Abstract
The efficacy of oncolytic adenoviruses (OAs) for cancer therapy has been limited by insufficient delivery to tumors after systemic injection and the propensity of OAs to induce the expression of immune checkpoints. To address these limitations, we use T cells to deliver OAs into tumors and engineer the OA to express a Cas9 system targeting the PDL1 gene encoding the immune checkpoint protein PD-L1. By cloaking OAs with cell membranes presenting T cell-specific antigens, we physically conjugated OAs onto T cell surfaces by antigen–receptor interaction. We tested the oncolytic virus–T cell chimera (ONCOTECH) via intravenous delivery in mouse cancer models, including models of melanoma, pancreatic adenocarcinoma, lung cancer and glioblastoma. In the melanoma model, the in vivo delivery of ONCOTECH resulted in a strong accumulation of OAs in tumor cells, where PD-L1 expression was reduced by 50% and the single administration of ONCOTECH enabled 80% survival over 70 days. Collectively, ONCOTECH represents a promising translational technology to combine virotherapy and cell therapy.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All data that support the findings of this study are available within the article and its Supplementary Information or from the corresponding author upon reasonable request. The raw data of tumor transcriptome sequencing have been deposited at the NCBI Gene Expression Omnibus under accession number: PRJNA939261 (ref. 54). Source data are provided with this paper.
References
Melcher, A., Harrington, K. & Vile, R. Oncolytic virotherapy as immunotherapy. Science 374, 1325–1326 (2021).
Twumasi-Boateng, K., Pettigrew, J. L., Kwok, Y. Y. E., Bell, J. C. & Nelson, B. H. Oncolytic viruses as engineering platforms for combination immunotherapy. Nat. Rev. Cancer 18, 419–432 (2018).
Tian, Y., Xie, D. & Yang, L. Engineering strategies to enhance oncolytic viruses in cancer immunotherapy. Signal Transduct. Target. Ther. 7, 117 (2022).
Killock, D. T-VEC oncolytic viral therapy shows promise in melanoma. Nat. Rev. Clin. Oncol. 12, 438 (2015).
Andtbacka, R. H. I. et al. Talimogene Laherparepvec improves durable response rate in patients with advanced melanoma. J. Clin. Oncol. 33, 25 (2015).
Breitbach, C. J. et al. Intravenous delivery of a multi-mechanistic cancer-targeted oncolytic poxvirus in humans. Nature 477, 99–102 (2011).
Nemunaitis, J. et al. Intravenous infusion of a replication-selective adenovirus (ONYX-015) in cancer patients: safety, feasibility and biological activity. Gene Ther. 8, 746–759 (2001).
Lichty, B. D., Breitbach, C. J., Stojdl, D. F. & Bell, J. C. Going viral with cancer immunotherapy. Nat. Rev. Cancer 14, 559–567 (2014).
Carlisle, R. C. et al. Human erythrocytes bind and inactivate type 5 adenovirus by presenting Coxsackie virus-adenovirus receptor and complement receptor 1. Blood 113, 1909–1918 (2009).
Sampath, P. et al. Crosstalk between immune cell and oncolytic vaccinia therapy enhances tumor trafficking and antitumor effects. Mol. Ther. 21, 620–628 (2013).
Lyons, M. et al. Adenovirus type 5 interactions with human blood cells may compromise systemic delivery. Mol. Ther. 14, 118–128 (2006).
Garcia-Carbonero, R. et al. Phase 1 study of intravenous administration of the chimeric adenovirus enadenotucirev in patients undergoing primary tumor resection. J. Immunother. Cancer 5, 71 (2017).
Ribas, A. et al. Oncolytic virotherapy promotes intratumoral T cell infiltration and improves anti-PD-1 immunotherapy. Cell 170, 1109–1119 (2017).
Liu, Z. et al. Rational combination of oncolytic vaccinia virus and PD-L1 blockade works synergistically to enhance therapeutic efficacy. Nat. Commun. 8, 14754 (2017).
Flies, D. B. & Chen, L. The new B7s: playing a pivotal role in tumor immunity. J. Immunother. 30, 251–260 (2007).
Keir, M. E., Butte, M. J., Freeman, G. J. & Sharpe, A. H. PD-1 and its ligands in tolerance and immunity. Annu. Rev. Immunol. 26, 677–704 (2008).
Wang, G. et al. An engineered oncolytic virus expressing PD-L1 inhibitors activates tumor neoantigen-specific T cell responses. Nat. Commun. 11, 1395 (2020).
Lin, C. et al. Intratumoral delivery of a PD-1–blocking scFv encoded in oncolytic HSV-1 promotes antitumor immunity and synergizes with TIGIT blockade. Cancer Immunol. Res. 8, 632–647 (2020).
Cole, C. et al. Tumor-targeted, systemic delivery of therapeutic viral vectors using hitchhiking on antigen-specific T cells. Nat. Med. 11, 1073–1081 (2005).
Evgin, L. et al. Oncolytic virus–mediated expansion of dual-specific CAR T cells improves efficacy against solid tumors in mice. Sci. Transl. Med. 14, eabn2231 (2022).
Zheng, N. et al. Induction of tumor cell autosis by myxoma virus-infected CAR-T and TCR-T cells to overcome primary and acquired resistance. Cancer Cell 40, 973–985 (2022).
Villa, N. Y. et al. Myxoma virus suppresses proliferation of activated T lymphocytes yet permits oncolytic virus transfer to cancer cells. Blood 125, 3778–3788 (2015).
Fares, J. et al. Neural stem cell delivery of an oncolytic adenovirus in newly diagnosed malignant glioma: a first-in-human, phase 1 clinical trial. Lancet Oncol. 22, 1103–1114 (2021).
Jazowiecka-Rakus, J. et al. Myxoma virus-loaded mesenchymal stem cells in experimental oncolytic therapy of murine pulmonary melanoma. Mol. Ther. Oncolytics 18, 335–350 (2020).
Lv, P. et al. Genetically engineered cell membrane nanovesicles for oncolytic adenovirus delivery: a versatile platform for cancer virotherapy. Nano Lett. 19, 2993–3001 (2019).
Chen, J. et al. Oncolytic adenovirus complexes coated with lipids and calcium phosphate for cancer gene therapy. ACS Nano 10, 11548–11560 (2016).
Aoyama, K. et al. Liposome-encapsulated plasmid DNA of telomerase-specific oncolytic adenovirus with stealth effect on the immune system. Sci. Rep. 7, 14177 (2017).
Keshavarz, M. et al. Oncolytic virus delivery modulated immune responses toward cancer therapy: challenges and perspectives. Int. Immunopharmacol. 108, 108882 (2022).
Atasheva, S. et al. Systemic cancer therapy with engineered adenovirus that evades innate immunity. Sci. Transl. Med. 12, eabc6659 (2020).
Pardoll, D. M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 12, 252–264 (2012).
Herbst, R. S. et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 515, 563–567 (2014).
Tumeh, P. C. et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 515, 568–571 (2014).
Hao, M. et al. Combination of metabolic intervention and T cell therapy enhances solid tumor immunotherapy. Sci. Transl. Med. 12, eaaz6667 (2020).
Guo, Y. et al. Metabolic reprogramming of terminally exhausted CD8+ T cells by IL-10 enhances anti-tumor immunity. Nat. Immunol. 22, 746–756 (2021).
Clarke, S. R. et al. Characterization of the ovalbumin-specific TCR transgenic line OT-I: MHC elements for positive and negative selection. Immunol. Cell Biol. 78, 110–117 (2000).
Mulcahy, L. A., Pink, R. C. & Carter, D. R. F. Routes and mechanisms of extracellular vesicle uptake. J. Extracell. Vesicles 3, 24641 (2014).
Nath, S. C. et al. Fluid shear stress promotes embryonic stem cell pluripotency via interplay between β-catenin and vinculin in bioreactor culture. Stem Cells 39, 1166–1177 (2021).
Mariuzza, R. A., Agnihotri, P. & Orban, J. The structural basis of T-cell receptor (TCR) activation: an enduring enigma. J. Biol. Chem. 295, 914–925 (2020).
Mardi, A. et al. Biological causes of immunogenic cancer cell death (ICD) and anti-tumor therapy; combination of oncolytic virus-based immunotherapy and CAR T-cell therapy for ICD induction. Cancer Cell Int. 22, 168 (2022).
Hillerdal, V. et al. Systemic treatment with CAR-engineered T cells against PSCA delays subcutaneous tumor growth and prolongs survival of mice. BMC Cancer 14, 30 (2014).
Wang, S. et al. Macrophage-tumor chimeric exosomes accumulate in lymph node and tumor to activate the immune response and the tumor microenvironment. Sci. Transl. Med. 13, eabb6981 (2021).
Tang, L. et al. Enhancing T cell therapy through TCR-signaling-responsive nanoparticle drug delivery. Nat. Biotechnol. 36, 707–716 (2018).
Stephan, M. T., Moon, J. J., Um, S. H., Bershteyn, A. & Irvine, D. J. Therapeutic cell engineering with surface-conjugated synthetic nanoparticles. Nat. Med. 16, 1035–1041 (2010).
Yi, J., Balagopalan, L., Nguyen, T., McIntire, K. M. & Samelson, L. E. TCR microclusters form spatially segregated domains and sequentially assemble in calcium-dependent kinetic steps. Nat. Commun. 10, 277 (2019).
Martins, F. et al. Adverse effects of immune-checkpoint inhibitors: epidemiology, management and surveillance. Nat. Rev. Clin. Oncol. 16, 563–580 (2019).
Naidoo, J. et al. Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann. Oncol. 27, 1362 (2016).
McLeod, H. L. et al. Assessment of adverse events and their ability to discriminate response to anti-PD-1/PD-L1 antibody immunotherapy. J. Clin. Oncol. 38, 103–104 (2020).
Burr, M. L. et al. CMTM6 maintains the expression of PD-L1 and regulates anti-tumour immunity. Nature 549, 101–105 (2017).
Doundna, J. A. The promise and challenge of therapeutic genome editing. Nature 578, 229–236 (2020).
Mattapally, S. et al. Human leukocyte antigen class I and II knockout human induced pluripotent stem cell-derived cells: universal donor for cell therapy. J. Am. Heart Assoc. 7, e010239 (2018).
Depil, S., Duchateau, P., Grupp, S. A., Mufti, G. & Poirot, L. ‘Off-the-shelf’ allogeneic CAR T cells: development and challenges. Nat. Rev. Drug Discov. 19, 185–199 (2020).
Wang, Y. et al. Engineering tumor-specific gene nanomedicine to recruit and activate T cells for enhanced immunotherapy. Nat. Commun. 14, 1993 (2023).
Badrinath, S. et al. A vaccine targeting resistant tumours by dual T cell plus NK cell attack. Nature 606, 992–998 (2022).
Chen, Y. et al. An oncolytic virus-T-cell chimera for cancer immunotherapy. https://www.ncbi.nlm.nih.gov/sra/PRJNA939261
Acknowledgements
This work was supported by the National Key Research and Development Program of China (2021YFA0909900, Y.P.; 2018YFA0901800, Y.P.), the National Natural Science Foundation of China (32261143727, Y.P.), the Pivotal Life Sciences Sponsorship Program (202203, Y.P.). We thank H. Yang, H. Yin, H. Yu, L. Si, J. Sheng, D. Li, Q. Chen and G. Huang for stimulating discussions; J. Pan and D. Wu of the Research and Service Center (College of Pharmaceutical Science, Zhejiang University) and L. Liu of the Core Facilities (School of Medicine, Zhejiang University) for technical support; and Y. Zhang and colleagues from the Ping Laboratory for assistance in experiments.
Author information
Authors and Affiliations
Contributions
Y.C. and X.C. designed and performed experiments and analyzed and interpreted data. W.B. helped with the construction of PDO. G.L. helped with the construction of the oncolytic adenovirus. W.W. discussed the manuscript. Y.P. designed the study, analyzed and interpreted data and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
Y.P. is a scientific cofounder of Ruidax. The other authors declare no competing interests.
Peer review
Peer review information
Nature Biotechnology thanks John Bell, Li Tang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1
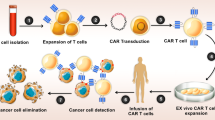
Scheme of genome editing of PDL1 by eOA effectively improved virotherapy and adoptive T-cell therapy. First, eOA encoding Cas9 editor and sgRNA targeting PDL1 was engineered, and eOA was further shielded by an engineered biological membrane presenting T cell-specific antigens in two ways to form M@eOA. Finally, M@eOA was physically attached onto carrier T cell surfaces by recognizing TCR or CAR through antigen-receptor interaction to form ONCOTECH. By virtue of tumor-targeting ability of T cells, ONCOTECH could reach tumor following the systemic administration, and eOAs were readily released once carrier T cells reach the surface of tumor cells and recognize their cognate tumor-specific antigens through competitive antigen-receptor interaction and membrane fusion. The OAs were then internalized by cancer cells and facilitate specific viral infection. After infection, oncolytic virus constructs encoding Cas9 editor could disrupt PDL1 gene of tumor cells to downregulate their PD-L1 expression, which greatly contributes to mitigating the immunosuppressive TME and promoting both T-cell therapy and oncolysis against tumors.
Extended Data Fig. 2 Tumor PDL1 analysis and Construction of engineered OA (eOA).
a, Overall survival of patients with melanoma, pancreatic cancer, or glioma with a high or low level of PDL1 analyzed by GEPIA. The source data was isolated from the TCGA. b, Representative images of PDL1 by immunohistochemistry (brown) of tumors from patients with pancreatic cancer, which were used to establish PDX model. The PDX mouse model was treated with saline, intratumorally (i.t.) injected with OA (1 × 1010 VP), i.v. administered with 1 × 107 CAR-T cells, and combination OA (i.t.) with CAR-T cell (i.v.). Scale bar 100 μm. n = 5 biological independent mice, per tumor was random analyzed from three IHC slices. Score 0 (-, negative), score 1 (+, weak), score 2 (++, moderate), score 3 (+++, strong). c, Gating strategy (upper) and representative flow cytometry plot of PDL1 expression (lower) in EpCAM+ tumor cells after treatment. d, PDL1 expression in B16OVA tumor-bearing mice after treatment. e, The quantitative analysis of GFP-positive cells infected for 48 hours with eOA at an increasing MOI at B16OVA and SW1990 cells. f, Western blot (upper) analysis of Cas9 protein level and indel mutation (lower) of PDL1 locus in B16OVA cells 48 hours after infection. g, Western blot analysis (upper) of Cas9 proteins and indel mutation (lower) of human PDL1 locus in SW1990 cells after 48 hours of eOA infection at increasing MOIs. h, Schematic illustration of eOA-PLK1 design and indel mutations of PLK1 locus in B16F10 cells after 48 hours of eOA infection at increasing MOIs. i, Quantitative analysis of viral copy numbers in different cell lines by qRT-PCR after 48 hours of infection with eOA. j, Cell viability of B16OVA, L929, SW1990, and HUVEC cells with the indicated treatments for 72 h. k, Western blot analysis of PDL1 level in B16OVA cells after treatment for 72 h. l. Cell viability of B16OVA cells after treatment for 72 h. m. Illustration of eOA treatment in C57BL/6 mice bearing B16OVA tumor (Upper). T7E1 (lower left) and western blot (lower right) assays for the analysis of PDL1 levels after treatment. n. Tumor volume (left) and survival curves after the indicated treatment (n = 5 biologically independent mice). Red arrows (f-h) denote cleaved DNA fragments. Data are presented as the mean ± s.d. (b-e,i,l) and s.e.m. (n). n = 3 (e,i), n = 5 (b, c, d, j), and n = 6 (l) biological independent samples. One-way ANOVA with Tukey’s post hoc analysis was used in (c, i, l), and two-way ANOVA with Bonferroni post hoc test was used in (n), and log-rank (Mantel-Cox) test used in survival curves (a,n), with P values indicated on the graphs.
Extended Data Fig. 3 Characterizations of T-M@eOA.
a, Dot blot and quantitative analysis of cancer cell membrane (CM) encapsulation of eOA (1 × 1010 VP) by anti-Ad5 antibody. CM@eOA was denatured by RIPA buffer. b, Cytometry analysis of surface-bound hybrid M obtained from CM mixed with the different weight percentage of DMG-PEG. c, Size and zeta potential analysis of different M@eOA formulations. M means CM hybrid with lipids mixture. d, TEM images of eOA and M@eOA. Scale bar 200 nm. e, Dot blot of hybrid membrane (M) encapsulation of eOA, M@eOA was denatured by RIPA buffer. f, Cytometry plots of T-M@eOA with different weight of M@eOA on T cells (1 × 107 cells). g, Representative cytometry plots and MFI analysis of M@eOA release from T-M@eOA after incubating with MHC-I SIINFEKL-tetramer for 30 min, eOA stained by TRITC. h, Representative CLSM images of T-M@eOA for carrier stability analysis for 48 h. M@eOA and T cells were labeled with DiI and Hoechst 33342, respectively. Scale bar 10 μm. i, Representative CLSM images of T-M@eOA on cross section (left) and reconstructed 3D model from the Z-stacks (right). Scale bar, 5 μm. j, Quantitative analysis of Ad5 antibody MFI on the surface of carrier T cells. k, Indel mutation of carrier T cells after being incubated at 37 °C for 3 days. l,m, Quantitative analysis of T cell viability (l) and relative eOA intensity (m) in T-M@eOA after incubation with or without stirring (100 rpm) at 37 °C for 24 h by 7-AAD and dot blot. n, Cytometry plots of T-M@eOA apoptosis by Annexin V-FITC and PI. T cell without M@eOA was used as control. o, Representative cytometry histograms of T cell proliferation stimulated by anti-CD3/CD28 beads with IL-2. p, Cell migration percentage of T cells and T-M@eOA stimulated by MCP-1 in a Transwell dish. q, ELISA analysis of IL-2 releases by T cells or T-M@eOA after Concanavalin A stimulation for 48 h. r, Representative GFP expression images of B16OVA treated with M@eOA or T-M@eOA for 24 h, The GFP-positive percentages were analyzed by cytometry. s, Representative images of B16OVA cells after indicated treatment for 48 h. Scale bar 50 μm. n = 3 (a-f, l-n, p-r), n = 5 (g, j) biological independent samples. Data are presented as mean ± s.d., unpaired two-tailed t-test was used in (m, n, q, r), one-way ANOVA with Tukey’s post hoc test was used in (b, g, j), two-way ANOVA with Bonferroni post hoc analysis was used in (l,p), with P values indicated on the graphs. NS denotes no significant difference (P > 0.05).
Extended Data Fig. 4 T-M@eOA therapy reshapes immunosuppressive TME in vivo.
a, Cytometry plots and the quantitative analysis of eOA-Cy7 in TCR Vα2+ carrier T cells in blood 6 h after i.v. injection. b, Quantitative analysis of organ distribution of Cy7-eOA and carrier DiD-OT-1 CD8+ T cells 24 h after i.v. administration in different organs. c, CLSM images of tumor sections after i.v. administration of T + M@eOA or T-M@eOA for 24 h. eOA were labeled by Cy7, and carrier T cells were labeled by DiD. Scale bar 200 μm. d, Representative B16OVA tumor section imaging after T-M@eOA-GFP treatment for 72 h. GFP expression was indicated in the eOA-GFP-infected cells (left). Blue signal, DAPI. Scale bar 300 μm. Quantitative analysis of GFP expression after eOA-GFP infection (right), n = 5 biological independent samples. e, Indel mutations of PDL1 locus in tumor after indicated treatments (upper), and different organs after T-M@eOA treatment (lower). f, Quantitative analysis of PDL1 expression in different type of cells. n = 5 biological independent mice. g, Gating strategy for the PDL1 expression in different subpopulations. Plots are representative of six experimental replicates. Data are presented as mean ± s.d. Paired two-tailed t-test was used in (a), one-way ANOVA with Tukey’s post hoc test was used in (f), with P values indicated on the graphs. NS denotes no significant difference (P > 0.05).
Extended Data Fig. 5 Immune composition of TME after T-M@eOA treatment.
a, Western blot analysis of cleaved caspase-3, caspase-1, and cleaved GSDMD protein levels. GAPDH was used as the control. b, Representative cytometry plots and quantitative analysis of CD80+CD86+ in CD11c+ dendritic cells in the lymph node. n = 5 biological independent mice. c-g, Representative cytometry plots of SIINFEKL-H-2Kb+ gated on CD11c+ cells in the lymph node (c), MHC-I SIINFEKL-tetramer-APC gated on CD3+CD8+TCR-Vα2- cells in the tumor (d), the ratio of M1 (CD86+CD206−)/M2 (CD86−CD206+) in TAMs (gated on CD45+CD11b+F4/80+ cells, e), the endogenous CD4+ and CD8+ T cells (gated on CD3+TCR-Vα2- cells, f), and T (Foxp3+ gated on CD3+CD4+ cells, g) in the tumor. h, Representative flow cytometry plots and quantitative analysis of killing effectors (IFNγ, GZMB) level in endogenous TCR-Vα2-CD8+ T cells. n = 5 biological independent samples. i, Representative flow cytometry plots of killing effectors (IFNγ, GZMB) level in intratumoral OT-1 TCR-Vα2+CD8+ T cells. j, Heat map analysis of the differentially expressed genes (DEGs) in B16OVA tumors after the indicated treatment (T cell, M@eOA, T-M@eOA), and saline group was used as the control. Red represents upregulation, and blue represents downregulation. Genes with absolute log2 (fold change) > 1 and an adjusted P < 0.05 were deemed to be significantly differentially expressed, differential gene expression was analyzed via DESeq based on the statistical model of negative binomial distribution. DEG names are listed on the right. k, Venn diagram from the DEGs heat maps of mice treated with T cells, M@eOA, and T-M@eOA (left). Quantitative analysis of the numbers of DEGs in the tumor after different treatment (right). Data represent mean ± s.d. P value is determined by one-way ANOVA with Tukey’s post hoc analysis (b) and two-way ANOVA with Bonferroni post hoc analysis (h), with P values indicated on the graphs.
Extended Data Fig. 6 Long-term immune effects in B16OVA-tumor bearing mice.
a, Timeline of the anti-tumor effect of T-M@eOA with NK cells or CD4+ T cells depletion in B16OVA-tumor bearing mice. b,c, Tumor volumes (b) and survival curves (c) of indicated treatments. n = 5 biological independent mice. d, Representative cytometry plots and the quantitative analysis of the effector memory T cells (CD3+CD8+CD44+CD62L−, Tem) and central memory T cells (CD3+CD8+CD44+CD62L+, Tcm) in the spleen of healthy mice (control) or tumor-bearing mice after the indicated treatment, control groups: healthy mice, saline treatment groups. n = 5 biological independent mice. e, Gating strategy of lymphoid cell populations after antibody treatment. The representative flow cytometry plots (upper) and quantitative analysis (lower) for the depletion of NK cells (f), CD4+ T cells (g) and CD8+ T cells (h) in C57BL/6 mice. Plots are representative of three experimental replicates. n = 3 biological independent mice. Data represent mean ± s.d. (d-h) and s.e.m. (b), P value is determined by two-way ANOVA with Bonferroni post hoc analysis (b, d, f-h), and survival curves (c) were analyzed by the Log-rank (Mantel-cox) test, with P values indicated on the graphs.
Extended Data Fig. 7 Biosafety analysis and additional in vivo anti-tumor experiments.
a, Comparison of anti-tumor efficacy in T-M@eOA (Extended Data Fig. 7f) with T(i.v.)+eOA(i.t.) (Extended Data Fig. 2n) treatment in B16OVA-tumor bearing mice. B16OVA tumor were s.c. inoculated at day -7, and treated with OT-1 CD8+ T (i.v.). b, The body temperature of mice after the indicated treatment. c, The inflammatory cytokine levels in the serum 7 days after the indicated treatments. d, In vivo biosafety analysis of lactate dehydrogenase (LDH), alkaline phosphatase (AKP/ALP), aspartate aminotransferase (AST), alanine transaminase (ALT) in the mice 7 days after the indicated treatment. e, Schematic illustration of the Cas9-expressing eOA and anti-PDL1 scFv expressing OA-aPDL1, and timeline of the anti-tumor effect with different OAs in a subcutaneous B16OVA tumor model. f, tumor volume (left) and survival curves (right) of B16OVA tumor-bearing mice after indicated treatment. g, Representative cytometry histograms of carrier OT-1 CD8+ T cells expansion by CFSE in the lung metastatic tumor. h, Representative cytometry plots and quantitative analysis of cytokines (IFNγ and GZMB) expressing carrier OT-1 CD8+ T cells in lung metastatic tumor. i, The photographs and corresponding H&E−stained slices of the lung tissue isolated from the mice bearing B16OVA tumors after the indicated treatment for 20 days. Scale bar 3 mm. n = 5 biological independent mice. Data are presented as the mean ± s.d. (b-d, h) and mean ± s.e.m. (a, f). One-way ANOVA with Tukey’s post hoc test were used in (c,d,g,h), and two-way ANOVA with Bonferroni post hoc test was used in (a, b, f), and Log-rank (Mantel-cox) test (survival curve), with P values indicated on the graphs.
Extended Data Fig. 8 Construction and characterizations of CAR-T-M@eOA in vitro and in vivo.
a, Dot blot and the quantitative analysis were performed to evaluate the hybrid membrane (LN229 CM: Lipids, wt ratio) encapsulation of eOA (1 × 1010 VP) analyzed by the anti-Ad5. M@eOA was denatured by RIPA buffer. b, Dot blot and the quantitative analysis were performed to evaluate the anti-GD2-CAR-T loading capacity of M@eOA by the anti-Ad5. M@eOA contained 100 μg A549 cell membrane (M) with 1 × 1010 VP eOA. c, Cytometry analysis of CAR-T-M@eOA with different weight of M@eOA on CAR-T cells (1 × 107 cells). d, Scheme of orthotopic LN229 glioblastoma treatment by anti-GD2-CAR-T-M@eOA in humanized NSG mice, LN229 cell membrane (M) that overexpress GD2 were used in M@eOA generation. e, f, Indel mutations of PDL1 locus (e) and western blot analysis of PDL1 (f) in LN229 tumor tissue after treatment. Red arrows denote cleaved DNA fragments. g, Quantitative analysis of Treg cells in CD4+ T cells. h, Cytokine profiles in the tumor supernatant by ELISA. i, Representative cytometry plots and quantitative analysis of GZMB and IFNγ expression in CD8+ CAR-T cells within tumors. j, k, Bioluminescence images (j) and survival curves (k) of LN229 orthotopic glioblastoma tumor-bearing humanized mice after treatments. l, Scheme of metastasis A549 tumor treatment by anti-EphA2-CAR-T-M@eOA in humanized NSG mice. NSG mice were humanized by HLA-A2+ PBMC (1 × 107 cells) before the tumor inoculation, and A549-Luc cells (1 × 105) were i.v. injected 7 days before treatment. A549 cell membrane (M) that overexpress EphA2 were used in M@eOA generation. m, n, Indel mutations of PDL1 locus (m) and western blot analysis of PDL1 (m) in the lung metastatic tumor tissue after the indicated treatment. Red arrows denote cleaved DNA fragments. o, Cytokine profiles in the tumor supernatant by ELISA. p, Quantitative analysis of GZMB+ cells in CD8+ CAR-T cells within tumor. q,r, Bioluminescence images (q) and survival curve (r) of metastatic A549 tumor-bearing humanized mice after the indicated treatment. n = 3 biological independent samples (a-c), n = 5 biological independent mice (g-k, o-r). Data are presented as the mean ± s.d. One-way ANOVA with Tukey’s post hoc test was used in (g, i, p), and log-rank (Mantel-Cox) test was used in survival curves (k, r), with P values indicated on the graphs.
Extended Data Fig. 9 Construction and characterization of anti-PSCA-CAR-T-MV@eOA in vitro and in vivo.
a, TEM image of MV@eOA. b, Quantification of MV/eOA (wt:VP) by dot blot. c, Dot blot and the quantitative analysis of CAR-T-MV@eOA by anti-ad5 antibody. MV@eOA contained 200 μg 293T-PSCA-MV with 1 × 1010 VP of eOA. d Quantitative analysis of the anti-PSCA-scFv occupied by MV@eOA on the surface of CAR-T cells by recombinant PSCA-FITC protein. e, Dot blot assay (top) and the quantitative analysis (bottom) of CAR-T-M@eOA stored at different culture temperatures with or without adding serum for 24 h. f, Quantitative analysis of PSCA mRNA level of PDAC tumor sample and corresponding peritumoral normal tissues by RT-qPCR (n = 5 biological independent PDX tumor derived from one human tumor sample). g, Western blot analysis of cleaved caspase 3, caspase 1, cleaved GSDMD in the PDX tumor tissue after the indicated treatment. h, Representative Treg (Foxp3+) percentage in human CD4+ T cells within PDX TME. i, j Representative cytometry plots of intratumoral activated CAR-T cells (hCD8+CFSE+GZMB+, i) and endogenous hCD8+CFSE-GZMB+ T cells (j) after the i.v. administration. k, Cytokine profiles of (TNFα, IFNγ) in the PDX tumor supernatant by ELISA. l, The change of body temperature of the humanized mice after the indicated treatment. n = 3 biological independent samples (a-e), and n = 5 biological independent mice (f, k, l). Data are presented as the mean ± s.d. One-way ANOVA with Tukey’s post hoc analysis was used in (e, f), and two-way ANOVA with Bonferroni post hoc test were used in (k, l), with P values indicated on the graphs. NS denotes no significant difference (P > 0.05).
Supplementary information
Supplementary Information
Supplementary Methods, References, Figs. 1–5, Tables 1–3 and unprocessed blots and gels for Supplementary figures.
Supplementary Data 1
Statistical data for Supplementary figures.
Supplementary Video 1
T-M@eOA recognized by B16OVA-mCherry cells.
Source data
Source Data Fig. 1
Unprocessed western blots and gels and statistical source data for Fig. 1.
Source Data Fig. 2
Unprocessed western blots and statistical source data for Fig. 2.
Source Data Fig. 3
Unprocessed western blots and statistical source data for Fig. 3.
Source Data Fig. 4
Unprocessed western blots and gels and statistical source data for Fig. 4.
Source Data Fig. 5
Unprocessed gels and statistical source data for Fig. 5.
Source Data Fig. 6
Unprocessed western blots and gels and statistical source data for Fig. 6.
Source Data Extended Data Fig. 2
Unprocessed western blots and gels and statistical source data for Extended Data Fig. 2.
Source Data Extended Data Fig. 3
Unprocessed western blots and gels and statistical source data for Extended Data Fig. 3.
Source Data Extended Data Fig. 4
Unprocessed gels and statistical source data for Extended Data Fig. 4.
Source Data Extended Data Fig. 5
Unprocessed western blots and statistical source data for Extended Data Fig. 5.
Source Data Extended Data Fig. 6
Statistical source data for Extended Data Fig. 6.
Source Data Extended Data Fig. 7
Statistical source data for Extended Data Fig. 7.
Source Data Extended Data Fig. 8
Unprocessed western blots and gels and statistical source data for Extended Data Fig. 8.
Source Data Extended Data Fig. 9
Unprocessed western blots and gels and statistical source data for Extended Data Fig. 9.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, Y., Chen, X., Bao, W. et al. An oncolytic virus–T cell chimera for cancer immunotherapy. Nat Biotechnol (2024). https://doi.org/10.1038/s41587-023-02118-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41587-023-02118-7
This article is cited by
-
Optimizing oncolytic virus design: a “Swiss army knife” approach to create a systemically delivered therapeutic
Signal Transduction and Targeted Therapy (2024)