Abstract
The success of chimeric antigen receptor (CAR) T cell therapy in treating several hematopoietic malignancies has been difficult to replicate in solid tumors, in part because of T cell exhaustion and eventually dysfunction. To counter T cell dysfunction in the tumor microenvironment, we metabolically armored CAR T cells by engineering them to secrete interleukin-10 (IL-10). We show that IL-10 CAR T cells preserve intact mitochondrial structure and function in the tumor microenvironment and increase oxidative phosphorylation in a mitochondrial pyruvate carrier-dependent manner. IL-10 secretion promoted proliferation and effector function of CAR T cells, leading to complete regression of established solid tumors and metastatic cancers across several cancer types in syngeneic and xenograft mouse models, including colon cancer, breast cancer, melanoma and pancreatic cancer. IL-10 CAR T cells also induced stem cell-like memory responses in lymphoid organs that imparted durable protection against tumor rechallenge. Our results establish a generalizable approach to counter CAR T cell dysfunction through metabolic armoring, leading to solid tumor eradication and long-lasting immune protection.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All data generated and supporting the findings of this study are available within the paper. scRNA-seq data for the mouse CAR T cells from this study have been deposited in the Gene Expression Omnibus under accession number GSE245517. scRNA-seq data for the hCAR T cells are available in the CNGB Sequence Archive of the China National GeneBank Database under accession number CNP0003547. Source data are provided with this paper. Additional information and materials will be made available upon reasonable request.
Code availability
The R code required to reproduce the scRNA-seq data shown in Figs. 4 and 6 and Extended Data Figs. 5 and 10 is available at the following repository: https://github.com/carmonalab/LiTang_IL10_CART.
References
Hou, A. J., Chen, L. C. & Chen, Y. Y. Navigating CAR-T cells through the solid-tumour microenvironment. Nat. Rev. Drug Discov. 20, 531–550 (2021).
Blank, C. U. et al. Defining ‘T cell exhaustion’. Nat. Rev. Immunol. 19, 665–674 (2019).
Wherry, E. J. & Kurachi, M. Molecular and cellular insights into T cell exhaustion. Nat. Rev. Immunol. 15, 486–499 (2015).
Wherry, E. J. et al. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity 27, 670–684 (2007).
Sen, D. R. et al. The epigenetic landscape of T cell exhaustion. Science 354, 1165–1169 (2016).
DePeaux, K. & Delgoffe, G. M. Metabolic barriers to cancer immunotherapy. Nat. Rev. Immunol. 21, 785–797 (2021).
Scharping, N. E. et al. Mitochondrial stress induced by continuous stimulation under hypoxia rapidly drives T cell exhaustion. Nat. Immunol. 22, 205–215 (2021).
Yu, Y.-R. et al. Disturbed mitochondrial dynamics in CD8+ TILs reinforce T cell exhaustion. Nat. Immunol. 21, 1540–1551 (2020).
Vardhana, S. A. et al. Impaired mitochondrial oxidative phosphorylation limits the self-renewal of T cells exposed to persistent antigen. Nat. Immunol. 21, 1022–1033 (2020).
Mo, F. et al. An engineered IL-2 partial agonist promotes CD8+ T cell stemness. Nature 597, 544–548 (2021).
Alizadeh, D. et al. IL15 enhances CAR-T cell antitumor activity by reducing mTORC1 activity and preserving their stem cell memory phenotype. Cancer Immunol. Res. 7, 759–772 (2019).
Guo, Y. et al. Metabolic reprogramming of terminally exhausted CD8+ T cells by IL-10 enhances anti-tumor immunity. Nat. Immunol. 22, 746–756 (2021).
Scarfò, I. & Maus, M. V. Unraveling the signaling balance of activation and exhaustion of CAR T cells. Cancer Cell 37, 143–144 (2020).
Mueller, S. N. & Rafi, A. High antigen levels are the cause of T cell exhaustion during chronic viral infection. Proc. Natl Acad. Sci. USA 106, 8623–8628 (2009).
Liu, X. & Peng, G. Mitochondria orchestrate T cell fate and function. Nat. Immunol. 22, 276–278 (2021).
Andreatta, M. et al. Interpretation of T cell states from single-cell transcriptomics data using reference atlases. Nat. Commun. 12, 2965 (2021).
Gattinoni, L. et al. Wnt signaling arrests effector T cell differentiation and generates CD8+ memory stem cells. Nat. Med. 15, 808–813 (2009).
Flynn, J. K. & Gorry, P. R. Stem memory T cells (Tscm)—their role in cancer and HIV immunotherapies. Clin. Transl. Immunol. 3, e20 (2014).
Buck, M. D. et al. Mitochondrial dynamics controls T cell fate through metabolic programming. Cell 166, 63–76 (2016).
Gattinoni, L. et al. A human memory T cell subset with stem cell-like properties. Nat. Med. 17, 1290–1297 (2011).
Herndler-Brandstetter, D. et al. KLRG1+ effector CD8+ T cells lose KLRG1, differentiate into all memory T cell lineages, and convey enhanced protective immunity. Immunity 48, 716–729.e8 (2018).
Andreatta, M. & Carmona, S. J. UCell: robust and scalable single-cell gene signature scoring. Comput. Struct. Biotechnol. J. 19, 3796–3798 (2021).
Kawalekar, O. U. et al. Distinct signaling of coreceptors regulates specific metabolism pathways and impacts memory development in CAR T cells. Immunity 44, 380–390 (2016).
Long, A. H. et al. 4-1BB costimulation ameliorates T cell exhaustion induced by tonic signaling of chimeric antigen receptors. Nat. Med. 21, 581–590 (2015).
Hurton, L. V. et al. Tethered IL-15 augments antitumor activity and promotes a stem-cell memory subset in tumor-specific T cells. Proc. Natl Acad. Sci. USA 113, E7788–E7797 (2016).
Wei, J. et al. Targeting REGNASE-1 programs long-lived effector T cells for cancer therapy. Nature 576, 471–476 (2019).
Zhang, Y. et al. Enhancing CD8+ T Cell Fatty Acid Catabolism within a Metabolically Challenging Tumor Microenvironment increases the Efficacy of Melanoma Immunotherapy. Cancer Cell 32, 377–391.e9 (2017).
Chowdhury, P. S., Chamoto, K., Kumar, A. & Honjo, T. PPAR-induced fatty acid oxidation in T cells increases the number of tumor-reactive CD8+ T cells and facilitates anti-PD-1 therapy. Cancer Immunol. Res. 6, 1375–1387 (2018).
Ma, X. et al. Interleukin-23 engineering improves CAR T cell function in solid tumors. Nat. Biotechnol. 38, 448–459 (2020).
Adachi, K. et al. IL-7 and CCL19 expression in CAR-T cells improves immune cell infiltration and CAR-T cell survival in the tumor. Nat. Biotechnol. 36, 346–351 (2018).
Avanzi, M. P. et al. Engineered tumor-targeted T cells mediate enhanced anti-tumor efficacy both directly and through activation of the endogenous immune system. Cell Rep. 23, 2130–2141 (2018).
Rafiq, S. et al. Targeted delivery of a PD-1-blocking scFV by CAR-T cells enhances anti-tumor efficacy in vivo. Nat. Biotechnol. 36, 847–858 (2018).
Lynn, R. C. et al. c-Jun overexpression in CAR T cells induces exhaustion resistance. Nature 576, 293–300 (2019).
Eyquem, J. et al. Targeting a CAR to the TRAC locus with CRISPR/Cas9 enhances tumour rejection. Nature 543, 113–117 (2017).
Miller, B. C. et al. Subsets of exhausted CD8+ T cells differentially mediate tumor control and respond to checkpoint blockade. Nat. Immunol. 20, 326–336 (2019).
Hanna, B. S. et al. Interleukin-10 receptor signaling promotes the maintenance of a PD-1 intTCF-1+CD8+ T cell population that sustains anti-tumor immunity. Immunity 54, 2825–2841.e10 (2021).
Cui, W., Liu, Y., Weinstein, J. S., Craft, J. & Kaech, S. M. An interleukin-21–interleukin-10–STAT3 pathway is critical for functional maturation of memory CD8+ T cells. Immunity 35, 792–805 (2011).
Laidlaw, B. J. et al. Production of IL-10 by CD4+ regulatory T cells during the resolution of infection promotes the maturation of memory CD8+ T cells. Nat. Immunol. 16, 871–879 (2015).
Bordon, Y. IL-15 provides breathing space for memory. Nat. Rev. Immunol. 12, 77 (2012).
Li, W. & Zhang, L. Rewiring mitochondrial metabolism for CD8+ T cell memory formation and effective cancer immunotherapy. Front. Immunol. 11, 1834 (2020).
Tschumi, B. O. et al. CART cells are prone to Fas- and DR5-mediated cell death. J. Immunother. Cancer 6, 71 (2018).
Brentjens, R. J. et al. Eradication of systemic B-cell tumors by genetically targeted human T lymphocytes co-stimulated by CD80 and interleukin-15. Nat. Med. 9, 279–286 (2003).
Jing, R. et al. Cas9-cleavage sequences in size-reduced plasmids enhance nonviral genome targeting of CARs in primary human T cells. Small Methods 5, 2100071 (2021).
Michelet, X. et al. Metabolic reprogramming of natural killer cells in obesity limits antitumor responses. Nat. Immunol. 19, 1330–1340 (2018).
Hao, Y. et al. Integrated analysis of multimodal single-cell data. Cell 184, 3573–3587.e29 (2021).
Andreatta, M., Berenstein, A. J. & Carmona, S. J. scGate: marker-based purification of cell types from heterogeneous single-cell RNA-seq datasets. Bioinformatics 38, 2642–2644 (2022).
Liberzon, A. et al. Molecular signatures database (MSigDB) 3.0. Bioinformatics 27, 1739–1740 (2011).
Yu, G., Wang, L.-G., Han, Y. & He, Q.-Y. clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS 16, 284–287 (2012).
Acknowledgements
We thank D. J. Irvine (Massachusetts Institute of Technology) for providing TRP-1 CAR and EGFRvIII CAR plasmids and 4T1-EGFRvIII-Luc cells, J. Auwerx (EPFL) for providing access to a Seahorse XFe96 Analyzer, and J. Shi and Y. Wang (Zhejiang University) for technical support for the orthotopic PDAC model. We acknowledge the EPFL CPG, Flow Cytometry Core Facility, Gene Expression Core Facility and Biological Electron Microscopy Facility and the University of Lausanne Center of Metabolomics Platform for technical assistance. We thank Y. Li, Y. Huang and C. Guo from the Core Facilities, Zhejiang University School of Medicine, for their technical support. This work was supported, in part, by the Swiss National Science Foundation (315230_173243, 316030_189686, 315230_204202, IZLCZ0_206035 and CRSII5_205930), Swiss Cancer Research Foundation (KFS-4600-08-2018), Kristian Gerhard Jebsen Foundation, the European Research Council under the ERC grant agreement MechanoIMM (805337), Anna Fuller Fund grant, Xtalpi, Inc., and EPFL. J.S. was funded by the National Natural Science Foundation of China grants 82161138028 and 31971324, Zhejiang Provincial Natural Science Foundation grant LR20H160003 and National Key R&D Program of China grant 2021YFA0909900. S.C. was supported by the Swiss National Science Foundation Ambizione grant 180010. P.R. was supported, in part, by grants from the Swiss National Science Foundation (310030_182735) and the Swiss Cancer League (KFS-4404-02-18). M.G. was supported by the Chinese Scholarship Council (201808320453). X.H. has received funding from the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement 754354 and was supported by the China Scholarship Council (201700260266).
Author information
Authors and Affiliations
Contributions
Y.Z., J.C., J.S., Y.G. and L.T. conceived the study. Y.Z., J.C., J.S., Y.G. and L.T. designed the experiments. Y.Z., J.C., M.A., B.F., Y.-Q.X., M.W., Y.W., M.G., X.H., P.R. and S.C. performed the experiments. Y.Z., J.C., J.S., Y.G. and L.T. analyzed the data and wrote the paper. All authors edited the paper.
Corresponding authors
Ethics declarations
Competing interests
Y.G., L.T. and Y.Z. are inventors on patents related to the technology described in this paper. L.T. and Y.G. are cofounders, share-holders and advisors for Leman Biotech. The interests of L.T. were reviewed and managed by EPFL. The other authors declare no competing interests.
Peer review
Peer review information
Nature Biotechnology thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 In vitro characterizations of IL-10 CAR-T cells prior to infusion.
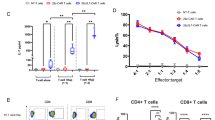
a-e, C57BL/6 mice were inoculated subcutaneously (s.c.) with MC38-HER2 colon cancer cells (3 × 105) and received intravenously (i.v.) adoptive cell transfer of HER2 CAR-T cells (3 × 106), untransduced T cells (Ctrl T, equivalent cell number infused), or phosphate buffered saline (PBS) on day 6. Mice were either monitored for tumor growth (n = 5 mice) or sacrificed on day 14 for the analysis of CAR-T cells (n = 10 mice). a, Experimental timeline. b–e, Shown are individual tumor growth curves (b), survival curves (c), frequencies of granzyme B+IFNγ+TNFα+ polyfunctional CAR-T cells in spleen and tumor (d), and MFI of PD-1 and TIM-3 (e). Indicated are numbers of tumor-free mice/total mice in each group (c). f, HER2 CAR or IL-10 HER2 CAR constructs were introduced via retroviral vectors. CAR expression levels were assessed by flow cytometry. Data are one representative of ten independent experiments. g, HER2 CAR-T or IL-10 HER2 CAR-T cells were co-cultured with MC38-HER2 tumor cells for 3 days (n = 3 biologically independent samples)). The culture supernatants were assessed for IL-10 concentration using an enzyme-linked immunosorbent assay (ELISA). h-q, CAR-T cells prior to infusion were examined for the expression levels of indicated markers by flow cytometry (n = 9 biologically independent samples for IL-7Rα, and n = 10 biologically independent samples for others). Shown are average MFI of CD44 (h), CD62L (i), Sca-1 (j), CD122 (k), IL-7Rα (l), KLRG-1 (m), PD-1 (n), TIM-3 (o), and CD69 (p) expression in HER2 CAR-T and IL-10 HER2 CAR-T cells. q, Average frequencies of viable CAR-T cells. All data represent the mean ± s.e.m. and are analyzed by two-tailed Student’s t-test (d, e, g-q), or one- or two-way ANOVA with Tukey’s multiple-comparisons test (b) or log-rank test (c). ns, not significant (P > 0.05). Data are one representative of two independent experiments.
Extended Data Fig. 2 IL-10 expression shows negligible effects on other immune cells or endogenous T cells in tumor.
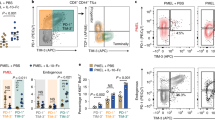
a-c, The experimental setting was the same as described in Fig. 1b (n = 5 mice). a, Counts of TCF-1+PD-1+TIM3−CD8+ HER2 CAR-T cells in tumors. b,c, Frequencies of IFNγ+Granzyme B+TNFα+ polyfunctional cells among PD-1+TCF-1+TIM3− (b) and PD-1+TCF-1−TIM3+ (c) CD8+ CAR-T cells. d, The experimental setting was the same as described in Fig. 1b (n = 10 mice). Counts of indicated immune cell subsets in the MC38-HER2 tumors from each treatment group. e-g, C57BL/6 mice were inoculated s.c. with MC38-HER2 colon cancer cells (1 × 106) and received i.v. adoptive transfer of IL-10 HER2 CAR-T cells (3 × 106) or HER2 CAR-T cells (3 × 106) on day 6 (n = 5 mice). e, Experimental timeline. f, Counts of endogenous T cells. g, Counts of Foxp3+CD25+CD4+ endogenous Tregs. h,i, The experimental setting is described in Fig. 1b. CAR-T cells in tumors were classified into several subpopulations based on the gating of CAR density (Fig. 1j). Shown are response curves of MFI of TNFα (h) and TIM-3 (i) as a function of CAR density (n = 4 mice). Indicated are P values. All data represent the mean ± s.e.m. and are analyzed by two-tailed Student’s t-test (a-c, h, i), or one-way ANOVA with Tukey’s multiple-comparisons test (d, f, g). Data are one representative of two independent experiments.
Extended Data Fig. 3 Preparation and in vitro characterizations of IL-10-secreting CD19 hCAR-T cells.
a, Schematic depicting constructs of CD19-targeted second-generation CD28-based human CAR (CD19 hCAR) and human IL-10-secreting CD19 hCAR (IL-10 CD19 hCAR). b, The expression levels of CD19 hCAR were analyzed by flow cytometry. Ctrl T, untransduced control human T cells. c, The culture supernatants were examined for the concentration of hIL-10 on day 8 after hCAR-T cell preparation (n = 12 biologically independent samples). d, e, CD19 hCAR-T and IL-10 CD19 hCAR-T cells were cocultured with PANC1-CD19 cells or Raji cells at different E:T ratios for 48 h (n = 3 biologically independent samples). Shown are the percentage of lysis of PANC1-CD19 cell (d) or Raji cell (e). f,g, The proliferation of CD19 hCAR and IL-10 CD19 hCAR-T cells after multiple weekly stimulation (indicated by arrows; n = 3) with irradiated NIH/3T3-CD19 (f) or PANC1-CD19 (g) cells (n = 3 biologically independent samples). All data represent the mean ± s.e.m. and are analyzed by two-tailed Student’s t-test. Data are one representative of two independent experiments.
Extended Data Fig. 4 IL-10 expression improved mitochondrial fitness and increased the levels of pyruvate production intermediates and TCA cycle intermediates in CAR-T cells.
The experimental setting is described in Fig. 2a. a, MFI of PD-1, TIM-3, and CD107a in MDR/MGhi and MDR/MGlo CAR-T cells in tumor (n = 10 mice). b, Quantification of roundness index of mitochondria in CAR-T cells as shown in Fig. 2i (n = 15 biologically independent samples). c, Representative contour plot showing CAR-T cells in tumor were classified into three subpopulations based on CAR density. d-f, The experimental setting is described in Fig. 3a-c (n = 3 biologically independent samples). d, Real-time analysis of ECAR. e, Average basal and maximal ECAR. f, Average basal and maximal OCR of HER2 CAR-T and IL-10 HER2 CAR-T cells in the presence or absence of IL-10 without antigen stimulation. g-j, C57BL/6 mice were inoculated s.c. with MC38-HER2 colon cancer cells (1 × 106), lymphodepleted, and received IL-10 HER2 CAR-T cells or HER2 CAR-T cells (3 × 106, i.v.) on day 6. On day 14, tumor-infiltrating CAR-T cells were sorted for metabolomics analysis (n = 3 biologically independent samples, each sample is pooled from n = 5 mice). g, Experimental timeline. h, Clustered heatmap of significantly altered metabolites (P < 0.05) in HER2 CAR-T and IL-10 HER2 CAR-T cells. The color blocks indicate relative metabolite levels, with high expression in red and low expression in green. i,j, Fold changes of phosphoenolpyruvate (i) and succinate (j) (relative to HER2 CAR-T cells). k,l, HER2 CAR-T or IL-10 HER2 CAR-T cells were cocultured with MC38-HER2 cells in the presence of indicated inhibitors (n = 3 biologically independent samples). Shown are relative CAR-T cell counts (k) and relative frequencies of granzyme B+IFNγ+TNFα+ polyfunctional CAR-T cells (l) (IL-10 HER2 CAR-T vs. HER2 CAR-T). Data are one representative of two independent experiments. All data represent the mean ± s.e.m. and are analyzed by two-tailed Student’s t-test (a, b, i, j) or by one- or two-way ANOVA with Tukey’s multiple-comparisons test (e, f, k, l).
Extended Data Fig. 5 IL-10-expressing HER2 CAR-T cells exhibit an expanded subpopulation of CD8+ terminally exhausted T cells with upregulated gene expression encoding cell cycling, cytotoxicity, and OXPHOS.
a, The experimental setting and timeline is described in Fig. 4. b, Heatmap representing the expression levels of representative marker genes in each cluster. c, Projection of IL-10 HER2 CAR-T cells and HER2 CAR-T cells into a reference atlas of tumor-infiltrating lymphocytes. Reference atlas states are indicated as colored cells. CD8+ terminally-exhausted T cell cluster was characterized as high expression of granzymes, multiple inhibitory receptors (Pdcd1, Ctla4, Lag3, Tigit, Havcr2/TIM-3, etc.) and Tox16. Shown are cell subtype compositions for each group. Black contour lines represent the density of projected cells. d, Gene signature scores for each group of samples (HER2 CAR-T and IL-10 HER2 CAR-T cells) for the indicated gene sets.
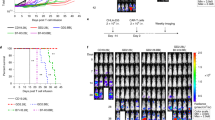
Extended Data Fig. 6 In vivo assessment of therapeutic efficacy and toxicity of IL-10 HER2 CAR-T cells in the MC38-HER2 tumor model.
a, Experimental setting is described in Fig. 5a. Shown are individual tumor growth curves. Indicated are numbers of tumor-free mice among the total number of mice in the group (a). b-e, The experimental setting was the same as described in Fig. 5a except another two treatment groups were added: HER2 CAR-T cells (3 × 106) in combination with IL-10 − Fc12 (20 µg × 5, equivalent to native IL-10 at the dose of 3.5 µg × 5) through i.v. or peritumoral (p.t.) administration (n = 5 mice). Shown are experimental timeline (b), average tumor growth curves (c), survival curves (d), and individual tumor growth curves (e). Indicated are numbers of tumor-free mice among the total number of mice in the group (d, e). f, Body weight of treated mice in both Fig. 5b and Extended Data Fig. 6c (n = 10 mice for the PBS, HER2 CAR-T, and IL-10 HER2 CAR-T groups, and n = 5 mice for the rest of the groups). g, The experimental setting was the same as described in Fig. 1b (n = 5 mice). Serum was collected and examined for the concentration of IL-1β, IL-6, IL-2, and IFNγ by ELISA. All data represent the mean ± s.e.m. and are analyzed by one-way ANOVA with Tukey’s multiple-comparisons test.
Extended Data Fig. 7 Preparation and efficacy assessment of TRP-1 CAR-T cells in the subcutaneous and orthotopic B16F10 tumor models.
a, Schematic depicting constructs of TRP-1-directed second-generation CD28-based CAR (TRP-1 CAR) and murine IL-10-secreting TRP-1 CAR (IL-10 TRP-1 CAR). b, The expression levels of TRP-1 CAR were examined by staining the c-Myc tag. Ctrl T, untransduced T cells as control. c, IL-10 TRP-1 CAR-T or TRP-1 CAR-T cells were co-cultured with B16F10 cells for 3 days (n = 3 biologically independent samples). The culture supernatants were examined for the concentration of IL-10 by ELISA. d, IL-10 TRP-1 CAR-T or TRP-1 CAR-T cells in the absence or presence of IL-10 (145 ng/ml) were cocultured with B16F10 cells at the E:T ratio of 0.5:1 for 48 h (n = 3 biologically independent samples). The percentage of tumor cell lysis was analyzed by flow cytometry. e,f, Experimental setting is described in Fig. 5d (n = 5 mice). Shown are individual tumor growth curves (e) and relative body weight (f). Indicated are numbers of tumor-free mice among the total number of mice in the group (e). g-j, C57BL/6 mice were inoculated intradermally (i.d.) with B16F10 cells (2 × 105) to establish an orthotopic melanoma model. Mice were then lymphodepleted, and received i.v. adoptive transfer of IL-10 TRP-1 CAR-T cells (3 × 106), or TRP-1 CAR-T cells (3 × 106) on day 6 (n = 7 mice). g, Experimental timeline. h-j, Shown are average tumor growth curves (h), mouse survival curves (i), and individual tumor growth curves (j) of the orthotopic B16F10 melanoma model. Indicated are numbers of tumor-free mice among the total number of mice in the group (i, j). All data represent the mean ± s.e.m. and are analyzed by two-tailed Student’s t-test (c), or by one- or two-way ANOVA with Tukey’s multiple-comparisons test (d, h), or by log-rank test (i).
Extended Data Fig. 8 Preparation and efficacy assessment of EGFRvIII CAR-T cells in the 4T1-EGFRvIII-Luc tumor model.
a, Schematic depicting constructs of EGFRvIII-directed second-generation CD28-based CAR (EGFRvIII CAR), and murine IL-10-secreting EGFRvIII CAR (IL-10 EGFRvIII CAR). b, The expression levels of EGFRvIII CAR were examined by staining the c-Myc tag. Ctrl T, untransduced T cells as control. c, EGFRvIII CAR-T or IL-10 EGFRvIII CAR-T cells were co-cultured with 4T1-EGFRvIIIl-Luc cells for 3 days (n = 5 biologically independent samples for EGFRvIII CAR-T, n = 10 biologically independent samples for EGFRvIII CAR-T). The culture supernatants were examined for the concentration of IL-10 by ELISA. d, IL-10 EGFRvIII CAR-T or EGFRvIII CAR-T cells in the presence or absence of IL-10 were cocultured with 4T1-EGFRvIII-Luc cells at the E:T ratio of 5:1 for 18 h (n = 4 biologically independent samples). CAR-T cells were then isolated for a seahorse assay. Shown are average basal OCR. e, IL-10 EGFRvIII CAR-T or EGFRvIII CAR-T cells in the absence or presence of IL-10 (145 ng/ml) were cocultured with 4T1-EGFRvIIIl-Luc cells at the E:T ratio of 0.5:1 for 48 h (n = 4 biologically independent samples). The percentage of tumor cell lysis was analyzed by flow cytometry. f, Experimental setting is described in Fig. 5f (n = 5 mice). Shown are relative body weight. g, BALB/c mice were i.v. inoculated with 4T1-EGFRvIII-Luc cancer cells (5 × 104), lymphodepleted, and received i.v. adoptive transfer of IL-10 EGFRvIII CAR-T cells (3 × 106), or EGFRvIII CAR-T cells (3 × 106) in the presence or absence of i.v. administered IL-10 (1 µg) on day 6 (n = 5 mice). On day 6, 9, 12, 15, and 18, the blood samples were collected and analyzed by flow cytometry. Shown are counts of viable EGFRvIII CAR-T cells in the peripheral blood of individual mouse. All data represent the mean ± s.e.m. and are analyzed by two-tailed Student’s t-test (c, d), or by one- or two-way ANOVA with Tukey’s multiple-comparisons test (e, g). Data are one representative of two independent experiments.
Extended Data Fig. 9 Efficacy assessment of hCAR-T cells in the subcutaneous Raji and PANC1-CD19 xenograft tumor models, and the orthotopic PANC1-CD19-Luc xenograft tumor model.
a, b, Experimental setting is described in Fig. 5i. a, b, Shown are individual tumor growth curves of the subcutaneous Raji model (a) and PANC1-CD19 model (b). Indicated are numbers of tumor-free mice among the total number of mice in the group (a, b). c-e, PANC1-CD19-Luc human epithelioid carcinoma cells (2 × 106) were implanted in the tail of the pancreas of NSG mice to establish an orthotopic human PDAC model. Mice were then received i.v. adoptive cell transfer of CD19 hCAR-T or IL-10 CD19 hCAR-T cells (1 × 105) on day 4 (n = 5 mice). c, Experimental timeline. d, Longitudinal bioluminescent imaging was performed to monitor orthotopic tumor growth. e, Average radiance (p/s/cm2/sr) of mice of different treatment groups. f-i, PANC1-CD19-Luc human epithelioid carcinoma cells (2 × 106) were orthotopically implanted in the tail of the pancreas of NSG mice. Mice were then received i.v. adoptive cell transfer of CD19 hCAR-T or IL-10 CD19 hCAR-T cells (5 × 105) on day 4 (n = 5 mice). On day 18, 28 and 50, the blood samples were collected and analyzed by flow cytometry. Survivors from treatment groups of CD19 hCAR-T and IL-10 CD19 hCAR-T were rechallenged orthotopically with PANC1-CD19-Luc (2 × 106) cells on day 50 post primary inoculation. Naive NSG mice were inoculated with the same number of tumor cells as controls. f, Experimental timeline. g, Shown are counts of viable hCAR-T cells in peripheral blood on day 18, 28 and 50. n = 10 mice pooled from two independent experiments. h, Longitudinal bioluminescent imaging was performed to monitor tumor growth post rechallenge (n = 5 mice). i, Individual radiance (p/s/cm2/sr) showing the rechallenged tumor growth of survivors from indicated treatment groups (n = 5 mice). All data represent the mean ± s.e.m. and are analyzed by two-tailed Student’s t-test (g, i), or by Mann–Whitney U test (e). Data are one representative of two independent experiments.
Extended Data Fig. 10 IL-10 expression promotes stemness in mouse and human CAR-T cells.
a, The experimental timeline for Fig. 6a-c. b-d, The experimental setting is described in Fig. 6d (n = 5 mice). Shown are frequencies of CD62LhiCD44lo cells among total CAR-T cells (b) and Sca-1+CD122+ cells among CD62LhiCD44lo CAR-T cells (c) in blood. d, Shown are the frequencies of IL-7Rα+KLRG-1– among total CAR-T cells in blood. e-i, BALB/c mice were inoculated with 4T1-EGFRvIII-Luc (5 × 104, i.v.), sublethally lymphodepleted by irradiation on day 5, and received i.v. adoptive transfer of IL-10 EGFRvIII CAR-T cells (3 × 106), or EGFRvIII CAR-T cells (3 × 106) on day 6 (n = 5 mice). On day 18, mice were killed for phenotype analyses of CAR-T cells in spleen and bone marrow by flow cytometry. e, Experimental timeline. f, h, Average frequencies of CD62LhiCD44lo cells among total CAR-T cells in spleen (f) and bone marrow (h). g, i, Average frequencies of Sca-1+CD122+ cells among CD62LhiCD44lo CAR-T cells in spleen (g) and bone marrow (i). j, The experimental setting is described in Fig. 6d. Shown are single-cell expression of key marker genes over the UMAP representation of the map. k-m, NSG mice were inoculated (s.c.) with PANC1-CD19 cells (2 × 106) and received i.v. adoptive cell transfers of CD19 hCAR-T cells (1 × 106) or IL-10 CD19 hCAR-T cells (1 × 106) on day 8 (n = 4 mice). On day 63, mice were killed for phenotype analyses of CAR-T cells in spleen and bone marrow by flow cytometry. k, Experimental timeline. l, m, Shown are counts of CD45RA+CD27+CD62L+CD95+CCR7+ human CD8+ CAR-T cells in spleen (l) and bone marrow (m). n, The experimental setting is described in Fig. 6k. Shown are single-cell expression of key marker genes over the UMAP representation of the map. All data represent the mean ± s.e.m. and are analyzed by two-tailed Student’s t-test (f, g, h, i, l, m), one-way ANOVA with Tukey’s multiple-comparisons test (b-d).
Supplementary information
Supplementary Information
Supplementary Fig. 1.
Supplementary Table 1
Metabolomics of HER2 CAR T cells in tumors.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 7
Statistical source data.
Source Data Extended Data Fig. 8
Statistical source data.
Source Data Extended Data Fig. 9
Statistical source data.
Source Data Extended Data Fig. 10
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhao, Y., Chen, J., Andreatta, M. et al. IL-10-expressing CAR T cells resist dysfunction and mediate durable clearance of solid tumors and metastases. Nat Biotechnol (2024). https://doi.org/10.1038/s41587-023-02060-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41587-023-02060-8
This article is cited by
-
Tonic-ing emissions and compatibility to turbocharge CAR-T
Nature Metabolism (2024)
-
Incorporating IL7 receptor alpha signaling in the endodomain of B7H3-targeting chimeric antigen receptor T cells mediates antitumor activity in glioblastoma
Cancer Immunology, Immunotherapy (2024)