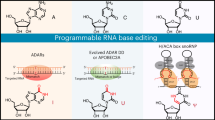
Abstract
RNA base editing refers to the rewriting of genetic information within an intact RNA molecule and serves various functions, such as evasion of the endogenous immune system and regulation of protein function. To achieve this, certain enzymes have been discovered in human cells that catalyze the conversion of one nucleobase into another. This natural process could be exploited to manipulate and recode any base in a target transcript. In contrast to DNA base editing, analogous changes introduced in RNA are not permanent or inheritable but rather allow reversible and doseable effects that appeal to various therapeutic applications. The current practice of RNA base editing involves the deamination of adenosines and cytidines, which are converted to inosines and uridines, respectively. In this Review, we summarize current site-directed RNA base-editing strategies and highlight recent achievements to improve editing efficiency, precision, codon-targeting scope and in vivo delivery into disease-relevant tissues. Besides engineered editing effectors, we focus on strategies to harness endogenous adenosine deaminases acting on RNA (ADAR) enzymes and discuss limitations and future perspectives to apply the tools in basic research and as a therapeutic modality. We expect the field to realize the first RNA base-editing drug soon, likely on a well-defined genetic disease. However, the long-term challenge will be to carve out the sweet spot of the technology where its unique ability is exploited to modulate signaling cues, metabolism or other clinically relevant processes in a safe and doseable manner.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Gray, M. W. Evolutionary origin of RNA editing. Biochemistry 51, 5235–5242 (2012).
Covello, P. & Gray, M. On the evolution of RNA editing. Trends Genet. 9, 265–268 (1993).
Yablonovitch, A. L., Deng, P., Jacobson, D. & Li, J. B. The evolution and adaptation of A-to-I RNA editing. PLoS Genet. 13, e1007064 (2017).
Zhang, P. et al. On the origin and evolution of RNA editing in metazoans. Cell Rep. 42, 112112 (2023).
Song, B., Shiromoto, Y., Minakuchi, M. & Nishikura, K. The role of RNA editing enzyme ADAR1 in human disease. Wiley Interdiscip. Rev. RNA 13, e1665 (2021).
Harris, R. S. & Dudley, J. P. APOBECs and virus restriction. Virology 479–480, 131–145 (2015).
Nishikura, K. A-to-I editing of coding and non-coding RNAs by ADARs. Nat. Rev. Mol. Cell Biol. 17, 83–96 (2016).
Vesely, C. & Jantsch, M. F. An I for an A: dynamic regulation of adenosine deamination-mediated RNA editing. Genes 12, 1026 (2021).
Pecori, R., Di Giorgio, S., Paulo Lorenzo, J. & Papavasiliou, F. N. Functions and consequences of AID/APOBEC-mediated DNA and RNA deamination. Nat. Rev. Genet. 23, 505–518 (2022).
Bass, B. L. & Weintraub, H. An unwinding activity that covalently modifies its double-stranded RNA substrate. Cell 55, 1089–1098 (1988).
Kim, U., Wang, Y., Sanford, T., Zeng, Y. & Nishikura, K. Molecular cloning of cDNA for double-stranded RNA adenosine deaminase, a candidate enzyme for nuclear RNA editing. Proc. Natl Acad. Sci. USA 91, 11457–11461 (1994).
Melcher, T. et al. RED2, a brain-specific member of the RNA-specific adenosine deaminase family. J. Biol. Chem. 271, 31795–31798 (1996).
Gerber, A., O’Connell, M. A. & Keller, W. Two forms of human double-stranded RNA-specific editase 1 (hRED1) generated by the insertion of an Alu cassette. RNA 3, 453–463 (1997).
Patterson, J. B. & Samuel, C. E. Expression and regulation by interferon of a double-stranded-RNA-specific adenosine deaminase from human cells: evidence for two forms of the deaminase. Mol. Cell. Biol. 15, 5376–5388 (1995).
George, C. X. & Samuel, C. E. Human RNA-specific adenosine deaminase ADAR1 transcripts possess alternative exon 1 structures that initiate from different promoters, one constitutively active and the other interferon inducible. Proc. Natl Acad. Sci. USA 96, 4621–4626 (1999).
Kawakubo, K. & Samuel, C. E. Human RNA-specific adenosine deaminase (ADAR1) gene specifies transcripts that initiate from a constitutively active alternative promoter. Gene 258, 165–172 (2000).
Wang, Y. et al. RNA binding candidates for human ADAR3 from substrates of a gain of function mutant expressed in neuronal cells. Nucleic Acids Res. 47, 10801–10814 (2019).
Chen, C. X. et al. A third member of the RNA-specific adenosine deaminase gene family, ADAR3, contains both single- and double-stranded RNA binding domains. RNA 6, 755–767 (2000).
Barraud, P., Banerjee, S., Mohamed, W. I., Jantsch, M. F. & Allain, F. H. A bimodular nuclear localization signal assembled via an extended double-stranded RNA-binding domain acts as an RNA-sensing signal for transportin 1. Proc. Natl Acad. Sci. USA 111, E1852–E1861 (2014).
Behm, M., Wahlstedt, H., Widmark, A., Eriksson, M. & Ohman, M. Accumulation of nuclear ADAR2 regulates A-to-I RNA editing during neuronal development. J. Cell Sci. 130, 745–753 (2017).
Maas, S. & Gommans, W. M. Identification of a selective nuclear import signal in adenosine deaminases acting on RNA. Nucleic Acids Res. 37, 5822–5829 (2009).
Fritz, J. et al. RNA-regulated interaction of transportin-1 and exportin-5 with the double-stranded RNA-binding domain regulates nucleocytoplasmic shuttling of ADAR1. Mol. Cell. Biol. 29, 1487–1497 (2009).
Matthews, M. M. et al. Structures of human ADAR2 bound to dsRNA reveal base-flipping mechanism and basis for site selectivity. Nat. Struct. Mol. Biol. 23, 426–433 (2016).
Tan, M. H. et al. Dynamic landscape and regulation of RNA editing in mammals. Nature 550, 249–254 (2017).
Peng, Z. et al. Comprehensive analysis of RNA-seq data reveals extensive RNA editing in a human transcriptome. Nat. Biotechnol. 30, 253–260 (2012).
Bazak, L. et al. A-to-I RNA editing occurs at over a hundred million genomic sites, located in a majority of human genes. Genome Res. 24, 365–376 (2014).
Levanon, E. Y. et al. Systematic identification of abundant A-to-I editing sites in the human transcriptome. Nat. Biotechnol. 22, 1001–1005 (2004).
Tang, Q. et al. Adenosine-to-inosine editing of endogenous Z-form RNA by the deaminase ADAR1 prevents spontaneous MAVS-dependent type I interferon responses. Immunity 54, 1961–1975 (2021).
Liddicoat, B. J. et al. RNA editing by ADAR1 prevents MDA5 sensing of endogenous dsRNA as nonself. Science 349, 1115–1120 (2015).
Maurano, M. et al. Protein kinase R and the integrated stress response drive immunopathology caused by mutations in the RNA deaminase ADAR1. Immunity 54, 1948–1960 (2021).
Mannion, NiamhM. et al. The RNA-editing enzyme ADAR1 controls innate immune responses to RNA. Cell Rep. 9, 1482–1494 (2014).
de Reuver, R. et al. ADAR1 prevents autoinflammation by suppressing spontaneous ZBP1 activation. Nature 607, 784–789 (2022).
Eisenberg, E. & Levanon, E. Y. A-to-I RNA editing — immune protector and transcriptome diversifier. Nat. Rev. Genet. 19, 473–490 (2018).
Lomeli, H. et al. Control of kinetic properties of AMPA receptor channels by nuclear RNA editing. Science 266, 1709–1713 (1994).
Sommer, B., Köhler, M., Sprengel, R. & Seeburg, P. H. RNA editing in brain controls a determinant of ion flow in glutamate-gated channels. Cell 67, 11–19 (1991).
Higuchi, M. et al. RNA editing of AMPA receptor subunit GluR-B: a base-paired intron–exon structure determines position and efficiency. Cell 75, 1361–1370 (1993).
Stefl, R. et al. The solution structure of the ADAR2 dsRBM–RNA complex reveals a sequence-specific readout of the minor groove. Cell 143, 225–237 (2010).
Thomas, J. M. & Beal, P. A. How do ADARs bind RNA? New protein–RNA structures illuminate substrate recognition by the RNA editing ADARs. BioEssays 39, 1600187 (2017).
Thuy-Boun, A. S. et al. Asymmetric dimerization of adenosine deaminase acting on RNA facilitates substrate recognition. Nucleic Acids Res. 48, 7958–7972 (2020).
Song, Y. et al. irCLASH reveals RNA substrates recognized by human ADARs. Nat. Struct. Mol. Biol. 27, 351–362 (2020).
Macbeth, M. R. et al. Inositol hexakisphosphate is bound in the ADAR2 core and required for RNA editing. Science 309, 1534–1539 (2005).
Liu, X. et al. Learning cis-regulatory principles of ADAR-based RNA editing from CRISPR-mediated mutagenesis. Nat. Commun. 12, 2165 (2021).
Schwartz, T., Rould, M. A., Lowenhaupt, K., Herbert, A. & Rich, A. Crystal structure of the Zα domain of the human editing enzyme ADAR1 bound to left-handed Z-DNA. Science 284, 1841–1845 (1999).
Placido, D., Brown, B. A., Lowenhaupt, K., Rich, A. & Athanasiadis, A. A left-handed RNA double helix bound by the Zα domain of the RNA-editing enzyme ADAR1. Structure 15, 395–404 (2007).
Ha, S. C. et al. The structures of non-CG-repeat Z-DNAs co-crystallized with the Z-DNA-binding domain, hZα ADAR1. Nucleic Acids Res. 37, 629–637 (2009).
Lau, P. P., Xiong, W. J., Zhu, H. J., Chen, S. H. & Chan, L. Apolipoprotein B mRNA editing is an intranuclear event that occurs posttranscriptionally coincident with splicing and polyadenylation. J. Biol. Chem. 266, 20550–20554 (1991).
Sharma, S. et al. APOBEC3A cytidine deaminase induces RNA editing in monocytes and macrophages. Nat. Commun. 6, 6881 (2015).
Teng, B., Burant Charles, F. & Davidson Nicholas, O. Molecular cloning of an apolipoprotein B messenger RNA editing protein. Science 260, 1816–1819 (1993).
Fossat, N. et al. C to U RNA editing mediated by APOBEC1 requires RNA-binding protein RBM47. EMBO Rep. 15, 903–910 (2014).
Blanc, V. et al. APOBEC1 complementation factor (A1CF) and RBM47 interact in tissue-specific regulation of C to U RNA editing in mouse intestine and liver. RNA 25, 70–81 (2019).
Snyder, E. M. et al. APOBEC1 complementation factor (A1CF) is dispensable for C-to-U RNA editing in vivo. RNA 23, 457–465 (2017).
Mehta, A. & Driscoll Donna, M. A sequence-specific RNA-binding protein complements APOBEC-1 to edit apolipoprotein B mRNA. Mol. Cell. Biol. 18, 4426–4432 (1998).
Shah, R. R. et al. Sequence requirements for the editing of apolipoprotein B mRNA. J. Biol. Chem. 266, 16301–16304 (1991).
Komor, A. C., Kim, Y. B., Packer, M. S., Zuris, J. A. & Liu, D. R. Programmable editing of a target base in genomic DNA without double-stranded DNA cleavage. Nature 533, 420–424 (2016).
Huang, X. et al. Programmable C-to-U RNA editing using the human APOBEC3A deaminase. EMBO J. 40, e108209 (2021).
Rees, H. A. & Liu, D. R. Base editing: precision chemistry on the genome and transcriptome of living cells. Nat. Rev. Genet. 19, 770–788 (2018).
Khosravi, H. M. & Jantsch, M. F. Site-directed RNA editing: recent advances and open challenges. RNA Biol. 18, 41–50 (2021).
Montiel-Gonzalez, M. F., Diaz Quiroz, J. F. & Rosenthal, J. J. C. Current strategies for site-directed RNA editing using ADARs. Methods 156, 16–24 (2019).
Vogel, P. & Stafforst, T. Critical review on engineering deaminases for site-directed RNA editing. Curr. Opin. Biotechnol. 55, 74–80 (2019).
Eggington, J. M., Greene, T. & Bass, B. L. Predicting sites of ADAR editing in double-stranded RNA. Nat. Commun. 2, 319 (2011).
Polson, A. G. & Bass, B. L. Preferential selection of adenosines for modification by double-stranded RNA adenosine deaminase. EMBO J. 13, 5701–5711 (1994).
Doherty, E. E. et al. ADAR activation by inducing a syn conformation at guanosine adjacent to an editing site. Nucleic Acids Res. 50, 10857–10868 (2022).
Kuttan, A. & Bass, B. L. Mechanistic insights into editing-site specificity of ADARs. Proc. Natl Acad. Sci. USA 109, E3295–3304 (2012).
Vogel, P. et al. Efficient and precise editing of endogenous transcripts with SNAP-tagged ADARs. Nat. Methods 15, 535–538 (2018).
Buchumenski, I. et al. Global quantification exposes abundant low-level off-target activity by base editors. Genome Res. 31, 2354–2361 (2021).
Wong, S. K., Sato, S. & Lazinski, D. W. Substrate recognition by ADAR1 and ADAR2. RNA 7, 846–858 (2001).
Schneider, M. F., Wettengel, J., Hoffmann, P. C. & Stafforst, T. Optimal guideRNAs for re-directing deaminase activity of hADAR1 and hADAR2 in trans. Nucleic Acids Res. 42, e87 (2014).
Diaz Quiroz, J. F. et al. Development of a selection assay for small guide RNAs that drive efficient site-directed RNA editing. Nucleic Acids Res. 51, e41 (2023).
Uzonyi, A. et al. Deciphering the principles of the RNA editing code via large-scale systematic probing. Mol. Cell 81, 2374–2387 (2021).
Xiang, X. et al. Enhancing CRISPR–Cas9 gRNA efficiency prediction by data integration and deep learning. Nat. Commun. 12, 3238 (2021).
Muhammad Rafid, A. H., Toufikuzzaman, M., Rahman, M. S. & Rahman, M. S. CRISPRpred(SEQ): a sequence-based method for sgRNA on target activity prediction using traditional machine learning. BMC Bioinformatics 21, 223 (2020).
Wang, J., Zhang, X., Cheng, L. & Luo, Y. An overview and metanalysis of machine and deep learning-based CRISPR gRNA design tools. RNA Biol. 17, 13–22 (2020).
Han, Y., He, F., Chen, Y., Liu, Y. & Yu, H. siRNA silencing efficacy prediction based on a deep architecture. BMC Genomics 19, 669 (2018).
Han, Y., He, F., Tan, X. & Yu, H. In 2017 IEEE International Conference on Bioinformatics and Biomedicine 16–21 (IEEE, 2017).
Merkle, T. et al. Precise RNA editing by recruiting endogenous ADARs with antisense oligonucleotides. Nat. Biotechnol. 37, 133–138 (2019).
Obika, S. et al. Synthesis of 2′-O,4′-C-methyleneuridine and -cytidine. Novel bicyclic nucleosides having a fixed C3, -endo sugar puckering. Tetrahedron Lett. 38, 8735–8738 (1997).
Koshkin, A. A. et al. LNA (locked nucleic acids): synthesis of the adenine, cytosine, guanine, 5-methylcytosine, thymine and uracil bicyclonucleoside monomers, oligomerisation, and unprecedented nucleic acid recognition. Tetrahedron 54, 3607–3630 (1998).
Vogel, P., Schneider, M. F., Wettengel, J. & Stafforst, T. Improving site-directed RNA editing in vitro and in cell culture by chemical modification of the guideRNA. Angew. Chem. Int. Ed. Engl. 53, 6267–6271 (2014).
Monian, P. et al. Endogenous ADAR-mediated RNA editing in non-human primates using stereopure chemically modified oligonucleotides. Nat. Biotechnol. 40, 1093–1102 (2022).
Doherty, E. E. et al. Rational design of RNA editing guide strands: cytidine analogs at the orphan position. J. Am. Chem. Soc. 143, 6865–6876 (2021).
Cho, S. W. et al. Analysis of off-target effects of CRISPR/Cas-derived RNA-guided endonucleases and nickases. Genome Res. 24, 132–141 (2014).
Zhou, C. et al. Off-target RNA mutation induced by DNA base editing and its elimination by mutagenesis. Nature 571, 275–278 (2019).
Rueter, S. M., Dawson, T. R. & Emeson, R. B. Regulation of alternative splicing by RNA editing. Nature 399, 75–80 (1999).
Licht, K. et al. Inosine induces context-dependent recoding and translational stalling. Nucleic Acids Res. 47, 3–14 (2019).
Vogel, P., Hanswillemenke, A. & Stafforst, T. Switching protein localization by site-directed RNA editing under control of light. ACS Synth. Biol. 6, 1642–1649 (2017).
Monteleone, L. R. et al. A bump–hole approach for directed RNA editing. Cell Chem. Biol. 26, 269–277 (2019).
Abudayyeh Omar, O. et al. A cytosine deaminase for programmable single-base RNA editing. Science 365, 382–386 (2019).
Cox, D. B. T. et al. RNA editing with CRISPR–Cas13. Science 358, 1019–1027 (2017).
Vallecillo-Viejo, I. C., Liscovitch-Brauer, N., Montiel-Gonzalez, M. F., Eisenberg, E. & Rosenthal, J. J. C. Abundant off-target edits from site-directed RNA editing can be reduced by nuclear localization of the editing enzyme. RNA Biol. 15, 104–114 (2018).
Katrekar, D. et al. Comprehensive interrogation of the ADAR2 deaminase domain for engineering enhanced RNA editing activity and specificity. eLife 11, e75555 (2022).
Katrekar, D. et al. In vivo RNA editing of point mutations via RNA-guided adenosine deaminases. Nat. Methods 16, 239–242 (2019).
Hanswillemenke, A., Kuzdere, T., Vogel, P., Jekely, G. & Stafforst, T. Site-directed RNA editing in vivo can be triggered by the light-driven assembly of an artificial riboprotein. J. Am. Chem. Soc. 137, 15875–15881 (2015).
Hanswillemenke, A. & Stafforst, T. Protocols for the generation of caged guideRNAs for light-triggered RNA-targeting with SNAP–ADARs. Methods Enzymol. 624, 47–68 (2019).
Stroppel, A. S., Lappalainen, R. & Stafforst, T. Controlling site-directed RNA editing by chemically induced dimerization. Chemistry 27, 12300–12304 (2021).
Rauch, S., Jones, K. A. & Dickinson, B. C. Small molecule-inducible RNA-targeting systems for temporal control of RNA regulation. ACS Cent. Sci. 6, 1987–1996 (2020).
Sinnamon, J. R. et al. Site-directed RNA repair of endogenous Mecp2 RNA in neurons. Proc. Natl Acad. Sci. USA 114, E9395–E9402 (2017).
Sinnamon, J. R. et al. Targeted RNA editing in brainstem alleviates respiratory dysfunction in a mouse model of Rett syndrome. Proc. Natl Acad. Sci. USA 119, e2206053119 (2022).
Sinnamon, J. R. et al. In vivo repair of a protein underlying a neurological disorder by programmable RNA editing. Cell Rep. 32, 107878 (2020).
Wang, D., Tai, P. W. L. & Gao, G. Adeno-associated virus vector as a platform for gene therapy delivery. Nat. Rev. Drug Discov. 18, 358–378 (2019).
Jiang, T. et al. Chemical modifications of adenine base editor mRNA and guide RNA expand its application scope. Nat. Commun. 11, 1979 (2020).
Kariko, K., Buckstein, M., Ni, H. & Weissman, D. Suppression of RNA recognition by Toll-like receptors: the impact of nucleoside modification and the evolutionary origin of RNA. Immunity 23, 165–175 (2005).
Judge, A. & MacLachlan, I. Overcoming the innate immune response to small interfering RNA. Hum. Gene Ther. 19, 111–124 (2008).
Kim, S. et al. CRISPR RNAs trigger innate immune responses in human cells. Genome Res. 28, 367–373 (2018).
Stafforst, T. & Schneider, M. F. An RNA–deaminase conjugate selectively repairs point mutations. Angew. Chem. Int. Ed. Engl. 51, 11166–11169 (2012).
Montiel-Gonzalez, M. F., Vallecillo-Viejo, I., Yudowski, G. A. & Rosenthal, J. J. C. Correction of mutations within the cystic fibrosis transmembrane conductance regulator by site-directed RNA editing. Proc. Natl Acad. Sci. USA 110, 18285–18290 (2013).
Keppler, A. et al. A general method for the covalent labeling of fusion proteins with small molecules in vivo. Nat. Biotechnol. 21, 86–89 (2003).
Craig, N. L. The mechanism of conservative site-specific recombination. Annu. Rev. Genet. 22, 77–105 (1988).
Sauer, B. Site-specific recombination: developments and applications. Curr. Opin. Biotechnol. 5, 521–527 (1994).
Stroppel, A. S. et al. Harnessing self-labeling enzymes for selective and concurrent A-to-I and C-to-U RNA base editing. Nucleic Acids Res. 49, e95 (2021).
Latifi, N., Mack, A. M., Tellioglu, I., Di Giorgio, S. & Stafforst, T. Precise and efficient C-to-U RNA base editing with SNAP-CDAR-S. Nucleic Acids Res. 51, e84 (2023).
Los, G. V. et al. HaloTag: a novel protein labeling technology for cell imaging and protein analysis. ACS Chem. Biol. 3, 373–382 (2008).
Chattopadhyay, S., Garcia-Mena, J., DeVito, J., Wolska, K. & Das, A. Bipartite function of a small RNA hairpin in transcription antitermination in bacteriophage lambda. Proc. Natl Acad. Sci. USA 92, 4061–4065 (1995).
Austin, R. J., Xia, T., Ren, J., Takahashi, T. T. & Roberts, R. W. Designed arginine-rich RNA-binding peptides with picomolar affinity. J. Am. Chem. Soc. 124, 10966–10967 (2002).
Montiel-González, M. F., Vallecillo-Viejo, I. C. & Rosenthal, JoshuaJ. C. An efficient system for selectively altering genetic information within mRNAs. Nucleic Acids Res. 44, e157 (2016).
Azad, M. T. A., Bhakta, S. & Tsukahara, T. Site-directed RNA editing by adenosine deaminase acting on RNA for correction of the genetic code in gene therapy. Gene Ther. 24, 779–786 (2017).
Bhakta, S., Azad, M. T. A. & Tsukahara, T. Genetic code restoration by artificial RNA editing of ochre stop codon with ADAR1 deaminase. Protein Eng. Des. Sel. 31, 471–478 (2018).
Tutucci, E. et al. An improved MS2 system for accurate reporting of the mRNA life cycle. Nat. Methods 15, 81–89 (2018).
Valegård, K. et al. The three-dimensional structures of two complexes between recombinant MS2 capsids and RNA operator fragments reveal sequence-specific protein–RNA interactions. J. Mol. Biol. 270, 724–738 (1997).
Bhakta, S. & Tsukahara, T. Double MS2 guided restoration of genetic code in amber (TAG), opal (TGA) and ochre (TAA) stop codon. Enzyme Microb. Technol. 149, 109851 (2021).
Rauch, S. et al. Programmable RNA-guided RNA effector proteins built from human parts. Cell 178, 122–134 (2019).
Marina, R. J., Brannan, K. W., Dong, K. D., Yee, B. A. & Yeo, G. W. Evaluation of engineered CRISPR–Cas-mediated systems for site-specific RNA editing. Cell Rep. 33, 108350 (2020).
Sharma, S. & Baysal, B. E. Stem-loop structure preference for site-specific RNA editing by APOBEC3A and APOBEC3G. PeerJ 5, e4136 (2017).
Tang, G. et al. Creating RNA specific C-to-U editase from APOBEC3A by separation of its activities on DNA and RNA substrates. ACS Synth. Biol. 10, 1106–1115 (2021).
Kannan, S. et al. Compact RNA editors with small Cas13 proteins. Nat. Biotechnol. 40, 194–197 (2021).
Xu, C. et al. Programmable RNA editing with compact CRISPR–Cas13 systems from uncultivated microbes. Nat. Methods 18, 499–506 (2021).
Xiao, Q. et al. Rescue of autosomal dominant hearing loss by in vivo delivery of mini dCas13X-derived RNA base editor. Sci. Transl. Med. 14, eabn0449 (2022).
Charlesworth, C. T. et al. Identification of preexisting adaptive immunity to Cas9 proteins in humans. Nat. Med. 25, 249–254 (2019).
Li, A. et al. AAV–CRISPR gene editing is negated by pre-existing immunity to Cas9. Mol. Ther. 28, 1432–1441 (2020).
Wagner, D. L. et al. High prevalence of Streptococcus pyogenes Cas9-reactive T cells within the adult human population. Nat. Med. 25, 242–248 (2019).
Hakim, C. H. et al. Cas9-specific immune responses compromise local and systemic AAV CRISPR therapy in multiple dystrophic canine models. Nat. Commun. 12, 6769 (2021).
Simhadri, V. L. et al. Prevalence of pre-existing antibodies to CRISPR-associated nuclease Cas9 in the USA population. Mol. Ther. Methods Clin. Dev. 10, 105–112 (2018).
Haapaniemi, E., Botla, S., Persson, J., Schmierer, B. & Taipale, J. CRISPR–Cas9 genome editing induces a p53-mediated DNA damage response. Nat. Med. 24, 927–930 (2018).
Ihry, R. J. et al. p53 inhibits CRISPR–Cas9 engineering in human pluripotent stem cells. Nat. Med. 24, 939–946 (2018).
Enache, O. M. et al. Cas9 activates the p53 pathway and selects for p53-inactivating mutations. Nat. Genet. 52, 662–668 (2020).
Tang, X.-Z. E., Tan, S. X., Hoon, S. & Yeo, G. W. Pre-existing adaptive immunity to the RNA-editing enzyme Cas13d in humans. Nat. Med. 28, 1372–1376 (2022).
Stafforst, T. Chemistry helps to bump off-target edits away. Cell Chem. Biol. 26, 151–152 (2019).
Zhou, W. et al. Expanding the binding specificity for RNA recognition by a PUF domain. Nat. Commun. 12, 5107 (2021).
Filipovska, A., Razif, M. F. M., Nygård, K. K. A. & Rackham, O. A universal code for RNA recognition by PUF proteins. Nat. Chem. Biol. 7, 425–427 (2011).
Han, W. et al. Programmable RNA base editing with a single gRNA-free enzyme. Nucleic Acids Res. 50, 9580–9595 (2022).
Reautschnig, P. et al. CLUSTER guide RNAs enable precise and efficient RNA editing with endogenous ADAR enzymes in vivo. Nat. Biotechnol. 40, 759–768 (2022).
Qu, L. et al. Programmable RNA editing by recruiting endogenous ADAR using engineered RNAs. Nat. Biotechnol. 37, 1059–1069 (2019).
Tassinari, V. et al. ADAR1 is a new target of METTL3 and plays a pro-oncogenic role in glioblastoma by an editing-independent mechanism. Genome Biol. 22, 51 (2021).
Teoh, P. J. et al. Aberrant hyperediting of the myeloma transcriptome by ADAR1 confers oncogenicity and is a marker of poor prognosis. Blood 132, 1304–1317 (2018).
Behroozi, J., Shahbazi, S., Bakhtiarizadeh, M. R. & Mahmoodzadeh, H. ADAR expression and copy number variation in patients with advanced gastric cancer. BMC Gastroenterol. 20, 152 (2020).
Chan, T. H. M. et al. ADAR-mediated RNA editing predicts progression and prognosis of gastric cancer. Gastroenterology 151, 637–650 (2016).
Herbert, A. ADAR and immune silencing in cancer. Trends Cancer 5, 272–282 (2019).
Salvetat, N. et al. Phosphodiesterase 8A to discriminate in blood samples depressed patients and suicide attempters from healthy controls based on A-to-I RNA editing modifications. Transl. Psychiatry 11, 255 (2021).
Breen, M. S. et al. Global landscape and genetic regulation of RNA editing in cortical samples from individuals with schizophrenia. Nat. Neurosci. 22, 1402–1412 (2019).
Barbon, A. & Magri, C. RNA editing and modifications in mood disorders. Genes 11, 872 (2020).
Chatterjee, B., Shen, C. J. & Majumder, P. RNA modifications and RNA metabolism in neurological disease pathogenesis. Int. J. Mol. Sci. 22, 11870 (2021).
Yi, Z. et al. Engineered circular ADAR-recruiting RNAs increase the efficiency and fidelity of RNA editing in vitro and in vivo. Nat. Biotechnol. 40, 946–955 (2022).
Katrekar, D. et al. Efficient in vitro and in vivo RNA editing via recruitment of endogenous ADARs using circular guide RNAs. Nat. Biotechnol. 40, 938–945 (2022).
Litke, J. L. & Jaffrey, S. R. Highly efficient expression of circular RNA aptamers in cells using autocatalytic transcripts. Nat. Biotechnol. 37, 667–675 (2019).
Lehmann, K. A. & Bass, B. L. The importance of internal loops within RNA substrates of ADAR1. J. Mol. Biol. 291, 1–13 (1999).
Wang, D. et al. Characterization of an MPS I-H knock-in mouse that carries a nonsense mutation analogous to the human IDUA-W402X mutation. Mol. Genet. Metab. 99, 62–71 (2010).
Wettengel, J., Reautschnig, P., Geisler, S., Kahle, P. J. & Stafforst, T. Harnessing human ADAR2 for RNA repair—recoding a PINK1 mutation rescues mitophagy. Nucleic Acids Res. 45, 2797–2808 (2016).
Fukuda, M. et al. Construction of a guide-RNA for site-directed RNA mutagenesis utilising intracellular A-to-I RNA editing. Sci. Rep. 7, 41478 (2017).
Heep, M., Mach, P., Reautschnig, P., Wettengel, J. & Stafforst, T. Applying human ADAR1p110 and ADAR1p150 for site-directed RNA editing—G/C substitution stabilizes guideRNAs against editing. Genes 8, 34 (2017).
Nose, K., Hidaka, K., Yano, T., Tomita, Y. & Fukuda, M. Short-chain guide RNA for site-directed A-to-I RNA editing. Nucleic Acid Ther. 31, 58–67 (2020).
Vickers, T. A. et al. Efficient reduction of target RNAs by small interfering RNA and RNase H-dependent antisense agents: a comparative analysis. J. Biol. Chem. 278, 7108–7118 (2003).
Vickers, T. A., Wyatt, J. R. & Freier, S. M. Effects of RNA secondary structure on cellular antisense activity. Nucleic Acids Res. 28, 1340–1347 (2000).
Enuka, Y. et al. Circular RNAs are long-lived and display only minimal early alterations in response to a growth factor. Nucleic Acids Res. 44, 1370–1383 (2016).
Shen, X. & Corey, D. R. Chemistry, mechanism and clinical status of antisense oligonucleotides and duplex RNAs. Nucleic Acids Res. 46, 1584–1600 (2018).
Crooke, S. T., Baker, B. F., Crooke, R. M. & Liang, X.-H. Antisense technology: an overview and prospectus. Nat. Rev. Drug Discov. 20, 427–453 (2021).
Crooke, S. T. et al. Integrated assessment of the clinical performance of GalNAc3-conjugated 2′-O-methoxyethyl chimeric antisense oligonucleotides: I. Human volunteer experience. Nucleic Acid Ther. 29, 16–32 (2018).
Viney, N. J. et al. Antisense oligonucleotides targeting apolipoprotein(a) in people with raised lipoprotein(a): two randomised, double-blind, placebo-controlled, dose-ranging trials. Lancet 388, 2239–2253 (2016).
Brown, D. A. et al. Effect of phosphorothioate modification of oligodeoxynucleotides on specific protein binding. J. Biol. Chem. 269, 26801–26805 (1994).
Weidner, D. A., Valdez, B. C., Henning, D., Greenberg, S. & Busch, H. Phosphorothioate oligonucleotides bind in a non sequence-specific manner to the nucleolar protein C23/nucleolin. FEBS Lett. 366, 146–150 (1995).
Flynn, L. L. et al. Single stranded fully modified-phosphorothioate oligonucleotides can induce structured nuclear inclusions, alter nuclear protein localization and disturb the transcriptome in vitro. Front. Genet. 13, 791416 (2022).
Crooke, S. T., Vickers, T. A. & Liang, X.-H. Phosphorothioate modified oligonucleotide–protein interactions. Nucleic Acids Res. 48, 5235–5253 (2020).
Dowdy, S. F. Overcoming cellular barriers for RNA therapeutics. Nat. Biotechnol. 35, 222–229 (2017).
Greulich, W. et al. TLR8 is a sensor of RNase T2 degradation products. Cell 179, 1264–1275 (2019).
Shen, W. et al. Chemical modification of PS–ASO therapeutics reduces cellular protein-binding and improves the therapeutic index. Nat. Biotechnol. 37, 640–650 (2019).
Freund, I., Eigenbrod, T., Helm, M. & Dalpke, A. H. RNA modifications modulate activation of innate Toll-like receptors. Genes 10, 92 (2019).
Stein, C. A. et al. Efficient gene silencing by delivery of locked nucleic acid antisense oligonucleotides, unassisted by transfection reagents. Nucleic Acids Res. 38, e3 (2009).
Seth, P. P. et al. Design, synthesis and evaluation of constrained methoxyethyl (cMOE) and constrained ethyl (cEt) nucleoside analogs. Nucleic Acids Symp. Ser. https://doi.org/10.1093/nass/nrn280 (2008).
Biscans, A. et al. Docosanoic acid conjugation to siRNA enables functional and safe delivery to skeletal and cardiac muscles. Mol. Ther. 29, 1382–1394 (2020).
Prakash, T. P. et al. Fatty acid conjugation enhances potency of antisense oligonucleotides in muscle. Nucleic Acids Res. 47, 6029–6044 (2019).
Wang, S., Allen, N., Prakash, T. P., Liang, X.-H. & Crooke, S. T. Lipid conjugates enhance endosomal release of antisense oligonucleotides into cells. Nucleic Acid Ther. 29, 245–255 (2019).
Brown, K. M. et al. Expanding RNAi therapeutics to extrahepatic tissues with lipophilic conjugates. Nat. Biotechnol. 40, 1500–1508 (2022).
Ali, S. et al. Design of a new cell penetrating peptide for DNA, siRNA and mRNA delivery. J. Gene Med. 24, e3401 (2021).
Paunovska, K., Loughrey, D. & Dahlman, J. E. Drug delivery systems for RNA therapeutics. Nat. Rev. Genet. 23, 265–280 (2022).
Nair, J. K. et al. Multivalent N-acetylgalactosamine-conjugated siRNA localizes in hepatocytes and elicits robust RNAi-mediated gene silencing. J. Am. Chem. Soc. 136, 16958–16961 (2014).
Brown, C. R. et al. Investigating the pharmacodynamic durability of GalNAc–siRNA conjugates. Nucleic Acids Res. 48, 11827–11844 (2020).
Debacker, A. J., Voutila, J., Catley, M., Blakey, D. & Habib, N. Delivery of oligonucleotides to the liver with GalNAc: from research to registered therapeutic drug. Mol. Ther. 28, 1759–1771 (2020).
Woolf, T. M., Chase, J. M. & Stinchcomb, D. T. Toward the therapeutic editing of mutated RNA sequences. Proc. Natl Acad. Sci. USA 92, 8298–8302 (1995).
Seth, P. P. et al. Short antisense oligonucleotides with novel 2′–4′ conformationaly restricted nucleoside analogues show improved potency without increased toxicity in animals. J. Med. Chem. 52, 10–13 (2009).
Eichert, A. et al. The crystal structure of an ‘all locked’ nucleic acid duplex. Nucleic Acids Res. 38, 6729–6736 (2010).
Petersen, M., Bondensgaard, K., Wengel, J. & Jacobsen, J. P. Locked nucleic acid (LNA) recognition of RNA: NMR solution structures of LNA:RNA hybrids. J. Am. Chem. Soc. 124, 5974–5982 (2002).
Brinkman, H. F., Jauregui Matos, V., Mendoza, H. G., Doherty, E. E. & Beal, P. A. Nucleoside analogs in ADAR guide strands targeting 5′-U̲ sites. RSC Chem. Biol. 4, 74–83 (2022).
Malik, T. N. et al. Regulation of RNA editing by intracellular acidification. Nucleic Acids Res. 49, 4020–4036 (2021).
Lomas, D. A. & Mahadeva, R. α1-antitrypsin polymerization and the serpinopathies: pathobiology and prospects for therapy. J. Clin. Invest. 110, 1585–1590 (2002).
Zheng, Y., Lorenzo, C. & Beal, P. A. DNA editing in DNA/RNA hybrids by adenosine deaminases that act on RNA. Nucleic Acids Res. 45, 3369–3377 (2017).
Iwamoto, N. et al. Control of phosphorothioate stereochemistry substantially increases the efficacy of antisense oligonucleotides. Nat. Biotechnol. 35, 845–851 (2017).
Kupryushkin, M. S., Pyshnyi, D. V. & Stetsenko, D. A. Phosphoryl guanidines: a new type of nucleic acid analogues. Acta Naturae 6, 116–118 (2014).
Pavlova, A. S. et al. An influence of modification with phosphoryl guanidine combined with a 2′-O-methyl or 2′-fluoro group on the small-interfering-RNA effect. Int. J. Mol. Sci. 22, 9784 (2021).
Kupryushkin, M. S. et al. Antisense oligonucleotide gapmers containing phosphoryl guanidine groups reverse MDR1-mediated multiple drug resistance of tumor cells. Mol. Ther. Nucleic Acids 27, 211–226 (2022).
Cross, R. Watch out, CRISPR. The RNA editing race is on. C&EN (25 March 2019).
Cross, R. Lilly taps ProQR for RNA editing. CEN Glob. Enterp. 99, 19 (2021).
Cross, R. RNA-editing race intensifies as big pharma buys in. C&EN (23 October 2021).
Reardon, S. A new twist on gene editing. Nature 578, 24–27 (2020).
Cox, D. B. T., Platt, R. J. & Zhang, F. Therapeutic genome editing: prospects and challenges. Nat. Med. 21, 121–131 (2015).
Teboul, L., Herault, Y., Wells, S., Qasim, W. & Pavlovic, G. Variability in genome editing outcomes: challenges for research reproducibility and clinical safety. Mol. Ther. 28, 1422–1431 (2020).
Zolotukhin, S. & Vandenberghe, L. H. AAV capsid design: a Goldilocks challenge. Trends Mol. Med. 28, 183–193 (2022).
Crooke, S. T., Witztum, J. L., Bennett, C. F. & Baker, B. F. RNA-targeted therapeutics. Cell Metab. 27, 714–739 (2018).
Acknowledgements
We thank the University of Tübingen, the Deutsche Forschungsgemeinschaft (STA 1053/3-1, STA 1053/3-2, STA 1053/7-1, STA 1053/10-1, STA 1053/11-1, STA 1053/14-1), the European Research Council (CoG no. 647328, PoC no. 101069246), the VolkswagenStiftung (grant no. 96 876) and the International Rett Syndrome Foundation (grant no. 3806) for continuous generous support for our RNA base-editing research program. All figures were created with https://biorender.com. Fig. 5a was reprinted (adapted) with permission from Doherty et al.80 (copyright 2023, American Chemical Society). Additionally, we thank J.M. Preuss and D.T. Hofacker for proofreading as well as the other members of the Stafforst laboratory for insightful discussions.
Author information
Authors and Affiliations
Contributions
L.S.P. and T.S. conceived, wrote and proofread the manuscript.
Corresponding author
Ethics declarations
Competing interests
L.S.P. is an inventor on patents related to RNA base editing. T.S. is a cofounder and shareholder of and consultant to AIRNA Bio and an inventor on patents related to RNA base editing.
Peer review
Peer review information
Nature Biotechnology thanks Gene Yeo and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Table 1
Comparative collection of tools.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pfeiffer, L.S., Stafforst, T. Precision RNA base editing with engineered and endogenous effectors. Nat Biotechnol 41, 1526–1542 (2023). https://doi.org/10.1038/s41587-023-01927-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41587-023-01927-0
This article is cited by
-
RNA editing enzymes: structure, biological functions and applications
Cell & Bioscience (2024)