Abstract
Uveal melanoma is a clinically distinct and particularly lethal subtype of melanoma originating from melanocytes in the eye. Here, we performed multi-region DNA sequencing of primary uveal melanomas and their matched metastases from 35 patients. We observed previously unknown driver mutations and established the order in which these and known driver mutations undergo selection. Metastases had genomic alterations distinct from their primary tumors; metastatic dissemination sometimes occurred early during the development of the primary tumor. Our study offers new insights into the genetics and evolution of this melanoma subtype, providing potential biomarkers for progression and therapy.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The raw sequencing data are available at the European Genome Phenome Archive under accession no. EGAD00001004453.
References
Bastian, B. C. The molecular pathology of melanoma: an integrated taxonomy of melanocytic neoplasia. Annu. Rev. Pathol. 9, 239–271 (2014).
Singh, A. D. & Topham, A. Survival rates with uveal melanoma in the United States: 1973–1997. Ophthalmology 110, 962–965 (2003).
Singh, A. D., Turell, M. E. & Topham, A. K. Uveal melanoma: trends in incidence, treatment, and survival. Ophthalmology 118, 1881–1885 (2011).
Kujala, E., Mäkitie, T. & Kivelä, T. Very long-term prognosis of patients with malignant uveal melanoma. Invest. Ophthalmol. Vis. Sci. 44, 4651–4659 (2003).
Dogrusöz, M. et al. The prognostic value of AJCC staging in uveal melanoma is enhanced by adding chromosome 3 and 8q status. Invest. Ophthalmol. Vis. Sci. 58, 833–842 (2017).
Augsburger, J. J., Corrêa, Z. M. & Shaikh, A. H. Effectiveness of treatments for metastatic uveal melanoma. Am. J. Ophthalmol. 148, 119–127 (2009).
Robertson, A. G. et al. Integrative analysis identifies four molecular and clinical subsets in uveal melanoma. Cancer Cell 32, 204–220.e15 (2017).
Harbour, J. W. et al. Recurrent mutations at codon 625 of the splicing factor SF3B1 in uveal melanoma. Nat. Genet. 45, 133–135 (2013).
Martin, M. et al. Exome sequencing identifies recurrent somatic mutations in EIF1AX and SF3B1 in uveal melanoma with disomy 3. Nat. Genet. 45, 933–936 (2013).
Harbour, J. W. et al. Frequent mutation of BAP1 in metastasizing uveal melanomas. Science 330, 1410–1413 (2010).
Curtin, J. A. et al. Distinct sets of genetic alterations in melanoma. N. Engl. J. Med. 353, 2135–2147 (2005).
Van Raamsdonk, C. D. V. et al. Frequent somatic mutations of GNAQ in uveal melanoma and blue naevi. Nature 457, 599–602 (2009).
Van Raamsdonk, C. D. et al. Mutations in GNA11 in uveal melanoma. N. Engl. J. Med. 363, 2191–2199 (2010).
Johansson, P. et al. Deep sequencing of uveal melanoma identifies a recurrent mutation in PLCB4. Oncotarget 7, 4624–4631 (2016).
Moore, A. R. et al. Recurrent activating mutations of G-protein-coupled receptor CYSLTR2 in uveal melanoma. Nat. Genet. 48, 675–680 (2016).
Wiesner, T. et al. Germline mutations in BAP1 predispose to melanocytic tumors. Nat. Genet. 43, 1018–1021 (2011).
Royer-Bertrand, B. et al. Comprehensive genetic landscape of uveal melanoma by whole-genome sequencing. Am. J. Hum. Genet. 99, 1190–1198 (2016).
Bagger, M. et al. Long-term metastatic risk after biopsy of posterior uveal melanoma. Ophthalmology 125, 1969–1976 (2018).
Kerick, M. et al. Targeted high throughput sequencing in clinical cancer settings: formaldehyde fixed-paraffin embedded (FFPE) tumor tissues, input amount and tumor heterogeneity. BMC Med. Genomics 4, 68 (2011).
Field, M. G. et al. Punctuated evolution of canonical genomic aberrations in uveal melanoma. Nat. Commun. 9, 116 (2018).
Talevich, E., Shain, A. H., Botton, T. & Bastian, B. C. CNVkit: genome-wide copy number detection and visualization from targeted DNA sequencing. PLoS Comput. Biol. 12, e1004873 (2016).
Vader, M. J. C. et al. GNAQ and GNA11 mutations and downstream YAP activation in choroidal nevi. Br. J. Cancer 117, 884–887 (2017).
Huang, J. L.-Y., Urtatiz, O. & Van Raamsdonk, C. D. Oncogenic G protein GNAQ induces uveal melanoma and intravasation in mice. Cancer Res. 75, 3384–3397 (2015).
Callejo, S. A., Dopierala, J., Coupland, S. E. & Damato, B. Sudden growth of a choroidal melanoma and multiplex ligation-dependent probe amplification findings suggesting late transformation to monosomy 3 type. Arch. Ophthalmol. 129, 958–960 (2011).
de Lange, M. J. et al. Heterogeneity revealed by integrated genomic analysis uncovers a molecular switch in malignant uveal melanoma. Oncotarget 6, 37824–37835 (2015).
Singh, N., Singh, A. D. & Hide, W. Inferring an evolutionary tree of uveal melanoma from genomic copy number aberrations. Invest. Ophthalmol. Vis. Sci. 56, 6801–6809 (2015).
Shain, A. H. & Pollack, J. R. The spectrum of SWI/SNF mutations, ubiquitous in human cancers. PLoS ONE 8, e55119 (2013).
Zingg, D. et al. The epigenetic modifier EZH2 controls melanoma growth and metastasis through silencing of distinct tumour suppressors. Nat. Commun. 6, 6051 (2015).
LaFave, L. M. et al. Loss of BAP1 function leads to EZH2-dependent transformation. Nat. Med. 21, 1344–1349 (2015).
Wilson, B. G. et al. Epigenetic antagonism between polycomb and SWI/SNF complexes during oncogenic transformation. Cancer Cell 18, 316–328 (2010).
Cassoux, N. et al. Genome-wide profiling is a clinically relevant and affordable prognostic test in posterior uveal melanoma. Br. J. Ophthalmol. 98, 769–774 (2014).
Turajlic, S. et al. Tracking cancer evolution reveals constrained routes to metastases: TRACERx Renal. Cell 173, 581–594.e12 (2018).
Vogelstein, B. et al. Cancer genome landscapes. Science 339, 1546–1558 (2013).
Reiter, J. G. et al. Minimal functional driver gene heterogeneity among untreated metastases. Science 361, 1033–1037 (2018).
Bremner, R. & Balmain, A. Genetic changes in skin tumor progression: correlation between presence of a mutant ras gene and loss of heterozygosity on mouse chromosome 7. Cell 61, 407–417 (1990).
Shain, A. H. et al. The genetic evolution of melanoma from precursor lesions. N. Engl. J. Med. 373, 1926–1936 (2015).
Shain, A. H. et al. Exome sequencing of desmoplastic melanoma identifies recurrent NFKBIE promoter mutations and diverse activating mutations in the MAPK pathway. Nat. Genet. 47, 1194–1199 (2015).
Shain, H. Compiled genetic summary of the evolution of 35 metastatic uveal melanomas. figshare https://doi.org/10.6084/m9.figshare.6845675.v1 (2019).
Shain, A. H. et al. Genomic and transcriptomic analysis reveals incremental disruption of key signaling pathways during melanoma evolution. Cancer Cell 34, 45–55.e4 (2018).
Schouten, J. P. et al. Relative quantification of 40 nucleic acid sequences by multiplex ligation-dependent probe amplification. Nucleic Acids Res. 30, e57 (2002).
Acknowledgements
We wish to acknowledge B. Vainer, who passed away during the course of the study, and H. Ugleholdt, for the collection and preparation of tissue samples used in this study. We thank M. Tolstrup Andersen and M. Klarskov Andersen for genetic assistance. We also thank S. Mirza for help with FISH. We acknowledge funding support from the following: Melanoma Research Foundation (A.H.S.); National Cancer Institute K22 (CA217997 to A.H.S.); Melanoma Research Alliance (A.H.S.); Dermatology Foundation (A.H.S.); Fight for Sight Denmark (M.M.B.); the Danish Eye Research Foundation (M.M.B.); the Research Fund of Rigshospitalet (M.M.B.); the Danish Medical Research Grant/Højmosegård Grant (M.M.B.); Research to Prevent Blindness (B.C.B.); National Cancer Institute award no. 1R35CA220481 (B.C.B.); and the Terri Patters Foundation (B.C.B.).
Author information
Authors and Affiliations
Contributions
A.H.S., M.M.B., B.C.B. and J.F.K. conceived the study. M.M.B., K.W., S.H. and J.F.K. provided the clinical samples. S.L. prepared the sequencing libraries. A.H.S. supervised mutation calling, CN inference and phylogenetic tree construction. A.H.S., M.M.B., R.Y., D.C., J.F.K. and B.C.B. interpreted the genetic data. M.M.B., B.C.B. and J.F.K. performed the validation assays including immunostaining, MLPA and FISH. S.V. and J.F.W. performed FISH. A.H.S., M.M.B., B.C.B. and J.F.K. wrote the manuscript. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
B.C.B. is a consultant for Lilly Inc.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Integrated supplementary information
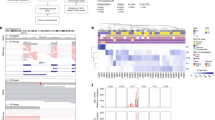
Supplementary Fig. 1 Stepwise deletion of CDKN2A and stepwise amplification of chromosomal arm 8q in case A11
. a-b, Probe-level copy number data for chromosome 9 (a) and chromosome 8 (b) in all melanoma regions from case A11. Log-scale ratios are shown on the y-axis (left side), but due to differences in tumor cellularity, these values are not comparable across tumors. We therefore also show absolute levels of copy number on the y-axis (right side) with extended dotted lines for each discrete level. Note the stepwise loss of CDKN2A (1 copy in melanoma2 and 0 copies in melanoma1 and the metastasis). Also note the stepwise gain of chromosomal arm 8q (5 copies in Mel2, 9 copies in Mel1, and 11 copies in the metastasis). c, Allelic imbalance, plotted on the y-axis as the deviation from a 0.5:0.5 split of reads, at each heterozygous SNP ordered by position across the genome (x-axis). Note that allelic imbalance corroborates the copy number alterations shown in Fig. 1c, including CNAs on chromosomes 3 and 8q (highlighted).
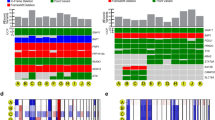
Supplementary Fig. 2 Gain-of-function mutations in the Gq signaling cascade are ubiquitous and undergo early selection; loss of wild-type GNAQ occurs later in a subset of uveal melanomas
. a, Examples of phylogenetic trees in which gain-of-function mutations in the Gq signaling cascade (highlighted in bold) underwent selection comparatively early (indicated by their truncal position). b, Examples of phylogenetic trees in which GNAQ mutations become hemi- or homo- zygous comparatively later (indicated by their branchial position in 4/6 trees). These events were exclusive to GNAQ (GNA11 mutations never underwent loss-of-heterozygosity in our cohort). The detailed evolution of each case is available in the supplementary dataset (see Methods).
Supplementary Fig. 3 SF3B1- and EIF1AX-mutant uveal melanomas acquire additional oncogenic mutations
. a, An example of an EIF1AX mutation occurring in conjunction with bi-allelic BAP1 mutations. As discussed, we distinguished this case from the ‘true’ SF3B1- and EIF1AX-driven tumors shown in b, though notably this case acquired a MED12NV40del mutation (a mutation not previously reported in uveal melanoma). b, Examples of ‘true’ EIF1AX- or SF3B1-driven uveal melanomas. Several of these uveal melanomas acquired additional pathogenic mutations during the course of evolution, including alterations not known to occur in uveal melanoma, such as PI3-kinase pathway mutations (cases A23 and A08) and bi-allelic CDKN2A alterations (cases A23 and A20).
Supplementary Fig. 4 Copy number inference over chromosomal arm 8q
. a-b, Probe level copy number data for chromosome 8 in case A61 (a) and case A06 (b). Log-scale ratios are shown on the y-axis (left side), but due to differences in tumor cellularity, these values are not comparable across tumors. Absolute levels of copy number are also shown (right side) and can be compared across tumors. c, Correlation between absolute copy number and allelic imbalance over chromosomal arm 8q. Absolute copy number was inferred from the amplitude of gain over chromosome arm 8q, as shown in a and b. The ratio of sequencing reads mapping to the major allele (more abundant allele) and minor allele was also measured (see Methods). The left panel shows the expected relationship between absolute copy number and major/minor allele ratio. Note that higher level amplifications can involve a single allele or both alleles (see example inset to the right; blue, major allele; red, minor allele), producing several possible combinations. The right panel shows the observed relationship between absolute copy number and major/minor allele ratio. d, Copy number estimates from sequencing depth (CNVkit) concord with copy number estimates from multiplex ligation-dependent probe amplification (MLPA). Copy number was estimated from 8 pieces of tissue over chromosomal arm 8q using both CNVkit and MLPA, and their relationship is summarized by their Pearson correlation coefficient (R value). Note that MLPA ratios become saturated at higher levels of copy number. e, Fluorescent in situ hybridization (FISH) over centromere 8 (left panel) and chromosomal arm 8q (right panel). The number of FISH signals over the chromosome 8 was counted from 50-300 cells, and the width of the black lines is proportional to the number of cells with a given copy number. Some cases have multiple black lines due to tissue heterogeneity (for example stromal cells and/or tumor subpopulations with distinct levels of 8q copy number). The copy number estimated from our next generation sequencing data is overlaid in red. *There were two melanoma areas in A73 with distinct copy number levels inferred from our sequencing data.
Supplementary Fig. 5 Gain of chromosomal arm 1q is common in uveal melanoma metastases
. Phylogenetic trees illustrating that gain of chromosomal arm 1q exclusively occurring in the metastasis. Refer to the supplementary dataset for the detailed evolution of each case.
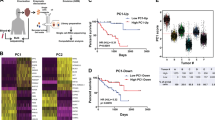
Supplementary Fig. 6 Correlations between phylogenetic features and clinical outcomes
. a, The left portion depicts a timeline of clinical events for each case: detection of the primary tumor (pink dot, time = 0), disease-free survival (dotted line), detection of metastasis (green dot), survival after metastases (solid line), and death (blue dot). The right portion shows the phylogenetic tree for each case, centered on the most genetically advanced portion of the primary tumor. b, Several measurements of the phylogenetic trees were taken (see legend at bottom of b) and recorded as variables a-f. We investigated correlations between these variables and disease-free survival, survival after metastasis, and overall survival from all 35 cases. The pairwise Pearson correlation coefficients (R-values), P-values, and adjusted P-values (corrected for multiple hypothesis testing) are shown in the three tables. The highest correlation was between variable ‘a’ and disease-free survival (highlighted as yellow). c, A scatterplot depicting variable ‘a’ versus disease-free survival is shown for all 35 cases. The Pearson correlation coefficient was significant as a single comparison, but it did not remain significant after account for multiple hypothesis testing.
Supplementary information
Supplementary Information
Supplementary Figs. 1–6
Supplementary Table 1
A summary of clinical, histopathologic, genetic and quality control metrics for each sequenced area in this study
Supplementary Table 2
Genes and bait intervals targeted for capture
Supplementary Table 3
Point mutations
Supplementary Table 4
Copy number segments
Rights and permissions
About this article
Cite this article
Shain, A.H., Bagger, M.M., Yu, R. et al. The genetic evolution of metastatic uveal melanoma. Nat Genet 51, 1123–1130 (2019). https://doi.org/10.1038/s41588-019-0440-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41588-019-0440-9
This article is cited by
-
How much do we know about the metastatic process?
Clinical & Experimental Metastasis (2024)
-
Uveal melanoma immunogenomics predict immunotherapy resistance and susceptibility
Nature Communications (2024)
-
INPP5A phosphatase is a synthetic lethal target in GNAQ and GNA11-mutant melanomas
Nature Cancer (2024)
-
TAP1, a potential immune-related prognosis biomarker with functional significance in uveal melanoma
BMC Cancer (2023)
-
BAP1 mutations inhibit the NF-κB signaling pathway to induce an immunosuppressive microenvironment in uveal melanoma
Molecular Medicine (2023)