differentiation. Cells are ordered from HSCs to mature granulocytes using pseudotime. DC1, diffusion component 1; DC2, diffusion component 2. h–j, Graphic representation of the roles of key chromatin regulatory complexes.
To rule out knockout-independent confounding patterns arising from neutral selection and amplification of individual LSK clones, we compared the distribution of 14 different nontargeting control (NTC) guides across hematopoietic lineages in seven separate experiments. Different NTCs demonstrated a homogeneous distribution with high correlation (Pearson correlation = 0.96) confirming a robust approach (Fig. 2c,d). Conversely, sgRNAs targeting the lineage-determining transcription factors Cebpa (myeloid), Klf1 (erythroid) and Pax5 (B cell) were absent from their cognate lineages, as expected (Fig. 2d). In line with the ex vivo bulk results, LOF of chromatin factors demonstrated strong lineage-specific patterns and marked functional diversity (Fig. 2e,f and Extended Data Fig. 3e). To characterize their roles, we used three metrics: (1) assessment of chromatin factor knockout enrichment and depletion across the different hematopoietic lineages; (2) analysis of the effect of chromatin factor knockouts at key lineage branching points: myeloid versus erythroid and monocyte versus granulocyte; and (3) analysis of the progression of chromatin factor knockouts along myeloid and erythroid differentiation trajectories using pseudotime analysis (Fig. 2f–h and Extended Data Figs. 4a–d and 5a).
Like ex vivo, we found that disruption of cohesin (Stag2 knockout) blocked differentiation, causing accumulation of progenitors and myeloid deficiency (Fig. 2e). In line with previous studies22, perturbation of the COMPASS H3K4 methyltransferases revealed marked functional diversity (Fig. 2e–h and Extended Data Fig. 4a–d). As reported23 and like our ex vivo screen, SET1 catalytic subunits (Setd1a, Setd1b) were required for erythropoiesis and their perturbation eradicated erythroid differentiation, leading to myeloid and megakaryocytic progenitor accumulation. Perturbation of the SET1 structural subunit Wdr82 partially phenocopied Setd1a and Setd1B knockouts, generating a marked accumulation of megakaryocyte progenitors. However, knockout also defined additional roles for Wdr82 as a B cell and granulocyte regulator, suggesting that Wdr82 cooperates with different chromatin complexes during hematopoiesis. LOF of MLL1 (Kmt2a and Men1 knockout) also blocked terminal granulocytic differentiation but further enhanced B cell priming. Finally, the H3K4 mono-methyltransferase MLL4 (Kmt2d knockout) functioned as a pleiotropic regulator of HSCs self-renewal, early myeloid branching and monocyte versus granulocyte specification. Collectively, these results demonstrate a lack of redundancy between H3K4 methyl writers during hematopoiesis.
Analysis of BAF perturbation patterns showed similar phenotypic diversity (Fig. 2e–g,i). Disruption of the canonical BAF (cBAF) member Smarcd2 confirmed the strong myeloid dependency found ex vivo, inducing an early erythroid skewing and accelerated erythropoiesis, a similar pattern to the Smarcd2 knockout mouse24. Alternatively, disruption of the noncanonical (ncBAF) complex, defined by Brd9, caused a major blockade of B cell development. Moreover, despite a mild myeloid priming defect for Brd9 knockout, these cells did not undergo terminal myeloid differentiation but accumulated at the progenitor stages. In stark contrast, disruption of the polybromo-associated BAF (pBAF)-defining subunit Pbrm1 prevented erythropoiesis, augmenting myeloid and B cell outputs. These results reveal very different roles for BAF subcomplexes during hematopoietic differentiation.
As predicted in the bulk screens, disruption of complexes with repressive functions, including NuRD (Chd4 and Rbbp4), ISWI (Smarca5) and heterochromatin repressors (Atf7ip, Setdb1), produced a similar pattern characterized by accelerated granulocytic versus erythroid and B cell trajectories (Fig. 2e–g,h,j and Extended Data Fig. 5a). This suggests that most epigenetic repressors act to safeguard the diversity of progenitor identities25,26 and, by limiting extensive myelopoiesis, ensure balanced lineage output. In addition, coRest (Rcor1, Hdac1) and NuRD (Chd4) complex repressive activity proved crucial for terminal erythropoiesis and its depletion induced the accumulation of aberrant erythroid cells, which expressed high levels of both mature and progenitor markers and were rarely found in the unperturbed scenario (Extended Data Fig. 5b–f). Finally, disruption of Kdm6a, Hmgbx4 and Ash1l led to other aberrant populations not present in the unperturbed state (Extended Data Fig. 5b–e).
Transcriptional analysis of chromatin factor perturbations provided a molecular basis for the lineage dependencies observed for BAF and SET/MLL members, where Pbrm1 knockout and Setd1a knockout caused downregulation of erythroid regulators but Smarcd2 knockout strongly reduced myeloid markers and transcription factors (Extended Data Fig. 6a). In addition, perturbations that blocked differentiation trajectories—Rcor1, Wdr82, Stag2 and Brd9 knockout—upregulated stem cell transcription factors (Hoxa7, Meis1) and surface markers (Kit, Cd34), highlighting that these alterations may facilitate leukemic transformation. By contrast, perturbations of chromatin repressors that show a clear myeloid bias did not significantly alter the balance between erythroid and granulocytic programs, suggesting that subtler and cumulative changes drive these phenotypes. Indeed, gene set enrichment analysis of these perturbations revealed a marked upregulation of inflammatory pathways (tumor necrosis-α or JAK/STAT) and Jun/Fos targets, mediators known to enhance myeloid lineage outputs under inflammatory stimuli27 (Extended Data Fig. 6b).
Together, these results demonstrate functional diversity of chromatin factors in hematopoiesis similar to that of transcription factors. Interestingly and unlike transcription factors, most chromatin factor dependencies cannot be simply explained by their gene expression levels and patterns (Extended Data Fig. 5g), suggesting that other mechanisms like posttranscriptional regulation, protein complex assembly or differential recruitment explain their functional diversity.
Chromatin factors regulate lineage-determining transcription factor accessibility
Intrigued by these findings, we sought to elucidate the interactions between chromatin factors and transcription factors that may explain the observed lineage-specific dependencies. To this end, we CRISPR-engineered the knockout of ten strong lineage-dependent chromatin factors in multipotent progenitors and induced both lineage priming and myeloid differentiation. Thereafter, we used an assay for transposase-accessible chromatin with sequencing (ATAC-seq) to perform transcription factor footprinting analysis28 and define synergistic or antagonistic connections between chromatin factors and lineage-determining transcription factors that explain chromatin factor lineage dependencies (Fig. 3a–d and Extended Data Fig. 7a). In line with their in vivo effects, disruption of repressive factors (Rbbp4, Hdac3 and Setdb1) resulted in increased accessibility of transcription factors like Cebps, Hlf29 and AP-1 (ref. 30) that drive myelopoiesis on inflammation. This suggests that these repressive complexes attenuate a myeloid transcription factor-mediated program triggered by inflammatory cytokines. Conversely, myeloid-dependent chromatin factors (MLL4/Kmt2d, Smarcd2/cBAF and Wdr82) regulate chromatin accessibility around the binding sites of key myeloid-determining transcription factors (Cebp, Pu.1, Spib). Disruption of ncBAF (Brd9 knockout) demonstrated milder effects on myeloid transcription factor binding site accessibility, in line with its subtler in vivo effects. Interestingly, we detected different degrees of specificity of chromatin factor–transcription factor connections, where MLL4 (Kmt2d knockout) regulated the accessibility of a more restricted set of myeloid transcription factors (Cebp, AP-1 and Hlf) but Wdr82 mediates the accessibility of a larger transcription factor repertoire. Finally, we interrogated the dynamics of these effects using a time series analysis in Wdr82 and Kmt2d knockouts (Extended Data Fig. 7b–d). Wdr82 knockout induced reduction in myeloid transcription factor accessibility at early time points (day 3), followed by increased mega-erythroid accessibility at later time points (days 5–7), suggesting that Wdr82 directly interacts with myeloid transcription factors and that its depletion indirectly enhances mega-erythroid transcription factors activity as a secondary effect. In general, we could demonstrate that the effects on TF-motif accessibility are not related to a decreased TF expression following CF-KO (Supplementary Fig. 6), suggesting that specific interactions between chromatin factors and lineage-determining TFs coordinately drive lineage differentiation.
a, Schema of the experiment; LSK progenitors were collected from Cas9-GFP mice, transduced with a single chromatin factor or NTC control gRNA, placed into differentiation medium for 7 days and ATAC-seq was performed and analyzed to infer the interacting synergistic and antagonistic transcription factors. b, Chromatin accessibility for representative myeloid and mega-erythroid loci after disruption of specific chromatin factors. The accessibility profiles correspond to two merged replicate experiments. The S100a9, Ly6g, Hbba and Pf4 loci coordinates are chr3:90685583–90703276, chr15:75134355–75144437, chr7:103869996–103874051 and chr5:90771031–90773155, respectively. The y axis ranges for all knockouts in the same regions are 0.02–1.5, 0.02–1, 0.02–2 and 0.02–4, respectively. c, Volcano plot showing differentially bound transcription factor motifs (estimated by TOBIAS) in Wdr82 and Hdac3 knockouts under lineage priming conditions. Transcription factor motifs demonstrating gained and lost accessibility in each chromatin factor knockouts (compared to NTCs) are shown in green and purple, respectively. n = 2 biologically independent experiments. d, Heatmap summarizing the effect of ten chromatin factor knockouts on transcription factor motif footprints estimated by TOBIAS. n = 2 biologically independent experiments. Dot color and size relate to the log2 fold change and the −log10(Padj) value, respectively28.
ncBAF is required for terminal myeloid differentiation
Ex vivo chromatin footprinting of BAF perturbation could not explain the sequential requirement of cBAF versus ncBAF complexes in, respectively, early myeloid priming and myeloid maturation. We speculated that this may reflect incomplete recapitulation of myeloid progression in our ex vivo system; therefore, we used an in vivo system to further dissect the roles of BAF subcomplexes in myelopoiesis (Fig. 4a). First, using Perturb-seq at a later time point (28 days), we detected a massive expansion of Brd9 knockout clones that mapped predominantly to the multipotent (HSC) and myeloid progenitor (GMP) compartments (Fig. 4b,c). This confirmed that Brd9 is required for full myeloid maturation and revealed that Brd9 disruption conferred a competitive growth advantage to progenitors.
a, Schema of the in vivo perturbation of cBAF (Smarcd2 sgRNA) and ncBAF (Brd9 sgRNA); in vivo transplantation of LSK cells transduced with a library of Smarcd1, Smarcd2, Brd9 and NTC guides, into irradiated recipient mice and lineage-negative cells were collected and sorted at either 14 or 28 days for Perturb-seq as in Fig. 2. b, Proportions of cells with a specific sgRNA at 14 and 28 days after transplant. c, UMAP showing the distribution of Brd9 knockout and control (NTC sgRNA) cells at 28 days after transplant. d, Schema of the in vivo ATAC-seq experiment; Brd9 knockout LSK were generated and transplanted as in a and collected at day 28 for the ATAC-seq analysis. e, The ATAC-seq signal at myeloid progenitor (top) and differentiated (bottom) loci. Coordinates: Hoxa7, Hoxa9, Hoxa10: chr6:52214971–52236669, chr11:18912448–19036437; Meis1: chr6:88186822–8820729; Gata2: chr11:87788022–87796472; Mpo: chr3:90651905–90703284; S100a7, S100a8, S100a9: chr14:56098019–56107286; Ctsg: chr14:56098019–56107286. f, Volcano plot showing differentially bound transcription factor motifs (estimated by TOBIAS) between control (NTC) and Brd9 knockout GMPs. n = 2 biologically independent experiments. g, Genome browser tracks showing ATAC-seq, Cebpa ChIP–seq and Cebpe ChIP–seq in wild-type (WT) myeloid progenitors (GMPs). Loci coordinates are the same as in Fig. 4e. h,i, Quantification of accessibility changes between Brd9 knockout and control (NTC) GMPs. h, MA plot showing loci overlapping with Cebpa binding (red). i, Box plots showing accessibility loss (statistically tested using a two-sided Kolmogorov–Smirnov test, n = 2) at Cebpa (n = 11,316, statistic = 0.86, P = 1 × 10−323) and Cebpe sites (n = 10,409, statistic = 0.85, P = 1 × 10−323). The box plot displays the median as the center line of the box, with the box representing the distribution’s 25th (minima) and 75th (maxima) percentiles. The whiskers extend up to 1.5 times the interquartile range (IQR) (Q3–Q1) from the minima and maxima. j, Schema of the ChIP–seq analysis. k, Heatmaps showing specific binding of Smarcb1 (cBAF) and Brd9 (ncBAF) in myeloid progenitors (GMPs) and bone marrow monocytes. Two merged independent ChIP–seq experiments were used. l, Transcription factor motif enrichment measured by HOMER at the cBAF and ncBAF sites between progenitor (GMP) and mature (monocytes) myeloid cells. The axes represent the transcription factor motif odds-ratio (OR). All colored transcription factor motifs have Padj < 0.001. The analysis was performed with two independent experiments per population.
Chromatin accessibility analysis of Brd9 knockout myeloid progenitors (Fig. 4d) demonstrated an aberrant pattern, with a marked loss of accessibility at the myeloid maturation loci (Mpo, S100a7, S100a8, S100a9 and Ctsg) and at the motifs of Cebp and AP-1, transcription factors mediating terminal myeloid maturation (Fig. 4e,f). By contrast, progenitor loci (Hoxa7, Hoxa9, Hoxa10, Gata2, Meis1) and progenitor-associated transcription factor motifs, including GATA2, demonstrated increased accessibility (Fig. 4e,f). ChIP–seq of Cebpa and Cebpe in freshly sorted GMPs validated the motif analysis derived from ATAC-seq (Fig. 4g–i), confirming that Brd9 knockout leads to reduced accessibility at the binding sites of these two progranulocytic transcription factors.
Finally, corroborating these results, ChIP–seq analysis of Brd9 in myeloid progenitors (GMPs) and mature myeloid cells (monocytes) showed a strong enrichment for the Cebp and AP-1 (ATF4) motifs in mature myeloid cells and highlighted a switch in the transcription factor partnership of Brd9, from a broader spectrum in GMPs to a Cebp-AP-1-centric association in mature myeloid cells (Fig. 4j–l). We expanded this approach to other chromatin factor complexes and hematopoietic lineages to highlight lineage-specific transcription factor–chromatin factor interactions with regulatory potential; for example, a strong connection between Brd9 binding and the Ebf1 motif may explain the strong B cell dependency of ncBAF complex members (Extended Data Fig. 8a–e).
Together, these results demonstrate that specific transcription factor–chromatin factor interactions mediate lineage specification in vivo. In particular, myeloid maturation is governed by a transcription factor–chromatin factor switch, where ncBAF and Brd9 initially complex with a broad range of transcription factor partners before specifically interacting with Cebp factors for terminal differentiation. Furthermore, Brd9 loss induces a preleukemia-like phenotype of differentiation block and retention of a leukemia-associated transcriptional program, related, at least in part, to the failure to transition from ‘progenitor transcription factor programs’ to later differentiation programs.
Chromatin factors enforce differentiation blockade in leukemia
Having extensively dissected chromatin factor function in normal hematopoiesis, we decided to explore their roles in the aberrantly blocked differentiation states typical of leukemia. To this end, we chose an aggressive Npm1c and Flt3-ITD model, driven by the two most common co-occurring mutations in AML that synergize to generate a highly corrupted chromatin landscape, which recapitulates many aspects of human AML with the same genotype31. We isolated primary leukemia cells from Npm1c, Flt3-ITD Cas9 mice, cultured them and used cellular indexing of transcriptomes and epitopes sequencing (CITE-seq) to interrogate their differentiation status (Fig. 5a,b). This identified a core of leukemia stem cell-like cells with high levels of stem cell transcripts (Bcat1, Sox4) and markers (Cd34) and also subpopulations with more differentiated transcriptomes resembling granulocytic, erythroid, basophilic and megakaryocytic states, which demonstrated decreased fitness when isolated and grown in liquid cultures and clonogenic assays (Fig. 5c,d and Extended Data Fig. 9a–e).
a, UMAP projection of single-cell transcriptomes from Npm1c and Flt3-ITD primary leukemia. The color-coded clusters correspond to cells with specific signatures: leukemic stem cells (LSC, green), granulocyte-like (Gra.1 and Gra.2, Gran-perturbed, blue), erythroid-like (Ery.1 and Ery.2, red) and basophil-like (Baso-like, yellow). The analysis integrates datasets from six different Perturb-seq experiments. b, mRNA-derived and CITE-seq-derived expression of lineage makers in the differentiated leukemia subpopulations. c,d, Clonogenic (c) and proliferation (d) assays for differentiated leukemia subpopulations, isolated according to the strategy in Extended Data Fig. 9e. Colonies were counted after 7 days of culture in methylcellulose. Proliferation and clonogenic values were obtained from n = 4 biologically independent experiments. ****P < 0.0001 (two-way analysis of variance (ANOVA)). The error bars are the s.e.m., the midpoints show the mean. e, Enrichment analyses of specific chromatin factor knockouts across differentiated leukemia subpopulations. Dot color and size relate to the log2(OR) and the percentage of significant enrichments versus NTCs, respectively. The analysis is based on measurements for two sgRNAs per chromatin factor target. All values are shown in Supplementary Table 6. Gra.P1 and Gra.P2, granulocyte-like. f, Perturbed growth curves for leukemic chromatin factor knockouts. The assay measures the change in the proportion of blue fluorescent protein (BFP) BFP sgRNA-expressing cells over time, n = 4 biologically independent experiments. All ***P < 0.001, except for Smarcd1 versus NTC (day 9) where ***P = 0.0009 (two-way ANOVA). The error bars are the s.e.m., the midpoints show the mean. g, FACS analysis of mega-erythroid (CD55) and myeloid (CD11b, Gr1) surface differentiation markers in leukemia cells depleted for cBAF (Smarcb1 and Smarcd2 knockout) and MLL (Kmt2a knockout) components. h, FACS analysis of myeloid surface differentiation markers (CD11b, Gr1) in leukemia cells treated with increasing doses of Men1 inhibitor (revumenib). Raw data can be found in Supplementary Data 1.
Importantly, Perturb-seq analysis of 50 chromatin factor knockouts uncovered latent trajectories toward such differentiated endpoints (Fig. 5e and Extended Data Fig. 9f). Specifically, the depletion of MLL1-COMPASS (Kmt2a and Men1 knockouts), NCoR (Hdac3 knockout), ncBAF (Brd9 and Smarcd1-knockouts), Prmt5 and the heterochromatin regulators Setdb1 and Atf7ip induced transition toward several granulocytic states. In contrast, LOF of cBAF (Smarcd2 and Smarcb1 knockouts), MLL4-COMPASS (Kmt2d knockout) and Prmt1 pushed leukemia toward basophil and erythroid fates. Interestingly, unlike their roles in normal hematopoiesis, disruption of the repressive factors Smarca5, Chd4 and Rbbp4 induced the same erythroid trajectory. Finally, like the normal setting, LOF of Wdr82 generated a megakaryocytic state, which was absent from the unperturbed scenario (Extended Data Fig. 9g).
Analysis of the growth dynamics of several individual chromatin factor knockouts revealed a marked defect in cell growth, indicating that the differentiation caused by their depletion leads to leukemic exhaustion (Fig. 5e,f). This was especially pronounced for cBAF and COMPASS members, revealing Npm1c and Flt3-ITD leukemia to be highly dependent on the epigenetic activities regulated by these complexes. However, of interest and unlike previous reports for MLL-driven leukemias32,33, our Npm1c and Flt3-ITD model did not show vulnerability to Brd9 (ncBAF) disruption, highlighting that different leukemia mutations produce specific chromatin states that are variably dependent on individual chromatin factors.
Of note, our analysis found potential therapeutic targets in Npm1c and Flt3ITD leukemia, including Prmt1 (Fig. 5e and Extended Data Fig. 9h), which may be amenable to therapeutic exploitation. As a proof of principle for the therapeutic implications of our approach, treatment of the cells with the clinical grade menin inhibitor (revumenib, previously known as SNDX-5613), which is currently producing promising results in a clinical trial in KMT2A-mutated and NPM1-mutated AML (AUGMENT-01; ClinicalTrials.gov registration: NCT04065399) (ref. 34), recapitulated the Men1 and Kmt2a knockout single-cell phenotype to induce a dose-dependent granulocytic differentiation and decrease in proliferation (Fig. 5g,h and Supplementary Fig. 7).
These collective findings demonstrate how leukemias hijack chromatin factors involved in homeostatic differentiation to aberrantly block latent differentiation pathways and how this can be therapeutically exploited to facilitate leukemia exhaustion.
Chromatin factors engage in corrupted transcription factor interactions in leukemia
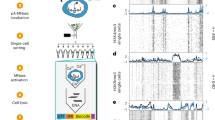
Finally, to interrogate the molecular mechanisms that underpin the requirement for the cBAF and COMPASS–MLL complexes in Npm1c and Flt3-ITD AML, and how these differ from normal hematopoiesis, we compared the genome-wide binding patterns of Smarcb1 (an exemplar of cBAF), Kmt2a (COMPASS-MLL1) and Kmt2d (COMPASS-MLL4) using ChIP–seq across leukemia, normal myeloid progenitors (GMP) and mature myeloid subsets (Fig. 6a). Of note, these analyses demonstrated marked redistribution of the cBAF and COMPASS–MLL1/MLL4 complexes on leukemia induction (Fig. 6a and Extended Data Fig. 10b–d), identifying three major binding patterns: (1) leukemic-specific, enriched in molecular functions such as tyrosine kinase signaling related to the Flt3-ITD mutation; (2) common to leukemia and myeloid progenitors; and (3) normal-specific.
a, Heatmaps showing ChIP–seq signal for cBAF (Smarcb1), MLL (Kmt2a) and MLL4 (Kmt2d) in leukemia (Npm1c and Flt3-ITD), in vivo myeloid progenitors (GMPs), in vivo monocytes and ex vivo-derived primary monocytes. n = 2 independent ChIP–seq experiments per factor. b, Motif enrichment analysis of cBAF, MLL and MLL4 binding patterns specific for leukemia (leukemic), common between leukemia and myeloid progenitors (common), and specific for normal myeloid cells (normal). c, Box plot showing the Stat5a, Runx1 and Runx2 binding signal (ChIP–seq) at the leukemic, common and normal loci defined in Fig. 6a (n = 2). The number of loci comprising the leukemic, common and normal categories are 2,019, 581 and 2,361 for Smarcb1; 2,427, 2,346 and 5,789 for Kmt2a; and 3,144, 810 and 4,129 for Kmt2d. d, Genome browser tracks showing the ChIP–seq signal for Smarcb1, Kmt2a, Kmt2d, Stat5a and Runx2 in leukemia, and ATAC-seq for control and chromatin factor-depleted leukemia cells. The chosen loci are leukemic-specific. n = 2 independent experiments. The green highlighted regions shown identify chromatin factor–transcription factor binding and altered accessibility on chromatin factor knockout. e, Box plots showing changes in chromatin accessibility at leukemic loci bound by Stat5a, Runx1 and Runx2 on depleting specific chromatin factors. cBAF, n = 2 independent experiments. Smarcd2 knockout: n = 1,385, 1,050, 1,711; statistic = 0.73, 0.75, 0.76; P = 5 × 10−15, 0, 0. Kmt2a knockout: n = 1,288, 695, 1,427; statistic = 0.78, 0.86, 0.78; P = 0, 9 × 10−16, 0. Kmt2d knockout: n = 1,482, 990, 1,913; statistic = 0.70, 0.70, 0.69; P = 2 × 10−15, 0, 0. The decay in accessibility was tested statistically using a two-sided Kolmogorov–Smirnov test. f, Growth curves for Cas9-leukemic cells expressing NTC and anti-Stat5a sgRNAs, n = 3 independent experiments. ***P < 0.001 (two-way ANOVA). The error bars are the s.e.m., the midpoints show the mean. The box plots in c and e display the median and the distribution’s 25th (minima) and 75th (maxima) percentiles. The whiskers extend up to 1.5 times the IQR (Q3–Q1) from the minima and maxima.
Motif analysis across the three binding patterns revealed subverted leukemic transcription factor–chromatin factor interactions (Fig. 6b), specifically between Stat and Runx transcription factors, and MLL1 and MLL4 and cBAF complexes. In contrast, Pu.1 and IRF factors were associated with cBAF specifically in normal cells. ChIP–seq profiling of Stat5a, Runx1 and Runx2 confirmed the motif analysis, demonstrating cobinding of these transcription factors and BAF or MLL1 and MLL4 complexes at key pro-leukemia genes including Hoxa7 and Hoxa9 (Fig. 6c,d and Extended Data Fig. 10e). Moreover, chromatin accessibility profiling of leukemia cells disrupted for cBAF (Smarcd2 knockout), MLL1 (Kmt2a knockout) or MLL4 (Kmt2d knockout) showed that these chromatin factors are required to maintain optimal accessibility at the Stat5a and Runx2 binding loci (Fig. 6d,e and Extended Data Fig. 10f). Finally, LOF of Stat5a in Npm1c and Flt3-ITD cells significantly reduced their proliferative fitness, confirming the importance of these transcription factor–chromatin factor switches for leukemic maintenance (Fig. 6f). Taken together, these findings demonstrate how individual chromatin factors required for normal myeloid lineage determination engage in corrupted interactions with alternative transcription factor partners to promote differentiation blockade, thereby maintaining cellular fitness in AML.
Discussion
In this study, we generated a detailed lineage dependency map for chromatin factors during hematopoiesis (Fig. 7a,b). We uncovered a remarkable phenotypic diversity that, for some chromatin factors, phenocopies key lineage-determining transcription factor functions (i.e. Smarcd2/Cebpa or Pbrm1/Klf1). Demonstrating the complex nature of chromatin factor regulation, we showed highly divergent roles for complexes that mediate the same, or very similar, epigenetic activities, including the different COMPASS H3K4 methyltransferases or the various BAF subcomplexes. The lack of redundancy among COMPASS complexes has been described in other systems35, suggesting lineage-specific requirements for H3K4 methylation deposition by particular COMPASS members or regulation via catalytic-independent roles36,37. In addition, reshaping of BAF complexes regulates cellular fates in pancreatic B cells38, and here we show evidence for another switch, from cBAF to ncBAF, which regulates myeloid differentiation, ensuring full lineage progression. Of note, Brd9 and ncBAF perturbation led to the accumulation of myeloid progenitors with a preleukemic gene expression program, mimicking the aberrant splicing of BRD9 that results in its degradation, a process mechanistically implicated in the AML precursor lesion, myelodysplastic syndrome39. Lastly, in stark contrast to functional diversity for some chromatin factors, we observed a common function for different chromatin repressors as attenuators of excessive granulopoiesis, suggesting repressive chromatin factors as a key buffering mechanism in the interplay between inflammatory signaling and chromatin state.
a, Roadmap of chromatin factor requirements for major hematopoietic cell fate decisions, identifying individual chromatin factors required for specific lineages and the transcription factor families they interact with to orchestrate these decisions. b, Table explaining the roles of specific chromatin factor–transcription factor complexes. c, Examples of how chromatin factor function is hijacked in leukemia, where cBAF-, MLL4- and MLL1-containing complexes block rather than facilitate hematopoietic differentiation. d, Examples of ‘transcription factor switches’ that mechanistically underpin the different functions of chromatin factors in normal and malignant hematopoiesis.
What then underlies such chromatin factor specificity? Inspired by previous studies40, we demonstrated that specific transcription factor–chromatin factor interactions mediate lineage diversification via the regulation of local accessibility and thus the binding site specificity of key lineage-determining transcription factors. However, as the chromatin factors investigated include a large number of proteins with diverse functions (remodelers, epigenetic readers, epigenetic writers, epigenetic erasers), we think it unlikely that a simple ‘one-size-fits-all’ mechanism governs chromatin factor–transcription factor interactions. We believe it more probable that the interactions are usually directed by transcription factors, which physically recruit specific chromatin factors to induce lineage-specific chromatin configurations41,42,43,44,45. However, an alternative and non-mutually exclusive explanation could be a sequential model, where specific chromatin factors, already deployed through multivalent interactions with epigenetic modifications, regulate subsequent transcription factor activity by modulating the chromatin state at the transcription factor binding sites. Specifically, we propose this as a possible mechanism whereby chromatin repressors attenuate myeloid pro-inflammatory transcription factor responses.
Regardless of the specific detail, central to the chromatin factor–transcription factor collaboration is the chromatin factor-mediated regulation and maintenance of locus accessibility and thus transcription factor binding46. As evidenced in our dynamic ATAC-seq studies, early alterations in accessibility are observed even after knockout of methyltransferase complex components (Kmt2d and Wdr82) that lack chromatin remodeling activity. Therefore, these data also inform interactions among chromatin factors with regulatory potential; the changes in accessibility suggest that the MLL4 and SET1 complexes recruit chromatin remodelers47, probably BAF members, an observation reinforced by the strong knockout phenocopy observed between Kmt2d and several cBAF subunits. However, whether this recruitment involves direct protein–protein interactions, or via an indirect mechanism mediated by a local pattern of histone methylation that, in turn, recruits remodelers via their own reader modules48, will require further investigation.
Finally, by studying chromatin factor requirement in AML, we highlight corrupted roles for certain chromatin factors in malignant hematopoiesis. Here, they alter their normal lineage regulatory role to conversely block differentiation in leukemia, reiterating that this blockade is an active process required for leukemic fitness (Fig. 7c). In characterizing these patterns, we also identified leukemic dependencies that may be amenable to inhibition or targeting, including Prmt1, Hdac3, Setdb1 and Kmt2d. Furthermore, we identified that these altered roles relate to leukemia-specific transcription factor–chromatin factor interactions, including COMPASS and BAF factors that rewire their transcription factor networks toward Runx and Stat transcription factors (Fig. 7d). These observations also have clinical implications; transcription factors and chromatin factors have pleiotropic effects across multiple tissues; however, targeting leukemia-specific transcription factor–chromatin factor interactions, important only for leukemia cells, will likely have much lower toxicity and higher specificity. This can be achieved chemically or through synthetic approaches49,50 and requires not only a detailed structural understanding of specific interactions, but also knowledge of the mechanisms governing individual transcription factor–chromatin factor associations51. Our study provides a blueprint to expand such approaches to leukemia.
Our approach combining large-scale CRISPR screening with downstream single-cell analysis in vivo could be readily deployed to assess the role of other classes of proteins in hematopoiesis or adapted to other organ and tumor systems. However, several limitations must be considered. First, our currently limited ex vivo differentiation readout could be supplemented by the use of other cytokine cocktails that permit interrogation of lymphoid lineages52. In addition, our in vivo approach does not completely reflect steady-state hematopoiesis, but more regenerative hematopoiesis in the after transplant setting. Thus, some of the roles described for individual chromatin factors, including the blockade of granulopoiesis by several repressors, may differ under steady-state conditions. Screening in homeostasis could be achieved by combining inducible Cas9 systems53,54,55 that permit inducible LOF of specific factors in steady-state conditions, after transplantation full reconstitution and a return to homeostatic hematopoiesis.
Taken together, the results of this study show that chromatin factors constitute a specific regulatory layer that should be accorded equal weighting with transcription factors when studying cell fate decisions. It lays the basis for additional, in-depth interrogation of specific chromatin factor–transcription factor interactions and functions, using multidisciplinary approaches ranging from in vivo functional approaches to protein–protein interactions, which we feel are warranted to further elucidate chromatin factor–transcription factor functions.
Methods
Mouse models
C57BL/6J (strain 000664, The Jackson Laboratory) and B6J.129(Cg)-Gt(ROSA)26Sortm1.1(CAG-cas9*/-EGFP)Fezh/J (strain no. 026179, The Jackson Laboratory) were used for all experimental procedures. The Npm1c/Flt3-ITD/Cas9 model has been extensively described previously31,57,58. The maximal tumor size allowed by the Home Office license for this project and authorized by the Animal Welfare Ethical Review Body at the University of Cambridge is 1.2 cm in diameter; however, as the animals developed a liquid not a solid tumor, in none of the experiments was this tumor size exceeded. Housing conditions were: 12h–12h dark–light cycle, at a temperature of 21 ± 1 °C and 40% humidity.
Bulk CRISPR screens
CRISPR library construction
sgRNA-CRISPR libraries (Supplementary Table 2) were ordered from Integrated DNA Technologies and cloned using a Gibson assembly mastermix (New England Biolabs) in the CRISPR sequencing (CRISP-seq) backbone (catalog no. 85707, Addgene).
Cloning of individual sgRNAs
Individual oligonucleotides were cloned in the CRISP-seq backbone (Supplementary Table 2) using a Golden-Gate reaction with a 100-ng vector backbone, 1 μl annealed sgRNA oligonucleotides, 1 μl Esp3I (New England Biolabs) and 1 μl T4 DNA Ligase (New England Biolabs) using the following program: 10× (5 min at 37 °C, 10 min at 22 °C), 30 min at 37 °C and 15 min at 75 °C. Individual colonies were picked and grown in lysogeny broth and ampicillin overnight. Plasmids were isolated with the ZymoPURE MiniPrep Kit (Zymo Research) and sequenced using a U6 forward primer (Supplementary Table 8).
Lentiviral production
HEK 293T (catalog no. 12022001-DNA-5UG, Sigma-Aldrich) cells were transfected with the CRISP-seq vectors, pMD2-G (plasmid no. 12259, Addgene) and psPAX2 (plasmid no. 12260, Addgene) using Lipofectamine 3000 according to the manufacturer’s protocol. After 10 h, media was replaced with Opti-MEM (Thermo Fisher Scientific) plus 1% penicillin-streptomycin (Thermo Fisher Scientific). The viral supernatant was collected 48 h after transfection, filtered using 0.45-μM filters and concentrated with a 100 Kda Centricon at 3,000g at 4 °C.
Isolation of murine hematopoietic progenitors (LSKs) from bone marrow
Femur, tibia, ileum, humerus, sternum and scapula were collected from 12–14-week-old C57BL/6J and ROSA-Cas9 mice (equal ratio of males and females), crushed in cold autoMACS Running Buffer and filtered through a 70-μM strainer. Erythrocytes were lysed and c-Kit+ cells were enriched using mouse CD117 magnetic beads (Miltenyi Biotec). The c-Kit-enriched fraction was stained with anti-lineage (B220, CD3, CD11b, Gr1, Ter-119), anti-CD117 (c-Kit) and anti-Sca1 (Supplementary Table 9). LSK cells were FACS-sorted in 1 ml DMEM/F-12 (Thermo Fisher Scientific) + 1× penicillin-streptomycin, centrifuged at 350g for 10 min and processed for ex vivo screens (see below). Dilution for all antibodies was 1:100, except 1:50 for CD34. An exemplar gating strategy can be found in Supplementary Fig. 1.
Ex vivo CRISPR screens cultures
After FACS sorting, multipotent (LSK) or myeloid (GMP) progenitors were resuspended at 250 cells per μl in DMEM/F-12 plus 1% penicillin-streptomycin-glutamine (Gibco), polyvinyl alcohol (PVA) 87% hydrolyzed (P8136, 363081 or 363146), 1× insulin-transferrin-selenium-ethanolamine (Gibco), 1× HEPES (Gibco), 100 ng ml−1 mouse TPO (PeproTech) and 10 ng ml−1 mouse SCF (PeproTech) and plated in 96-well plates with 25,000 cells (100 μl) per well. Immediately after plating, cells were transduced with the CRISPR libraries to reach a multiplicity of infection of approximately 20%. After 12 h, 2.5 volumes of fresh medium were added. Then, 48 h after infection, cells were transferred (1,000 cells per ml) to the ‘screen media’ and cultured.
Stem cell versus differentiation
Growth time was 7 days. The medium was complete DMEM/F-12, 1% penicillin-streptomycin-glutamine (Gibco), PVA 87% hydrolyzed (P8136, 363081 or 363146), 1× insulin-transferrin-selenium-ethanolamine (Gibco), 1× HEPES (Gibco), 100 ng ml−1 mouse TPO (PeproTech) and 10 ng ml−1 mouse SCF (PeproTech)59.
Lineage priming
Growth time was 5 days. The medium was complete DMEM/F-12, 1% penicillin-streptomycin-glutamine (Gibco), PVA 87% hydrolyzed (P8136, 363081 or 363146), 1× insulin-transferrin-selenium-ethanolamine (Gibco), 1× HEPES (Gibco), 100 ng ml−1 mouse TPO (PeproTech) and 10 ng ml−1 mouse SCF (PeproTech) + 1 ng ml−1 mouseFlt3L (PeproTech) and 1 U ml−1 Epo (R&D Systems).
Myeloid differentiation
Growth time was 4 days. The medium was IMDM (Thermo Fisher Scientific), 20% FCS (Thermo Fisher Scientific), 1% penicillin-streptomycin-glutamine (Gibco), 10 ng ml−1 mouse GM-CSF (PeproTech), 10 ng ml−1 mouse SCF (PeproTech), 5 ng ml−1 mouse G-CSF (PeproTech), 5 ng ml−1 mouse interleukin-3 (IL-3) (PeproTech), 5 ng ml−1 mouse interleukin-6 (IL-6) (PeproTech), 5 ng ml−1 mouse interleukin-5 (IL-5) (PeproTech), 5 ng ml−1 mouse Flt3L (PeproTech), 2 ng ml−1 mouse TPO (PeproTech) and 2 U ml−1 Epo (R&D Systems).
Terminal myeloid differentiation
Growth time was 2 days. The medium was IMDM, 20% FCS, 1% penicillin-streptomycin-glutamine (Gibco), 10 ng ml−1 mouse GM-CSF (PeproTech), 10 ng ml−1 mouse SCF (PeproTech), 5 ng ml−1 mouse G-CSF (PeproTech), 5 ng ml−1 mouse IL-3 (PeproTech), 5 ng ml−1 mouse IL-6 (PeproTech), 5 ng ml−1 mouse IL-5 (PeproTech), 5 ng ml−1 mouse Flt3L (PeproTech).
CRISPR FACS readouts
Cultures were collected and stained with the readout-specific antibody cocktails (below and Supplementary Table 9) plus a viability marker (TOPRO or propidium iodide). Cas9 (GFP+) and non-Cas9 (GFP−) populations were sorted in 1.5 ml PBS + 0.1% BSA (Thermo Fisher Scientific).
Stem cell differentiation
Multipotent progenitor (Lin−c-Kit+Sca-1+). Differentiated (Lin−c-Kit+Sca-1−).
Lineage priming
Myeloid progenitor (Lin−c-Kit+Sca-1+FcγRIII+). Mega-erythroid progenitor (Lin−c-Kit+Sca-1+FcγRIII+).
Myeloid differentiation
Mature myeloid (FcγRIII+CD11b+). Non-myeloid (FcγRIII−CD11b−).
Terminal myeloid differentiation
Mature myeloid (CD11b+Gr1+). Immature myeloid (CD11b−Gr1−). A dilution of 1:100 was used for all antibodies. Exemplar gating strategies can be found in Supplementary Figs. 2 and 3.
Bulk CRISPR library preparation, sequencing and preprocessing
Sorted cells were lysed in 40 μl of 0.2% SDS and 2 μl of proteinase K (New England Biolabs) at 42 °C for 30 min. Then, genomic DNA (gDNA) was isolated with a 2× solid-phase reversible immobilization (SPRI) cleanup and NGS libraries were prepared from purified gDNA with a two-step PCR protocol using 2× KAPA HiFi Master Mix (Roche): first PCR: 10 μM Read1-U6 and Read2 scaffold primer mix (Supplementary Table 2); 3 min at 98 °C; 20× (10 s at 98 °C, 10 s at 62 °C, 25 s at 72 °C); 2 min at 72 °C. Second PCR: 10 μM P5 and P7 index mix: 3 min at 98 °C; 10× (10 s at 98 °C, 10 s at 62 °C, 25 s at 72 °C); 2 min at 72 °C.
Libraries were purified with 1× SPRI cleanup and sequenced at 10 M reads per sample (paired-end 50 bp in a NextSeq 1000 system). Raw data were processed with bcl2fastq (v.2.20) into FASTQ files and then processed using a custom script (see 00_NR_CRISPR_extract.pi in the analysis code)60 to isolate the 20-mer protospacers; then, they were mapped using Bowtie2 (v.2.3.4.2) using an index file containing the sgRNA sequences (Supplementary Table 2). Raw counts can be found in Supplementary Data 2.
Computational analysis of FACS-based CRISPR screens
Analyses were performed in R (v.4.0.2). Biological replicates were merged by summing the counts. Aggregated counts were normalized by calculating normalizing factors using the function calcNormFactors from edgeR (v.3.32.1) (ref. 61) on nontargeting guide counts. Counts were transformed to counts per million (CPM) and log2-normalized using limma (v.3.46.0) (ref. 62). A raw lineage score comparing pairs of populations (A and B) was then calculated by subtracting the log2 CPMs of population A from population B, for each library of guides. To assess significance, we next calculated the probability of observing a given score in the Cas9 data given the non-Cas9 data, where no effective knockout occurs. For each comparison and each library, we centered and scaled the Cas9 data based on the mean and standard deviation calculated from the non-Cas9 data. The resulting normalized scores were used to calculate the probabilities of observing values as extreme (two-sided) using the function pnorm. The resulting probabilities represent the probability of an observed value given a background distribution but with the important difference to P values that in our analyses the background distribution was not based on replicates but on the non-Cas9 data. We next corrected these probabilities for multiple testing using the function p.adjust with the Benjamini–Hochberg method and selected values smaller than 0.05 as significant.
Validation of single candidates with flow cytometry
Cas9 progenitor cells (LSKs) were transduced with CRISPR sequencing lentiviral vectors, cultured and stained using the conditions described above, and analyzed with a FACSAria. FACS data were analyzed with FlowJo v.10.8.0 (FlowJo LLC).
Perturb-seq
Perturb-seq libraries
For each target, we cloned the top two performing sgRNAs in the lenti-Perturb-seq-BFP vector, which we built by modifying the original lenti-CRISPR-BFP vector by replacing the original sgRNA scaffold for a sgRNA scaffold containing the 10× capture-sequenced CR1Cs1 (ref. 63). Lentiviral particles were prepared as specified for bulk screens.
In vivo Perturb-seq
We performed seven experiments with 10–15 factors and two nontargeting control sgRNAs per batch. In each batch, 300,000 LSKs were isolated from 12–14-week-old ROSA26-Cas9 mice (equal ratio of males and females), and transduced with the Perturb-seq library to reach 10% infection (>1,000× coverage). After transduction, cells were left to recover for 36 h in stem cell medium: DMEM/F-12 plus 1% penicillin-streptomycin-glutamine (Gibco), PVA 87% hydrolyzed (P8136, 363081 or 363146), 1× insulin-transferrin-selenium-ethanolamine (Gibco), 1× HEPES (Gibco), 100 ng ml−1 mouse TPO (PeproTech) and 10 ng ml−1 mouse SCF (PeproTech)59. Then, cell number and viability were assessed with the Cellometer K2 Image Cytometer (Nexcelom Bioscience) and 50,000 viable cells were transplanted to each irradiated (902 cGy, 1 min) 12-week-old adult B6.SJL-Ptprca Pepcb/BoyJ (CD45.1) mice (strain no. 002014, The Jackson Laboratory) via tail injection.
After 2 weeks, mice were euthanized, c-Kit+ cells were isolated and stained with TOPRO (viability), anti-lineage (CD3, CD19, Ter119, CD11b, Gr1) and anti-CD117 (c-Kit) antibodies (1:100 dilution). Then, we gated GFP+ (Cas9) and BFP+ (sgRNA) cells, and FACS-sorted lineage− and lineage+c-Kit+ fractions. Cells from each of these gates were processed in the Chromium Controller to reach 500 cells per sgRNA. An exemplar gating strategy can be found in Supplementary Fig. 4.
Perturb-seq in leukemia
A total of 0.25–0.5 × 106 double-mutant (DM) Cas9 cells were transduced with Perturb-seq libraries using retronectin-mediated infection (Takara Bio) and maintained in culture for 6 days. Transduced, BFP+ and 7-AAD− (BD Biosciences) live cells were FACS-sorted (BD Influx, BD Biosciences). Finally, 16,000 live cells (cell number and viability assessed with the Cellometer K2 Image Cytometer) were processed in a 10× scRNA-seq partition aiming at a final coverage of 500 single cells per sgRNA.
Perturb-seq library preparation
Single-cell libraries were generated using the Chromium Next GEM Single Cell 3′ Reagent Kits v.3.1 (Dual Index) using the manufacturer’s recommended protocol. The resulting libraries were sequenced in a NovaSeq system to a final coverage of 50,000 reads per cell for 3′ Gene Expression libraries and 5,000 reads per cell for CRISPR Feature Barcode libraries.
CITE-seq
CITE-seq was performed on 2 × 106 DM Cas9 murine leukemic cells, stained with TotalSeq-B antibodies (BioLegend) for CD11b, Ly6C, CD115, CD14, CD150, CD48, CD34, CD117, CD55, CD41, CD326 and FcγRI (Supplementary Table 9) according to the manufacturer’s protocol. Stained cells were FACS-sorted for 7-AAD− (BD Biosciences) live cells (BD Influx; BD Biosciences).
scRNA-seq libraries were prepared at the Cancer Research UK Cambridge Institute Genomics Core Facility using the Chromium Single Cell 3′ Library & Gel Bead Kit v.3.1, Chromium Chip G Kit and Chromium Single Cell 3′ Reagent Kits v.3.1 User Guide (part no. CG000317 for CITE-seq). Libraries were sequenced in a NovaSeq system with a final coverage of 50,000 reads per cell for 3′ gene expression libraries and 5,000 reads per cell for antibody Feature Barcode libraries.
Perturb-seq and CITE-seq analysis
Analyses were performed in R (v.4.0.2) unless otherwise stated.
Basic processing and alignment
Raw reads were processed and aligned to the GRCm38/mm10 reference genome assembly (GENCODE vM23/Ensembl 98) using cellranger count (v.6.1.1).
Quality control and integration
Starting with the ‘filtered’ data matrix from cellranger, additional quality control and processing was performed. First, low-quality cells were filtered based on the number of detected genes, unique molecular identifiers (UMIs) and the percentage of mitochondrial reads using Seurat (v.4.0.0) (ref. 64). For each sample, the 90th percentile of cells was calculated based on the number of detected UMIs and the number of detected genes. Cells with less than 20% of the 90th percentile (and less than 500 genes and 1,000 UMIs as minimum cutoffs) were removed. Cells with more than 10% of mitochondrial reads were also removed. Second, cell cycle phases were inferred using the function CellCycleScoring from Seurat. Third, gRNAs were assigned to cells using the gRNA matrix provided by cellranger. In the case of multiple detected gRNAs per cell, guides matching 75% or more of the reads per cell were used. If no guide matched 75% or more of reads in a cell, this cell was left unassigned. Fourth, data were aligned across samples and cell cycle effects were removed using the function align_cds from Monocle 3 (v.0.2.3.0) (ref. 65). Finally, UMAP projection and clustering was performed using the functions reduce_dimension and cluster_cells from Monocle 3.
Cell type assignment (in vivo and ex vivo)
Cell types were predicted using the package singleR (v.1.4.1), based on a dataset from Izzo and colleagues56 and a dataset from the packages CytoTRACE (v.0.3.3) (ref. 66). SingleR67 was run using the Wilcoxon method for differential analysis. Cells in clusters with more than 80% of cells predicted as granulocytes, granulocyte progenitors or immature B cells in the bone marrow dataset from CytoTRACE were assigned based on this dataset. All other clusters were assigned based on the predictions by Ninkovic et al.45. Eosinophils and basophils were combined in one label. Cells predicted as erythrocytes were further split into MEPs, erythroid progenitors or erythrocytes based on a comparison of gene signatures with external datasets68 and on the expression of key marker genes: (1) low Gata2 and high Gata1, Epor and Klf1 marked the transition from MEPs to erythroid progenitors; (2) induction of Hba-a1 and Hbb-b1, increased Tfrc expression, enrichment of S and G1 cell cycle signatures mark the transition from erythroid progenitors to erythrocytes. Finally, cells coexpressing high levels of marker genes from the two distinct mature lineages (erythroid and myeloid) were removed as probable doublets or cells with contamination of ambient RNA.
Next, cells constituting less than 10% of a cluster were reassigned to the majority in each cluster. MEPs with Gata2 expression greater than Gata1 expression were labeled as early MEPs. MEP clusters with strong cell cycle phase signatures were labeled accordingly. A cluster of MEPs harboring predominantly Rcor1 knockouts was labeled as ‘erythroid perturbed’.
Cell type enrichment analyses
To test differences in the distributions of knockouts and NTCs, we tested the enrichment of knockouts compared to NTCs within each cell type. Clusters with fewer than five NTCs or less than 25% NTCs were removed from this analysis. A Fisher’s exact test was used with the function fisher.test. Enrichment was tested against each NTC separately. P values were adjusted using the function p.adjust with the Benjamini–Hochberg method.
Viability analysis
Counts of cells harboring knockouts at day 14 were transformed to CPMs and normalized to the number of cells harboring NTCs. The normalized cell counts were compared to the equally normalized read counts in the gRNA pool before cell infection, resulting in a log fold change that represents viability. Thus, negative or positive values represent an enrichment or loss, respectively of knockout-harboring cells at day 14 (after cell infection) relative to NTCs.
Cross-projection of ex vivo and leukemia samples to in vivo data
To project ex vivo and leukemia samples onto the in vivo data, we adapted the ProjecTILs algorithm (v.2.0.2) (ref. 69) predicting UMAP coordinates for each cell from the ex vivo and leukemia samples based on the UMAP coordinates of in vivo cells using a k-nearest neighbor approach with k = 20 neighbors.
Differential expression analysis
We performed differential expression comparing cells with chromatin factor knockouts to NTCs using nebula (v.1.1.8). We removed clusters with fewer than 31 cells and genes with fewer than 21 reads. We ran nebula70 with default parameters, testing differences of knockouts to NTCs with fixed effects (parameter ‘pred’) and adding sample information as random effects (parameter ‘id’). For genes, where the algorithm did not converge, we reran nebula with the ‘negative binomial lognormal mixed model’ model. P values were corrected for multiple testing using the function p.adjust with the Benjamini–Hochberg method. Gene set enrichment was performed using the function fgsea from the fgsea package71.
Pseudotime trajectory analysis
Pseudotime for the different lineage trajectories was identified using diffusion maps72 applied to the NTC population, using the SCANPY (v.1.9.1) (ref. 73) functions tl.diffmap and tl.dpt. Perturbed cells were then mapped to their nearest k = 15 nontargeting cells in the principal component analysis (PCA) space, considering the first n = 8 principal components, and then assigned the mean pseudotime value across these cells. PCA cutoffs were found via elbow plots by assessing the variance accounted for in the first n principal components. Each branch was extracted for separate analysis using the aforementioned cell labels (Fig. 2a) and pseudotime was scaled to the unit interval. We plotted perturbations with crucial biological significance, which incidentally showed visually striking distribution differences compared to the nontargeting cell population.
The CITE-seq read counts obtained from cellranger were normalized to log CPMs and then scaled. Perturb-seq data in leukemia were processed in the same way as the in vivo data, as described above. Enrichment analyses were performed on clusters instead of cell types.
Chromatin accessibility analysis of chromatin factor knockouts
Isolation of progenitors, CRISPR LOF and ex vivo differentiation
A total of 20,000 Cas9-LSK cells were transduced with the lenti-CRISPR-BFP virus, expressing the top performing sgRNA against each chromatin factor and cultured for 48 h under multipotent conditions (detailed above). Then, cells were stimulated with cytokine cocktails for lineage priming or myeloid differentiation for 5 days. For the time-course experiment, cells were perturbed and immediately grown for 3, 5 and 7 days under lineage priming or myeloid conditions. Finally, the CRISPR edited progeny was FACS-sorted (BFP+GFP+) into 1× PBS + 0.5% BSA and collected by centrifugation for ATAC-seq.
ATAC-seq was performed according to the Fast-ATAC protocol described in Corces et al.74. Briefly, 50,000 sorted cells were centrifuged at 500g for 7 min and resuspended into 25 μl Tagmentation Mix: 1× TD buffer (FC-121-1030, Illumina), 0.01% digitonin (Sigma-Aldrich), 0.1% Tween-20 (Sigma-Aldrich) and 0.1% NP-40 (Thermo Fisher Scientific). Tagmentation was performed at 37 °C for 30 min with agitation at 1,000 rpm. After the tagmentation reaction, 2 μl proteinase K, NaCl (150 mM final concentration) and SDS (0.3% final concentration) were added and the samples were incubated at 50 °C for 30 min. Then, gDNA was purified with SPRI Beads (Beckman Coulter) added at a 2× ratio; tagmented genomic regions were amplified using PCR with the KAPA Master Mix (Roche) and 5 μM P5 and P7 Nextera Indexing Primers (Supplementary Table 8) using the following program: 5 min at 72 °C, 2 min at 98 °C, 8× (98 °C for 20 s, 60 °C for 30 s, 72 °C for 1 min) and 5 min at 72 °C. The ATAC-seq libraries were sequenced at 50 million reads (paired-end 50 bp) on a NextSeq 1000 system.
Data processing and analysis
Based on the ATAC-seq nf-core pipeline, we ran Trim Galore (v.0.6.6) (ref. 75) with Cutadapt (v.3.4) (ref. 76) using the default parameters to trim low-quality and adapter sequences. We then aligned these reads to the GRCm38/mm10 reference genome assembly with decoy sequences using Bowtie2 (v.2.3.4.2) (ref. 77) with the following parameters: -X 1000 --no-discordant --no-mixed --very-sensitive. Then, we removed duplicated regions with Picard (v.2.25.4) (Broad Institute, https://broadinstitute.github.io/picard/), noninteresting chromosomes (for example, chrM, chrUn) and blacklisted regions included in the ENCODE blacklist (v.2.0) (ref. 65). Finally, we removed the Tn5 adapters with alignmentSieve (v.3.5.1) (ref. 78) (--ATACshift parameter) and indexed the final BAM files with SAMtools (v.1.3.1) (ref. 68). These BAM files were then processed to CPM-scaled BigWig files with bamCoverage (v.3.5.1) (ref. 79).
To identify the ATAC peaks, we pooled replicates and converted the paired BAM files to single-read BED format using the function bamToBed from BEDTools (v.2.27.1) (refs. 80,81). Then, we used MACS (v.2.2.7.1) (ref. 82) with the parameters --broad -f BED --keep-dup all --nomodel --shift -75 --extsize 150 to call peaks. To compare peak strength between conditions, we generated a unified peak set for all experiments, ending with, respectively, 376,658 and 207,724 peaks in LSKs and Npm1c or Flt3-ITD murine leukemic cells (DM cells), respectively. We then annotated these consensus peaks with the function annotatePeaks from HOMER (v.4.10) (ref. 83), counted the reads on them with featureCounts (v.2.0.1) (ref. 84) and calculated adjusted CPM values with the edgeR (v.3.34.1) trimmed mean of M-values method85. Additionally, we used DESeq2 (v.1.32.0) (ref. 73) to measure the fold change between conditions, defining the peaks with absolute log2(fold change) values greater than 0.75 and Padj values lower than 0.01 as differentially enriched. Finally, we filtered out peaks with fewer than two CPMs or ten reads in the compared conditions (knockout versus WT).
Motif analysis
To look for differential transcription factor motif enrichment between knockout and WT, we used TOBIAS (v.0.13.2) (ref. 28). Following the program guidelines, we generated a consensus set of peaks with all the peaks called previously in both the control and compared knockout sample. We then renamed and formatted HOMER’s list of vertebrate known motifs to make it suitable for TOBIAS. After generating the transcription factor footprint BigWig files (using the function ATACorrect with the parameters --read_shift 0 0 and the function ScoreBigwig with default parameters), we computed the differentially bound motifs with the function BINDetect.
Additionally, we used the output transcription factor binding coordinates of each of the motifs to measure the gain or loss of transcription factor union to chromatin for each chromatin factor knockout.
ChIP–seq analysis of normal and leukemic populations
Isolation of in vivo hematopoietic cells from bone marrow
Bone marrow cells were collected from 12–14-week-old C57BL6 mice as described above and stained for the isolation of the following cells: GMP: lineage− (CD3, CD19, CD11b, Gr1, Ter119, B220), c-Kit+, Sca-1−, FcγRIII+, CD34+; MEP: lineage− (CD3, CD19, CD11b, Gr1, Ter119, B220), c-Kit+, Sca-1−, FcγRIII−, CD34−; monocytes: CD3−, CD19−, Ter119−, CD11b+; B cell: CD3−, CD19+, Ter119−, CD11b−; erythroid cells were FACS-sorted from the spleens of 12-week-old C57BL6 mice as CD3−, CD19−, Ter119+, CD11b−, Gr1−. Dilution was 1:100 for all antibodies, except 1:50 for CD34 cells. Cells were sorted in PBS + 0.1% BSA and cross-linked immediately after sorting. The FACS antibodies are shown in Supplementary Table 9.
Isolation of leukemic cells
Npm1c/Flt3-ITD/Cas9 DM cells were generated from lineage-depleted bone marrow cells of primary transgenic mice after leukemia onset (female, 12 weeks old) as described previously57,58. Cells were maintained in XVIVO-20 medium (Lonza) supplemented with 5% FCS, 1% penicillin-streptomycin-glutamine, mouse SCF 50 ng ml−1 (PeproTech), mouse IL-3 10 ng ml−1 (PeproTech) and mouse IL-6 10 ng ml−1 (R&D Systems), in a 37 °C and 5% CO2 atmospheric environment. Npm1c/Flt3-ITD/Cas9 DM cells were passaged every 2 days and cultured for a short time (passages 3–5) to maintain the original leukemic properties.
Cross-linking
Freshly sorted normal cells or early passage (3–5) leukemic cells were cross-linked at room temperature with 3 mM ethylene glycol bis(succinimidyl succinate), disuccinimidyl glutarate and dimethyl adipimidate (Thermo Fisher Scientific) for 20 min followed by 1% formaldehyde (Thermo Fisher Scientific) for another 5 min. Then, glycine was added to 125 mM and incubated for 5 min to quench the cross-linkers. Finally, cells were pelleted at 750g for 7 min, washed twice with cold 0.5% BSA/PBS containing 1× cOmplete Protease Inhibitors (Roche) and flash frozen at −80 °C.
Chromatin immunoprecipitation
Cross-linked cells were thawed and resuspended in 1.5 ml ice-cold cell lysis buffer (10 mM HEPES, pH 7.5, 10 mM NaCl, 0.2% NP-40 (Thermo Fisher Scientific)) plus cOmplete Protease Inhibitors for 10 min on ice. Then, nuclei were pelleted at 5,000g for 7 min, resuspended in sonication buffer (0.5% SDS, 5 mM EDTA) and pelleted again at 8,000g, then resuspended in 50–100 μl sonication buffer and sonicated for five cycles (30 s ON, 30 s OFF) in a Bioruptor Nano (Diagenode). Then, chromatin extracts were diluted in four volumes of ChIP dilution buffer (25 mM HEPES, 185 mM NaCl, 1.25% Triton X-100 plus cOmplete Protease Inhibitors) and incubated with the relevant antibodies (Supplementary Table 10) at 4 °C for 10–12 h. The following day, 25 μl Magna ChIP Protein A + G (Merck Millipore) were added and incubated for 3 h at 4 °C. Bead-bound chromatin was washed twice with radioimmunoprecipitation assay (RIPA) buffer (10 mM Tris-Cl, pH 8, 150 mM NaCl, 0.1% SDS, 1% Triton X-100, 1 mM EDTA), twice with RIPA-500 buffer (10 mM Tris-Cl, pH 8, 500 mM NaCl, 0.1% SDS, 1% Triton X-100, 1 mM EDTA), twice with LiCl buffer (10 mM Tris-Cl, pH 8, 550 mM LiCl, 0.5% sodium deoxycholate, 0.5% NP-40, 1 mM EDTA) and once with TE buffer. ChIPped DNA was reverse-cross-linked by 30 min incubation with 2 μl proteinase K in 50 μl ChIP elution buffer (10 mM Tris-Cl, pH 8, 300 mM NaCl, 0.2 mM EDTA, 0.4% SDS) at 55 °C followed by 1-h incubation at 68 °C. Finally, the ChIPped DNA was purified with a 2.2× SPRI cleanup and quantified using the Qubit dsDNA HS Assay Kit (Thermo Fisher Scientific).
Every ChIP–seq experiment was performed in replicate except for Kmt2d and Kmt2a in early myeloid (GMP) and erythroid (MEP) progenitors. All attempts at replication were successful except for a failed Smarcb1 ChIP–seq experiment in MEPs, which was removed from the analysis and substituted by a third ChIP–seq experiment to reach n = 2.
Preparation of ChIP–seq libraries
ChIP–seq libraries were prepared from 0.5–10 ng of ChIPped DNA using the Next Ultra II kit (New England Biolabs) following the manufacturer’s instructions. ChIP–seq libraries were sequenced to 100 million reads per sample (paired-end 50 bp) in a NextSeq 1000 system and demultiplexed using bcl2Fastq (v.2.20).
ChIP–seq data processing and analysis
Based on the ChIP–seq nf-core pipeline86, we first processed the FASTQ files to BAM files as described for ATAC-seq, skipping the Tn5 adapter removal. (The statistics for each ChIP–seq experiment are detailed in Supplementary Table 11). Next, to identify the peaks for each sample, we pooled replicates and used MACS with the parameters -f BAMPE --keep-dup all. To compare peak strength between cell types, we generated a unified peak set per chromatin factor (Brd9, Kmt2a, Kmt2d and Smarcb1). We then followed the steps explained in the ATAC-seq data processing and analysis section to annotate the peaks and calculate the CPM reads on them. Finally, we measured the fold change between cell types using DESeq2 (ref. 85) to get peaks with an absolute log2(fold change) greater than 0.75 and a Padj lower than 0.01. Normalized ChIP–seq peak counts can be found in the Supplementary Data 3–6.
Motif analysis in ChIP–seq peaks
We first generated a list of cell type-specific peak coordinates for each of the analyzed chromatin factors. To do so, we selected all peaks that were significantly enriched or depleted in pairwise comparisons of GMP, myeloid, MEP, erythroid and B cell on each of the chromatin factors. Then, we clustered and manually curated these coordinates to get a list of cell type-specific peaks per chromatin factor. Enrichment of transcription factors in cell type-specific peak coordinates was then analyzed using the function findMotifsGenome from HOMER (v.4.10). For each chromatin factor, cell type-specific peaks were compared to all peaks found across all five cell types and all four chromatin factors as background. Motif enrichment analyses were centered on the 100 bp surrounding the peak summit.
Comparison of normal and leukemic patterns
To identify transcription factor switches in leukemia, we defined subsets of peaks that were gained in DM AML cells (leukemic), shared in DM and GMP cells (common) and not present in DM cells but present in GMPs and monocytes (normal). Gained peaks were defined by log2(fold change) values greater than 1, lost peaks by log2(fold change) values smaller than −1, and shared peaks by absolute log2(fold change) values lower than 0.5. The subsetting was done per chromatin factor in a consensus peak dataset of Smarcb1, Kmt2a and Kmt2d. Similar to motif analysis in ChIP–seq peaks section, we looked for enrichment of transcription factors in each of the subsets using the function findMotifsGenome from HOMER; all peaks from the consensus (across all subsets) were used as the background set.
To measure the Stat5a binding signal over the chromatin factor-bound sites, we collected Stat5a ChIP–seq sequencing data at the same consensus coordinates stated above and computed the CPM values as described in the ATAC-seq data processing and analysis section.
Functional assays in leukemia
Cell transduction and sorting of differentiated populations
Npm1/Flt3-ITD/Cas9 DM murine cells were transduced using a retronectin-transduction protocol (Takara Bio) with lenti-Perturb-seq-BFP vectors targeting Smarcb1, Smarcd2, Brd9 and Smarcd1, Kmt2a, Kmt2d, Wdr82, Hdac3, Setdb1, Stat5a or NTC. Then, growth was monitored by flow cytometry (LSRFortessa II; BD Biosciences) of BFP+ cells in culture at 2, 6, 9 and 13 days after transduction. Fold change in BFP+ cells at each time point relative to the proportion of BFP+ cells at day 2 was calculated. Immunophenotypic analysis was performed by flow cytometry at 6 days by staining with anti-CD11b, anti-Ly6G/Ly6C (Gr1) and anti-CD55 (Supplementary Table 9). The proportions of granulocyte-like (CD11bhighGr-1+) and erythroid/basophil-like (CD55high) were quantified with FlowJo (v.10.8.1). P values were calculated using a two-way ANOVA or ratio-paired t-test (Prism v.9.1, GraphPad Software).
Clonogenic and cell proliferation assays of DM AML cells
A total of 2 × 106 DM murine leukemic cells were stained for CD11b, Gr-1, CD55, CD41 and CD34 (Supplementary Table 9). Granulocyte-like (CD11bhighGr-1+), erythroid/basophil-like (CD55highCD41−) and CD34+ fractions were subsequently FACS-sorted (BD Influx; BD Biosciences). One thousand cells of each sorted population were seeded in 1 ml methylcellulose medium (M3434, STEMCELL Technologies) supplemented with recombinant mouse SCF (PeproTech) and mouse IL-3 (PeproTech) with recombinant IL-6 and Epo (R&D Systems) in duplicate. Methylcellulose cultures were maintained at 37 °C and 5% CO2; total colony forming units (CFUs) were enumerated 7 days later. Photographs of colonies were obtained with the STEMvision instrument and software (STEMCELL Technologies). Data are presented as a fold change of average CFUs per 1,000 cells seeded, relative to the CD34+ fraction. Ten thousand granulocyte-like, erythroid/basophil-like or CD34+ DM cells were also maintained in standard culture conditions for 21 days, and the number of cells was counted every 7 days. The total cell number is presented as a fold to the corresponding CD34+ counterpart for each time point. P values were calculated using a two-way ANOVA (Prism v.9.1, GraphPad Software).
Statistics and reproducibility
No statistical method was used to predetermine sample size. The lineage scores (bulk CRISPR screens) of the top 200 hits were validated in replicate screens. Likewise, Perturb-seq was performed in replicate for the top 40 chromatin factors. Epigenetic profiling (ATAC-seq and ChIP–seq) was performed in two biologically independent replicates (except for Kmt2d and Kmt2a ChIP–seq in MEPs) following a common practice in the field. Growth curves of chromatin factor knockouts were calculated from three to four independent experimental batches. Immunophenotypic patterns derived from chromatin factor perturbation were replicated at least in two biologically independent experiments.
Data exclusion
No data points were excluded from any of the analyses except for one ChIP–seq experiment (Smarcb1 in MEPs), which was excluded from the analysis due to a low signal-to-noise ratio. This data point was substituted by another ChIP–seq experiment to reach n = 2.
Randomization
Cell-based assays (screens and validations) and mouse allocation were randomized; proper batch designs were ensured to avoid confounding effects. In the Perturb-seq analysis, we used several NTCs across all experimental batches. The investigators were not blinded to allocation during experiments and outcome assessment.
Ethical compliance
Murine ethical compliance was fulfilled under the Guidelines of the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committees at the University of Navarra, Spain, and the Animal Welfare Ethical Review Body at the University of Cambridge, UK. Research in the UK was conducted under Home Office license PP3042348.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Bulk expression patterns of hematopoietic populations: Gene Expression Omnibus (GEO) accession no. GSE60103. Single-cell expression patterns of hematopoiesis: GEO accession no. GSE124822. Perturb-seq datasets (in vivo, ex vivo and leukemic): GEO accession no. GSE213511. Chromatin accessibility of CF-knockouts: GEO accession no. GSE213506. ChIP–seq datasets of chromatin factors (in vivo, ex vivo and leukemic): GEO accession no. GSE213507. Source data are provided with this paper.
Code availability
Analysis code is available in a dedicated GitHub repository at https://github.com/csbg/tfcf (ref. 87).
References
Stadhouders, R., Filion, G. J. & Graf, T. Transcription factors and 3D genome conformation in cell-fate decisions. Nature 569, 345–354 (2019).
Ding, Y., Liu, Z. & Liu, F. Transcriptional and epigenetic control of hematopoietic stem cell fate decisions in vertebrates. Dev. Biol. 475, 156–164 (2021).
Al-Mousawi, J. & Boskovic, A. Transcriptional and epigenetic control of early life cell fate decisions. Curr. Opin. Oncol. 34, 148–154 (2022).
Valencia, A. M. & Kadoch, C. Chromatin regulatory mechanisms and therapeutic opportunities in cancer. Nat. Cell Biol. 21, 152–161 (2019).
Laurenti, E. & Göttgens, B. From haematopoietic stem cells to complex differentiation landscapes. Nature 553, 418–426 (2018).
Orkin, S. H. & Zon, L. I. Hematopoiesis: an evolving paradigm for stem cell biology. Cell 132, 631–644 (2008).
Cedar, H. & Bergman, Y. Epigenetics of haematopoietic cell development. Nat. Rev. Immunol. 11, 478–488 (2011).
Wilson, N. K. et al. Combinatorial transcriptional control in blood stem/progenitor cells: genome-wide analysis of ten major transcriptional regulators. Cell Stem Cell 7, 532–544 (2010).
Downing, J. R. The core-binding factor leukemias: lessons learned from murine models. Curr. Opin. Genet. Dev. 13, 48–54 (2003).
Robb, L. et al. The scl gene product is required for the generation of all hematopoietic lineages in the adult mouse. EMBO J. 15, 4123–4129 (1996).
Rosenbauer, F. & Tenen, D. G. Transcription factors in myeloid development: balancing differentiation with transformation. Nat. Rev. Immunol. 7, 105–117 (2007).
Papaemmanuil, E. et al. Genomic classification and prognosis in acute myeloid leukemia. N. Engl. J. Med. 374, 2209–2221 (2016).
Michalak, E. M. et al. The roles of DNA, RNA and histone methylation in ageing and cancer. Nat. Rev. Mol. Cell Biol. 29, 573–589 (2019).
Cuartero, S. et al. Control of inducible gene expression links cohesin to hematopoietic progenitor self-renewal and differentiation. Nat. Immunol. 19, 932–941 (2018).
Sasca, D. et al. Cohesin-dependent regulation of gene expression during differentiation is lost in cohesin-mutated myeloid malignancies. Blood 134, 2195–2208 (2019).
Centore, R. C., Sandoval, G. J., Soares, L. M. M., Kadoch, C. & Chan, H. M. Mammalian SWI/SNF chromatin remodeling complexes: emerging mechanisms and therapeutic strategies. Trends Genet. 36, 936–950 (2020).
Laugesen, A. & Helin, K. Chromatin repressive complexes in stem cells, development, and cancer. Cell Stem Cell 14, 735–751 (2014).
Lai, A. & Wade, P. Cancer biology and NuRD: a multifaceted chromatin remodelling complex. Nat. Rev. Cancer 11, 588–596 (2011).
Vaicekauskaitė, I., Sabaliauskaitė, R., Lazutka, J. R. & Jarmalaitė, S. The emerging role of chromatin remodeling complexes in ovarian cancer. Int. J. Mol. Sci. 23, 13670 (2022).
Yu, L. et al. An erythroid-to-myeloid cell fate conversion is elicited by LSD1 inactivation. Blood 138, 1691–1704 (2021).
Gillespie, M. A. et al. Absolute quantification of transcription factors reveals principles of gene regulation in erythropoiesis. Mol. Cell 78, 960–974 (2020).
Kerimoglu, C. et al. KMT2A and KMT2B mediate memory function by affecting distinct genomic regions. Cell Rep. 20, 538–548 (2017).
Li, Y. Setd1a and NURF mediate chromatin dynamics and gene regulation during erythroid lineage commitment and differentiation. Nucleic Acids Res. 44, 7173–7188 (2016).
Priam, P. et al. SMARCD2 subunit of SWI/SNF chromatin-remodeling complexes mediates granulopoiesis through a CEBPɛ dependent mechanism. Nat. Genet. 49, 753–764 (2017).
Koide, S. et al. Setdb1 maintains hematopoietic stem and progenitor cells by restricting the ectopic activation of nonhematopoietic genes. Blood 128, 638–649 (2016).
Summers, A. R. et al. HDAC3 is essential for DNA replication in hematopoietic progenitor cells. J. Clin. Invest. 123, 3112–3123 (2013).
Paudel, S. et al. Regulation of emergency granulopoiesis during infection. Front. Immunol. 13, 961601 (2022).
Bentsen, M. et al. ATAC-seq footprinting unravels kinetics of transcription factor binding during zygotic genome activation. Nat. Commun. 11, 4267 (2020).
Gombart, A. F., Grewal, J. & Koeffler, H. P. ATF4 differentially regulates transcriptional activation of myeloid-specific genes by C/EBPε and C/EBPα. J. Leukoc. Biol. 81, 1535–1547 (2007).
Wahlestedt, M. et al. Critical modulation of hematopoietic lineage fate by hepatic leukemia factor. Cell Rep. 21, 2251–2263 (2017).
Yun, H. et al. Mutational synergy during leukemia induction remodels chromatin accessibility, histone modifications and three-dimensional DNA topology to alter gene expression. Nat. Genet. 53, 1443–1455 (2021).
Zhou, L., Yao, Q., Li, H. & Chen, J. Targeting BRD9 by I-BRD9 efficiently inhibits growth of acute myeloid leukemia cells. Transl. Cancer Res. 10, 3364–3372 (2021).
Hohmann, A. F. et al. Sensitivity and engineered resistance of myeloid leukemia cells to BRD9 inhibition. Nat. Chem. Biol. 12, 672–679 (2016).
Wang, E. S. et al. Preliminary data on a phase 1/2A first in human study of the menin-KMT2A (MLL) inhibitor KO-539 in patients with relapsed or refractory acute myeloid leukemia. Blood 136, 7–8 (2020).
Crump, N. T. & Milne, T. A. Why are so many MLL lysine methyltransferases required for normal mammalian development? Cell. Mol. Life Sci. 76, 2885–2898 (2019).
Douillet, D. Uncoupling histone H3K4 trimethylation from developmental gene expression via an equilibrium of COMPASS, Polycomb and DNA methylation. Nat. Genet. 52, 615–625 (2020).
Dorighi, K. M. et al. Mll3 and Mll4 facilitate enhancer RNA synthesis and transcription from promoters independently of H3K4 monomethylation. Mol. Cell 66, 568–576 (2017).
Wei, Z. Vitamin D switches BAF complexes to protect β cells. Cell 173, 1135–1149 (2018).
Inoue, D. et al. Spliceosomal disruption of the non-canonical BAF complex in cancer. Nature 574, 432–436 (2019).
Barisic, D., Stadler, M. B., Iurlaro, M. & Schübeler, D. Mammalian ISWI and SWI/SNF selectively mediate binding of distinct transcription factors. Nature 569, 136–140 (2019).
Liu, L. et al. Histone methyltransferase MLL4 controls myofiber identity and muscle performance through MEF2 interaction. J. Clin. Invest. 130, 4710–4725 (2020).
Sandoval, G. J. et al. Binding of TMPRSS2-ERG to BAF chromatin remodeling complexes mediates prostate oncogenesis. Mol. Cell 71, 554–566 (2018).
Vierbuchen, T. et al. AP-1 transcription factors and the BAF complex mediate signal-dependent enhancer selection. Mol. Cell 68, 1067–1082 (2017).
Huang, X. et al. OCT4 cooperates with distinct ATP-dependent chromatin remodelers in naïve and primed pluripotent states in human. Nat. Commun. 12, 5123 (2021).
Ninkovic, J. et al. The BAF complex interacts with Pax6 in adult neural progenitors to establish a neurogenic cross-regulatory transcriptional network. Cell Stem Cell 13, 403–418 (2013).
Schick, S. et al. Acute BAF perturbation causes immediate changes in chromatin accessibility. Nat. Genet. 53, 269–278 (2021).
Park, Y.-K. et al. Interplay of BAF and MLL4 promotes cell type-specific enhancer activation. Nat. Commun. 12, 1630 (2021).
Lange, M. et al. Regulation of muscle development by DPF3, a novel histone acetylation and methylation reader of the BAF chromatin remodeling complex. Genes Dev. 22, 2370–2384 (2008).
Holmes, A. G. et al. A MYC inhibitor selectively alters the MYC and MAX cistromes and modulates the epigenomic landscape to regulate target gene expression. Sci. Adv. 8, eabh3635 (2022).
Speltz, T. E. et al. Targeting MYC with modular synthetic transcriptional repressors derived from bHLH DNA-binding domains. Nat. Biotechnol. 41, 541–551 (2023).
Kadoch, C. Diverse compositions and functions of chromatin remodeling machines in cancer. Sci. Transl. Med. 11, eaay1018 (2019).
Trotman-Grant, A. C. et al. DL4-μbeads induce T cell lineage differentiation from stem cells in a stromal cell-free system. Nat. Commun. 12, 5023 (2021).
Lundin, A. et al. Development of an ObLiGaRe doxycycline inducible Cas9 system for pre-clinical cancer drug discovery. Nat. Commun. 11, 4903 (2020).
Bowling, S. et al. An engineered CRISPR–Cas9 mouse line for simultaneous readout of lineage histories and gene expression profiles in single cells. Cell 181, 1410–1422 (2020).
Dubrot, J. et al. In vivo screens using a selective CRISPR antigen removal lentiviral vector system reveal immune dependencies in renal cell carcinoma. Immunity 54, 571–585 (2021).
Izzo, F. et al. DNA methylation disruption reshapes the hematopoietic differentiation landscape. Nat. Genet. 52, 378–387 (2020).
Mupo, A. et al. A powerful molecular synergy between mutant nucleophosmin and Flt3-ITD drives acute myeloid leukemia in mice. Leukemia 27, 1917–1920 (2013).
Vassiliou, G. S. et al. Mutant nucleophosmin and cooperating pathways drive leukemia initiation and progression in mice. Nat. Genet. 43, 470–475 (2011).
Wilkinson, A. C. et al. Long-term ex vivo haematopoietic-stem-cell expansion allows nonconditioned transplantation. Nature 571, 117–121 (2019).
csbg/tfcf: published code. Zenodo https://doi.org/10.5281/zenodo.7891703 (2023).
Robinson, M. D., McCarthy, D. J. & Smyth, G. K. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26, 139–140 (2010).
Ritchie, M. E. et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 43, e47 (2015).
Replogle, J. M. et al. Combinatorial single-cell CRISPR screens by direct guide RNA capture and targeted sequencing. Nat. Biotechnol. 38, 954–961 (2020).
Hao, Y. et al. Integrated analysis of multimodal single-cell data. Cell 184, 3573–3587 (2021).
Trapnell, C. et al. The dynamics and regulators of cell fate decisions are revealed by pseudotemporal ordering of single cells. Nat. Biotechnol. 32, 381–386 (2014).
Gulati, G. S. et al. Single-cell transcriptional diversity is a hallmark of developmental potential. Science 367, 405–411 (2020).
Aran, D. et al. Reference-based analysis of lung single-cell sequencing reveals a transitional profibrotic macrophage. Nat. Immunol. 20, 163–172 (2019).
Lara-Astiaso, D. et al. Immunogenetics. Chromatin state dynamics during blood formation. Science 345, 943–949 (2014).
Andreatta, M. et al. Interpretation of T cell states from single-cell transcriptomics data using reference atlases. Nat. Commun. 12, 2965 (2021).
He, L. et al. NEBULA is a fast negative binomial mixed model for differential or co-expression analysis of large-scale multi-subject single-cell data. Commun. Biol. 4, 629 (2021).
Korotkevich, G. et al. Fast gene set enrichment analysis. Preprint at bioRxiv https://doi.org/10.1101/060012 (2021).
Haghverdi, L., Büttner, M., Wolf, F. A., Buettner, F. A. & Theis, F. J. Diffusion pseudotime robustly reconstructs lineage branching. Nat. Methods 13, 845–848 (2016).
Wolf, F. A., Angerer, P. & Theis, F. J. SCANPY: large-scale single-cell gene expression data analysis. Genome Biol. 19, 15 (2018).
Corces, M. R. et al. Lineage-specific and single-cell chromatin accessibility charts human hematopoiesis and leukemia evolution. Nat. Genet. 48, 1193–1203 (2016).
Krueger, F., James, F., Ewels, P., Afyounian, E. & Schuster-Boeckler, B. Zenodo FelixKrueger/TrimGalore: v0.6.7 https://doi.org/10.5281/zenodo.5127899 (2021).
Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 17, 10–12 (2011).
Langmead, B. & Salzberg, S. L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 4, 357–359 (2012).
Amemiya, H. M., Kundaje, A. & Boyle, A. P. The ENCODE blacklist: identification of problematic regions of the genome. Sci. Rep. 9, 9354 (2019).
Ramírez, F. et al. deepTools2: a next generation web server for deep-sequencing data analysis. Nucleic Acids Res. 44, W160–W165 (2016).
Danecek, P. et al. Twelve years of SAMtools and BCFtools. Gigascience 10, giab008 (2021).
Quinlan, A. R. & Hall, I. M. BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics 26, 841–842 (2010).
Zhang, Y. et al. Model-based analysis of ChIP-Seq (MACS). Genome Biol. 9, R137 (2008).
Heinz, S. et al. Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol. Cell 38, 576–589 (2010).
Liao, Y., Smyth, G. K. & Shi, W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 30, 923–930 (2014).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014).
Wolf, E. et al. Miz1 is required to maintain autophagic flux. Nat. Commun. 4, 2535 (2013).
Zhou, Y. et al. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat. Commun. 10, 1523 (2019).
Acknowledgements
We thank M. Wiederstein, University of Salzburg, for HPC support, K. Kania from the CRUK Genomics Core for assistance with single-cell methods and N. Cano and M. Dawes for superb administrative support. We thank D. Kent and A. Sebe-Pedrós for their constructive advice and discussions regarding the manuscript. The CRISPR sequencing-BFP backbone (plasmid no. 85707, Addgene) was produced by I. Amit, Weizmann Institute of Science. pMD2.G (plasmid no. 12259, Addgene) and psPAX2 (plasmid no. 12260, Addgene) was produced by D. Trono, École Polytechnique Fédérale de Lausanne. This project was funded by the ‘La Caixa’ Foundation (agreement no. LCF/PR/HR20/52400016, B.J.P.H., F.P.), Marie Skłodowska-Curie International Fellow (no. 886474, D.L.-A.), a European Hematology Association Junior Research Grant (D.L.-A.), Cancer Research UK Programme Grant (no. DRCRPG-Nov22/100014, B.J.P.H.), the European Research Council (no. 647685, B.J.P.H.), the Cancer Research UK Cambridge Major Centre (no. C49940/A25117, B.J.P.H.), a Kay Kendall Leukaemia Fund Junior Fellowship (no. KKL1440, N.N.), the National Institute for Health and Care Research (NIHR) Cambridge Biomedical Research Centre (no. BRC-1215-20014, B.J.P.H.), the UK Research and Innovation Medical Research Council (MRC) (no. MC_PC_17230, B.J.P.H.) supporting the Wellcome–MRC Cambridge Stem Cell Institute, Wellcome Trust (no. 203151/Z/16/Z, B.J.P.H.). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript. The views expressed are those of the authors and not necessarily those of the NIHR or the Department of Health and Social Care. For the purpose of open access, the author has applied a CC BY public copyright license to any author-accepted manuscript version arising from this submission.
Author information
Authors and Affiliations
Contributions
D.L.-A. and B.J.P.H. conceptualized the study. D.L.-A., A.G.-S., N.N., C.D.V., G.G., M.N.-A, T.B., J.Z., L.P.A.-A., F.M., N.T., I.A.C., C.K.L., D.A., A.L. and B.S. devised the methodology and data collection. D.L.-A., J.M.-E., T.G., J.P.T.-K., N.F. and B.J.P.H. carried out the data analysis. D.L.-A., J.M.-E., N.F. and B.J.P.H. visualized the data. D.L.-A., F.P. and B.J.P.H. acquired the funding. B.J.P.H. managed the project. D.L.-A., N.F. and B.J.P.H. supervised the project. D.L.-A. and B.J.P.H. wrote the original draft. D.L.-A., J.M.-E., N.F. and B.J.P.H. reviewed and edited the manuscript draft.
Corresponding authors
Ethics declarations
Competing interests
T.G. and J.P.T.-K. are employees receiving compensation from Relation Therapeutics. J.P.T.-K. is a founder of Relation Therapeutics. D.L.-A. is a consultant of Relation Therapeutics. The other authors declare no competing interests.
Peer review
Peer review information
Nature Genetics thanks Simon Haas, Thomas Milne, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Characterization of CRISPR Screen systems.
(a) Comparison of expression profiles of the different readout populations from our screens to lineage specific signatures from 3 different studies, named along the bottom of the graph. Comparisons are based on enrichment analyses between the screen signatures and the reference signatures. Dot colour and size relate to log2 odds ratio and -log10 adjusted p-value, respectively. CLP – Common Lymphoid Progenitor, MkP – Megakaryocyte Progenitor, IMP – Immature Myeloid Progenitor, Ery1-4 – Erythroblasts, Neu1-4 – Neutrophils, Mono1-3 – Monocytes. P-values were calculated using the Fisher’s exact test. (b) Comparison between the expression profiles of FACS-sorted populations from our ex vivo systems and a single-cell map of normal haematopoiesis56. Bulk transcriptomic signatures derived from FACS-sorted populations were projected on the single-cell map from Izzo and colleagues. (c) Example distribution of the CF lineage scores calculated from the Cas9 (green border) and Non-Cas9 (grey) populations. (d) Replicate analysis for 200 CFs screened in a second experiment under Self-renewal (top) and Lineage Priming conditions (bottom). (left) heatmaps showing correlation (Spearman) between two replicates, (right) scatter plots showing correlation (Spearman) between replicates. P-values are based on the algorithm AS 89 using the function cor.test in R. (e) Lineage scores for all hits. The color of each dot represents the aggregated lineage score. The size represents the number of significant guides, as per key to the right. All values are shown in Supplementary Table 3.
Extended Data Fig. 2 Validation of the effects of individual CF-KOs.
(a-b) Heatmap showing changes in representative populations for each CF-KO compared to a Non-Targeting Control (Fold-change in population abundances versus NTC) under lineage-priming (a) and Myeloid differentiation and terminal Myeloid maturation (b). The Myeloid master regulator Pu.1 (Spi1) was included as a positive control. Gates and values for the selected populations are derived from Supplementary Fig. 2c, d. (c-d) Exemplar FACS plots showing validation results for individual CF-KOs under lineage priming conditions (c) and Terminal Myeloid Differentiation (d). These validations were performed in different batches. Each batch included a Non-Targeting Control condition. All results were compared with the NTC included in each batch.
Extended Data Fig. 3 Characterization of the in vivo Perturb-seq system.
(a) Comparison of expression profiles from our in vivo single-cell clusters and external cell-type signatures from Izzo et al, 202056. (b) Number of cells per cell type. (c) UMAP projection of the Lineage- and Lineage+ ckit+ fractions. Color scale represents the number of cells in each area. (d) Number of cells with a sgRNAs targeting specific CFs. CF-KOs for which less than 50 cells (in red) were detected were removed from subsequent analysis. (e) Visualization of TF- and CF-KO patterns derived from in vivo Perturb-seq of Chromatin Regulatory Complexes during lineage specification. The distribution of NTCs is shown as background in grey in all plots. Cells are aggregated and the color of each area represents the density of cells in each area.
Extended Data Fig. 4 Extended in vivo Perturb-seq analysis of Chromatin Regulatory Complexes during lineage specification.
(a) Enrichment analyses of CF-KOs across 11 cellular states spanning the main hematopoietic lineages, all values are shown in Supplementary Table 4. Dot color and size relate to the log2 odds ratio and the percent of significant enrichments versus NTCs, respectively. The analysis is based on measurements of two aggregated sgRNAs per CF target. (b) CF-KO effects on early Myeloid versus Erythroid lineage branching, positive values (red) indicate CF-KOs leading to increased Myeloid outputs. (c) CF-KO effects on Myeloid versus Erythroid total outputs, positive values (red) indicate CF-KOs leading to increased Myeloid outputs. (d) CF-KO effects on Granulocyte versus Monocyte total outputs, positive values (red) indicate CF-KOs leading to increased monocytic outputs. (e) CF-KO effects on viability/survival of CF-KOs after 14 days post-transplant. Negative values (blue) indicate that cells with specific CF-KOs have growth/engraftment disadvantages a when compared to the Control (NTC harboring) cells. Positive values (red) indicate that cells with CF-KOs have growth advantages when compared to the Control (NTC harboring) cells.
Extended Data Fig. 5 Extended in vivo Perturb-seq analysis of Chromatin Regulatory Complexes during lineage specification.
(a) Heatmap summarizing trajectory analysis for CF-KOs along Myeloid and Erythroid branches ordered from HSCs to mature lineage using pseudotime. Colors are given by signed negative log10 p-values (for p<0.01) generated by a t-test between targeting and non-targeting control populations such that negative values correspond to reduced differentiation capability and positive values correspond to increased differentiation capability. (b-e) Analysis of aberrant cellular states generated after specific CF-KOs. (b) UMAP showing localization of 53 subclusters across the hematopoietic landscape. (c) Plot showing the abundance of CF-sgRNAs with respect to Control-sgRNAs across the 53 hematopoietic subclusters. Clusters deviating from the diagonal are rare or absent in the unperturbed scenario. (d) Enrichment of specific CF-KO cells in three representative aberrant subclusters: Erythroid-perturbed (cluster 26) and Granulocytic-Perturbed (clusters 45 and 51). (e) Marker genes of aberrant clusters. (f) Functions specific of the Erythroid-perturbed cluster (26). P-values were calculated by random sampling as implemented in the fgsea R package. (g) Barplots showing the expression levels of selected Chromatin and Transcription factors. The bars represent the normalized read counts taken from an RNA-seq dataset68.
Extended Data Fig. 6 Extended analysis of transcriptomic effects of CF-KOs.
(a) Analysis of the effect of Chromatin factor disruption (CF-KOs) on lineage specific expression patterns, comprising markers and transcription factors specific for progenitor, Myeloid, Erythroid, Megakaryocytic, Basophil and B-cell lineages. The color of each dot represents the log2 fold change (compared to NTCs), the size represents the –log10 adjusted p-value, as per key to the bottom right. P-values were calculated using negative binomial mixed models from the nebula R package. All values are shown in Supplementary Table 5. (b) Gene set enrichment analysis (GSEA) of differentially expressed genes in knockouts of factors belonging to repressive complexes. The color of each dot represents normalized enrichment score, the size represents the –log10 adjusted p-value. P-values were calculated by random sampling as implemented in the fgsea R package.
Extended Data Fig. 7 Extended analysis of effects of CF-KOs on chromatin accessibility and TF footprints.
(a) MA plots demonstrating differential accessibility analysis between selected CF-KOs and Control (NTC). Up- and down-regulated genomic loci are indicated in red and blue, respectively. (lower panel) Volcano Plots showing the differentially bound TF motifs (estimated by TOBIAS) between the same CF-KOs and Control (NTC). Gained and lost footprints are indicated in red and blue, respectively, n = 2 independent experiments. (b) Time-series analysis of chromatin accessibility dynamics under ex vivo priming conditions at day 3, 5 and 7, n=2 independent experiments. (c) Effect of Kmt2d- and Wdr82-KOs on the differential accessible patterns derived from the time-series analysis. n=8436, 19015, 9383, and 9275 for all conditions and days in the mid-late, lost, late, and transient clusters, respectively (n=2). Boxplots display the median and the distribution’s 25th (minima) and 75th (maxima) percentiles. The whiskers extend up to 1.5 times the interquartile range (Q3-Q1) from the minima and maxima. (d) Time-series analysis of differentially bound TF motifs (estimated by TOBIAS) under lineage priming conditions for Kmt2d- and Wdr82-KOs.
Extended Data Fig. 8 In vivo binding patterns of BAF and COMPASS complexes.
(a) CF binding at representative loci in Myeloid (GMP) and Erythroid (MEP) progenitors. Genomic Coordinates: Cebpa chr7:35,114,878-35,131,210; Cebpe chr14:54,702,383-54,717,520; Elane chr10:79,871,207-79,893,610 ; Gfi1b chr2:28,585,038-28,624,000; Hbb chr7:103,845,151-103,886,745; Car2 chr3:14,855,264-14,912,573. (b) Heatmaps showing lineage-specific binding patterns for each CF. (c) Heatmap showing joint analysis of Smarcb1/cBAF and Brd9/ncBAF binding in Myeloid progenitors (GMPs) and in mature Myeloid cells (monocytes). This analysis shows that Smarcb1/cBAF has widespread binding at early Myeloid stages while Brd9/ncBAF exhibits more presence in mature Myeloid cells (Monocytes). Strong overlap between cBAF and ncBAF complexes seems limited to few regions. (d) Representative binding tracks of Smarcb1 and Brd9 binding in GMP and Monocytes at progenitor loci (upper panel) and mature Myeloid loci (lower panel). (e) TF motif co-occurrence in lineage specific binding patterns of Smarcb1 (cBAF), Brd9 (ncBAF), Kmt2a (MLL) and Kmt2d (MLL4). TF motifs (discovered with HOMER) are sorted by their odds ratios (y-axis) in Kmt2a- Kmt2d- Brd9- and Smarcb1- lineage specific peaks: GMP, Mye (GMP & Monocytes), MEP, Ery (MEP & Erythrocytes) and B-cells. The color scale reflects the –log10 p-adjusted values for each TF motif.
Extended Data Fig. 9 Extended analysis of Chromatin Factor roles in Npm1c/Flt3-ITD Leukemia.
(a) UMAP showing original projection of Npm1c/Flt3-ITD single-cell transcriptomes. (b) UMAP projection of Npm1c/Flt3-ITD single-cell transcriptomes projected over the Hematopoietic in vivo map derived from bone marrow at 14-day post-transplant. (c) Scaled CITE-seq signal for 9 surface markers in leukemic cells. (d) Expression analysis of markers over the different leukemic clusters. According to their mRNA and Surface marker patterns these are classified into: Leukemic Stem Cells (LSC), GMP-like, Monocyte-like, Granulocyte-like, Basophil-like, Megakaryocyte-like and Erythroid-like. Clusters in red are absent in the unperturbed (NTC) cells. (e) Exemplar sorting strategy of leukemic subpopulations showing traits of differentiation into Granulocyte (Gran) or mixed Erythroid-Basophil populations. (f) Enrichment analyses of all CF-KOs across leukemia subpopulations. Disruption of factors highlighted in red induce differentiation pathways in leukemia. All values are shown in Supplementary Tables 6 and 7. (g). Plot showing the abundance of specific CF-sgRNAs with respect to Control-sgRNAs across the leukemic subclusters. Subclusters deviating from the diagonal are rare or absent in the unperturbed scenario. (h) Growth curves of Prmt1- and Prmt5-KO cells, n=3 biologically independent experiments. The cells expressing each sgRNA harbor a BFP reporter and, the assay measures the change in the proportion of BFP expressing cells over time. ***P<0.001 (Two Way ANOVA). Error bars are SEM.
Extended Data Fig. 10 Extended analysis of cBAF, MLL and MLL4 binding and TF-partnership in Npm1c/Flt3-ITD Leukemia.
(a) Correlation (spearman) analysis of CF binding patterns in Myeloid progenitors, Mature myeloid and leukemic populations. (b-d) Enrichment analysis of Smarcb1-, Kmt2a- and Kmt2d- bound loci specific of leukemia cells. Bar graphs show enriched terms across input gene lists, sorted by p-values. Targets connected to leukemia specific peaks (nearest TSS) were run in Metascape87. Functions with particularly high relevance for Npm1c/Flt3-ITD leukemia are highlighted in bold. (e) Genome-browser snapshots showing binding of cBAF/Smarcb1, MLL/Kmt2a and MLL4/Kmt2d on leukemic specific loci. (f) MA plots showing accessibility changes after acute depletion of Smarcd2-KO (cBAF), Kmt2a-KO (MLL) and Kmt2d-KO (MLL4) with respect to Control cells harbouring NTC sgRNAs. Color coding loci overlapping with Stat5a (red), Runx1(green) and Runx2 (blue).
Supplementary information
Supplementary Information
Supplementary Figs. 1–7.
Supplementary Tables
Supplementary Table 1: List of chromatin factors analyzed in FACS-based bulk CRISPR screens ex vivo. Supplementary Table 2: sgRNA sequences. Supplementary Table 3: Lineage scores in bulk CRISPR screens. Supplementary Table 4: In vivo Perturb-seq enrichment scores. Supplementary Table 5: In vivo Perturb-seq differential gene expression analysis. Supplementary Table 6: Leukemic Perturb-seq enrichment scores. Supplementary Table 7: Perturb-seq differential gene expression analysis in leukemia (comparisons of chromatin factor knockouts to NTCs). Supplementary Table 8: Primer list. Supplementary Table 9: FACS and CITE-seq antibodies. Supplementary Table 10: ChIP–seq antibodies. Supplementary Table 11: ChIP–seq sequencing statistics.
Supplementary Data 1
CRISPR screen raw sgRNA count tables.
Supplementary Data 2
Raw FACS data (FCS files) containing the source data for Fig. 5i. Cells treated with menin inhibitor and analyzed by flow cytometry to measure expression of myeloid differentiation markers.
Supplementary Data 3
Normalized ChIP–seq peak counts for Kmt2d.
Supplementary Data 4
Normalized ChIP–seq peak counts for Kmt2a.
Supplementary Data 5
Normalized ChIP–seq peak counts for Brd9.
Supplementary Data 6
Normalized ChIP–seq peak counts for Smarcb1.
Source data
Source Data Fig. 2
Data tables to reproduce the UMAPs in Figure 2.
Source Data Fig. 3
Transcription factor motif enrichment table (TOBIAS output).
Source Data Fig. 5
Data tables to reproduce the UMAP and FACS data of leukemic cells treated with the Men1 inhibitor.
Source Data Fig. 6
Matrix for the ChIP–seq heatmap and transcription factor motif enrichment values derived from HOMER.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lara-Astiaso, D., Goñi-Salaverri, A., Mendieta-Esteban, J. et al. In vivo screening characterizes chromatin factor functions during normal and malignant hematopoiesis. Nat Genet 55, 1542–1554 (2023). https://doi.org/10.1038/s41588-023-01471-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41588-023-01471-2