Abstract
Germ-free (GF) mice, which are depleted of their resident microbiota, are the gold standard for exploring the role of the microbiome in health and disease; however, they are of limited value in the study of human-specific pathogens because they do not support their replication. Here, we develop GF mice systemically reconstituted with human immune cells and use them to evaluate the role of the resident microbiome in the acquisition, replication and pathogenesis of two human-specific pathogens, Epstein–Barr virus (EBV) and human immunodeficiency virus (HIV). Comparison with conventional (CV) humanized mice showed that resident microbiota enhance the establishment of EBV infection and EBV-induced tumorigenesis and increase mucosal HIV acquisition and replication. HIV RNA levels were higher in plasma and tissues of CV humanized mice compared with GF humanized mice. The frequency of CCR5+ CD4+ T cells throughout the intestine was also higher in CV humanized mice, indicating that resident microbiota govern levels of HIV target cells. Thus, resident microbiota promote the acquisition and pathogenesis of two clinically relevant human-specific pathogens.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Brestoff, J. R. & Artis, D. Commensal bacteria at the interface of host metabolism and the immune system. Nat. Immunol. 14, 676–684 (2013).
Chung, H. et al. Gut immune maturation depends on colonization with a host-specific microbiota. Cell 149, 1578–1593 (2012).
Robinson, C. M. & Pfeiffer, J. K. Viruses and the microbiota. Annu. Rev. Virol. 1, 55–69 (2014).
Ubeda, C., Djukovic, A. & Isaac, S. Roles of the intestinal microbiota in pathogen protection. Clin Transl. Immunol. 6, e128 (2017).
Baldridge, M. T. et al. Commensal microbes and interferon-lambda determine persistence of enteric murine norovirus infection. Science 347, 266–269 (2015).
Cortez, V. et al. Astrovirus infects actively secreting goblet cells and alters the gut mucus barrier. Nat. Commun. 11, 2097 (2020).
Robinson, C. M., Jesudhasan, P. R. & Pfeiffer, J. K. Bacterial lipopolysaccharide binding enhances virion stability and promotes environmental fitness of an enteric virus. Cell Host Microbe 15, 36–46 (2014).
Jones, M. K. et al. Enteric bacteria promote human and mouse norovirus infection of B cells. Science 346, 755–759 (2014).
Kane, M. et al. Successful transmission of a retrovirus depends on the commensal microbiota. Science 334, 245–249 (2011).
Kuss, S. K. et al. Intestinal microbiota promote enteric virus replication and systemic pathogenesis. Science 334, 249–252 (2011).
Wilks, J. & Golovkina, T. Influence of microbiota on viral infections. PLoS Pathog. 8, e1002681 (2012).
Ponte, R. et al. Reversing gut damage in HIV infection: using non-human primate models to instruct clinical research. eBioMedicine 4, 40–49 (2016).
Kennedy, E. A., King, K. Y. & Baldridge, M. T. Mouse microbiota models: comparing germ-free mice and antibiotics treatment as tools for modifying gut bacteria. Front. Physiol. 9, 1534 (2018).
Odumade, O. A., Hogquist, K. A. & Balfour, H. H. Jr. Progress and problems in understanding and managing primary Epstein-Barr virus infections. Clin. Microbiol. Rev. 24, 193–209 (2011).
MacMahon, E. M. et al. Epstein-Barr virus in AIDS-related primary central nervous system lymphoma. Lancet 338, 969–973 (1991).
Zhang, L. et al. Interferon regulatory factor 7 is associated with Epstein-Barr virus-transformed central nervous system lymphoma and has oncogenic properties. J. Virol. 78, 12987–12995 (2004).
Raab-Traub, N. Epstein-Barr virus and nasopharyngeal carcinoma. Semin. Cancer Biol. 3, 297–307 (1992).
zur Hausen, H. et al. EBV DNA in biopsies of Burkitt tumours and anaplastic carcinomas of the nasopharynx. Nature 228, 1056–1058 (1970).
Weiss, L. M., Movahed, L. A., Warnke, R. A. & Sklar, J. Detection of Epstein-Barr viral genomes in Reed-Sternberg cells of Hodgkin’s disease. N. Engl. J. Med. 320, 502–506 (1989).
Cohen, J. I., Fauci, A. S., Varmus, H. & Nabel, G. J. Epstein-Barr virus: an important vaccine target for cancer prevention. Sci. Transl. Med. 3, 107fs107 (2011).
UNAIDS Data 2021. UNAIDS https://www.unaids.org/sites/default/files/media_asset/JC3032_AIDS_Data_book_2021_En.pdf (2021).
Brenchley, J. M. & Douek, D. C. HIV infection and the gastrointestinal immune system. Mucosal Immunol. 1, 23–30 (2008).
Brenchley, J. M. et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat. Med. 12, 1365–1371 (2006).
Dillon, S. M. et al. An altered intestinal mucosal microbiome in HIV-1 infection is associated with mucosal and systemic immune activation and endotoxemia. Mucosal Immunol. 7, 983–994 (2014).
Guadalupe, M. et al. Severe CD4+ T-cell depletion in gut lymphoid tissue during primary human immunodeficiency virus type 1 infection and substantial delay in restoration following highly active antiretroviral therapy. J. Virol. 77, 11708–11717 (2003).
Mutlu, E. A. et al. A compositional look at the human gastrointestinal microbiome and immune activation parameters in HIV infected subjects. PLoS Pathog. 10, e1003829 (2014).
Estes, J. D. et al. Defining total-body AIDS-virus burden with implications for curative strategies. Nat. Med. 23, 1271–1276 (2017).
Busman-Sahay, K., Starke, C. E., Nekorchuk, M. D. & Estes, J. D. Eliminating HIV reservoirs for a cure: the issue is in the tissue. Curr. Opin. HIV AIDS 16, 200–208 (2021).
Rogala, A. R., Oka, A. & Sartor, R. B. Strategies to dissect host-microbial immune interactions that determine mucosal homeostasis vs. intestinal inflammation in gnotobiotic Mice. Front. Immunol. 11, 214 (2020).
Murer, A. et al. MicroRNAs of Epstein-Barr virus attenuate T-cell-mediated immune control in vivo. mBio 10, e01941-18 (2019).
Antsiferova, O. et al. Adoptive transfer of EBV specific CD8+ T cell clones can transiently control EBV infection in humanized mice. PLoS Pathog. 10, e1004333 (2014).
Pender, M. P., Csurhes, P. A., Pfluger, C. M. & Burrows, S. R. CD8 T cell deficiency impairs control of Epstein–Barr virus and worsens with age in multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 83, 353–354 (2012).
Kovarova, M. et al. HIV pre-exposure prophylaxis for women and infants prevents vaginal and oral HIV transmission in a preclinical model of HIV infection. J. Antimicrob. Chemother. 71, 3185–3194 (2016).
Wahl, A. et al. Breast milk of HIV-positive mothers has potent and species-specific in vivo HIV-inhibitory activity. J. Virol. 89, 10868–10878 (2015).
Wahl, A. et al. Human breast milk and antiretrovirals dramatically reduce oral HIV-1 transmission in BLT humanized mice. PLoS Pathog. 8, e1002732 (2012).
Chateau, M. L., Denton, P. W., Swanson, M. D., McGowan, I. & Garcia, J. V. Rectal transmission of transmitted/founder HIV-1 is efficiently prevented by topical 1% tenofovir in BLT humanized mice. PLoS ONE 8, e60024 (2013).
Ochsenbauer, C. et al. Generation of transmitted/founder HIV-1 infectious molecular clones and characterization of their replication capacity in CD4 T lymphocytes and monocyte-derived macrophages. J. Virol. 86, 2715–2728 (2012).
Sender, R., Fuchs, S. & Milo, R. Revised estimates for the number of human and bacteria cells in the body. PLoS Biol. 14, e1002533 (2016).
Doolittle, J. M. & Webster-Cyriaque, J. Polymicrobial infection and bacterium-mediated epigenetic modification of DNA tumor viruses contribute to pathogenesis. mBio 5, e01015-14 (2014).
Imai, K. et al. The periodontal pathogen Porphyromonas gingivalis induces the Epstein-Barr virus lytic switch transactivator ZEBRA by histone modification. Biochimie 94, 839–846 (2012).
Westphal, E. M., Blackstock, W., Feng, W., Israel, B. & Kenney, S. C. Activation of lytic Epstein-Barr virus (EBV) infection by radiation and sodium butyrate in vitro and in vivo: a potential method for treating EBV-positive malignancies. Cancer Res. 60, 5781–5788 (2000).
Westphal, E. M. et al. Induction of lytic Epstein-Barr virus (EBV) infection in EBV-associated malignancies using adenovirus vectors in vitro and in vivo. Cancer Res. 59, 1485–1491 (1999).
Chien, Y. C. et al. Serologic markers of Epstein-Barr virus infection and nasopharyngeal carcinoma in Taiwanese men. N. Engl. J. Med. 345, 1877–1882 (2001).
Wen, Y., Xu, H., Han, J., Jin, R. & Chen, H. How does Epstein-Barr virus interact with other microbiomes in EBV-driven cancers? Front. Cell. Infect. Microbiol. 12, 852066 (2022).
Kashyap, D., Baral, B., Jakhmola, S., Singh, A. K. & Jha, H. C. Helicobacter pylori and Epstein-Barr virus coinfection stimulates aggressiveness in gastric cancer through the regulation of gankyrin. mSphere 6, e0075121 (2021).
Walter, B. L. et al. Role of low CD4 levels in the influence of human immunodeficiency virus type 1 envelope V1 and V2 regions on entry and spread in macrophages. J. Virol. 79, 4828–4837 (2005).
de Roda Husman, A. M., Blaak, H., Brouwer, M. & Schuitemaker, H. CC chemokine receptor 5 cell-surface expression in relation to CC chemokine receptor 5 genotype and the clinical course of HIV-1 infection. J. Immunol. 163, 4597–4603 (1999).
Reynes, J. et al. CD4+ T cell surface CCR5 density as a determining factor of virus load in persons infected with human immunodeficiency virus type 1. J. Infect. Dis. 181, 927–932 (2000).
Ostrowski, M. A. et al. Expression of chemokine receptors CXCR4 and CCR5 in HIV-1-infected and uninfected individuals. J. Immunol. 161, 3195–3201 (1998).
Reynes, J. et al. CD4 T cell surface CCR5 density as a host factor in HIV-1 disease progression. AIDS 15, 1627–1634 (2001).
Yang, X. et al. High CCR5 density on central memory CD4+ T cells in acute HIV-1 infection is mostly associated with rapid disease progression. PLoS ONE 7, e49526 (2012).
Meijerink, H. et al. The number of CCR5 expressing CD4+ T lymphocytes is lower in HIV-infected long-term non-progressors with viral control compared to normal progressors: a cross-sectional study. BMC Infect. Dis. 14, 683 (2014).
Weissman, D. et al. Interleukin-2 up-regulates expression of the human immunodeficiency virus fusion coreceptor CCR5 by CD4+ lymphocytes in vivo. J. Infect. Dis. 181, 933–938 (2000).
Yang, Y. F. et al. IL-12 as well as IL-2 upregulates CCR5 expression on T cell receptor-triggered human CD4+ and CD8+ T cells. J. Clin. Immunol. 21, 116–125 (2001).
Valentin, A. et al. Dual effect of interleukin 4 on HIV-1 expression: implications for viral phenotypic switch and disease progression. Proc. Natl Acad. Sci. USA 95, 8886–8891 (1998).
Patterson, B. K. et al. Regulation of CCR5 and CXCR4 expression by type 1 and type 2 cytokines: CCR5 expression is downregulated by IL-10 in CD4-positive lymphocytes. Clin. Immunol. 91, 254–262 (1999).
Claireaux, M. et al. Low CCR5 expression protects HIV-specific CD4+ T cells of elite controllers from viral entry. Nat. Commun. 13, 521 (2022).
McBrien, J. B. et al. Robust and persistent reactivation of SIV and HIV by N-803 and depletion of CD8+ cells. Nature 578, 154–159 (2020).
Nixon, C. C. et al. Systemic HIV and SIV latency reversal via non-canonical NF-κB signalling in vivo. Nature 578, 160–165 (2020).
Denton, P. W. et al. Systemic administration of antiretrovirals prior to exposure prevents rectal and intravenous HIV-1 transmission in humanized BLT mice. PLoS ONE 5, e8829 (2010).
Denton, P. W. et al. One percent tenofovir applied topically to humanized BLT mice and used according to the CAPRISA 004 experimental design demonstrates partial protection from vaginal HIV infection, validating the BLT model for evaluation of new microbicide candidates. J. Virol. 85, 7582–7593 (2011).
Wahl, A. et al. Predicting HIV pre-exposure prophylaxis efficacy for women using a preclinical pharmacokinetic-pharmacodynamic in vivo model. Sci. Rep. 7, 41098 (2017).
Hayes, C. L. et al. Commensal microbiota induces colonic barrier structure and functions that contribute to homeostasis. Sci. Rep. 8, 14184 (2018).
Smith, P. M. & Garrett, W. S. The gut microbiota and mucosal T cells. Front. Microbiol. 2, 111 (2011).
Round, J. L. & Mazmanian, S. K. The gut microbiota shapes intestinal immune responses during health and disease. Nat. Rev. Immunol. 9, 313–323 (2009).
Niess, J. H. & Adler, G. Enteric flora expands gut lamina propria CX3CR1+ dendritic cells supporting inflammatory immune responses under normal and inflammatory conditions. J. Immunol. 184, 2026–2037 (2010).
Umesaki, Y., Setoyama, H., Matsumoto, S. & Okada, Y. Expansion of alpha beta T-cell receptor-bearing intestinal intraepithelial lymphocytes after microbial colonization in germ-free mice and its independence from thymus. Immunology 79, 32–37 (1993).
Macpherson, A. J. & Harris, N. L. Interactions between commensal intestinal bacteria and the immune system. Nat. Rev. Immunol. 4, 478–485 (2004).
Kernbauer, E., Ding, Y. & Cadwell, K. An enteric virus can replace the beneficial function of commensal bacteria. Nature 516, 94–98 (2014).
Krych, L., Hansen, C. H., Hansen, A. K., van den Berg, F. W. & Nielsen, D. S. Quantitatively different, yet qualitatively alike: a meta-analysis of the mouse core gut microbiome with a view towards the human gut microbiome. PLoS ONE 8, e62578 (2013).
Park, J. C. & Im, S. H. Of men in mice: the development and application of a humanized gnotobiotic mouse model for microbiome therapeutics. Exp. Mol. Med. 52, 1383–1396 (2020).
Ivanov, I. I. et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell 139, 485–498 (2009).
Alameddine, J. et al. Faecalibacterium prausnitzii skews human DC to prime IL10-producing T cells through TLR2/6/JNK signaling and IL-10, IL-27, CD39, and IDO-1 induction. Front. Immunol. 10, 143 (2019).
Daharsh, L., Zhang, J., Ramer-Tait, A. & Li, Q. A double humanized BLT-mice model featuring a stable human-like gut microbiome and human immune system. J. Vis. Exp. https://doi.org/10.3791/59773 (2019).
Singh, M. et al. Minocycline attenuates HIV-1 infection and suppresses chronic immune activation in humanized NOD/LtsZ-scidIL-2Rγnull mice. Immunology 142, 562–572 (2014).
Nahui Palomino, R. A. et al. Extracellular vesicles from symbiotic vaginal lactobacilli inhibit HIV-1 infection of human tissues. Nat. Commun. 10, 5656 (2019).
Wahl, A. et al. Precision mouse models with expanded tropism for human pathogens. Nat. Biotechnol. 37, 1163–1173 (2019).
Wahl, A. et al. SARS-CoV-2 infection is effectively treated and prevented by EIDD-2801. Nature 591, 451–457 (2021).
Melkus, M. W. et al. Humanized mice mount specific adaptive and innate immune responses to EBV and TSST-1. Nat. Med. 12, 1316–1322 (2006).
Akkina, R. et al. Small animal models for human immunodeficiency virus (HIV), hepatitis B, and tuberculosis: proceedings of an NIAID workshop. Curr. HIV Res. 18, 19–28 (2020).
Council, O. D., Swanson, M. D., Spagnuolo, R. A., Wahl, A. & Garcia, J. V. Role of semen on vaginal HIV-1 transmission and maraviroc protection. Antimicrob. Agents Chemother. 59, 7847–7851 (2015).
Denton, P. W. et al. Generation of HIV latency in humanized BLT mice. J. Virol. 86, 630–634 (2012).
Honeycutt, J. B. et al. T cells establish and maintain CNS viral infection in HIV-infected humanized mice. J. Clin. Invest. 128, 2862–2876 (2018).
Honeycutt, J. B. et al. HIV-1 infection, response to treatment and establishment of viral latency in a novel humanized T cell-only mouse (TOM) model. Retrovirology 10, 121 (2013).
Honeycutt, J. B. et al. Macrophages sustain HIV replication in vivo independently of T cells. J. Clin. Invest. 126, 1353–1366 (2016).
Olesen, R. et al. ART influences HIV persistence in the female reproductive tract and cervicovaginal secretions. J. Clin. Invest. 126, 892–904 (2016).
Shanmugasundaram, U. et al. Efficient inhibition of HIV replication in the gastrointestinal and female reproductive tracts of humanized BLT mice by EFdA. PLoS ONE 11, e0159517 (2016).
Sun, Z. et al. Intrarectal transmission, systemic infection, and CD4+ T cell depletion in humanized mice infected with HIV-1. J. Exp. Med. 204, 705–714 (2007).
Wang, L. X. et al. Humanized-BLT mouse model of Kaposi’s sarcoma-associated herpesvirus infection. Proc. Natl Acad. Sci. USA 111, 3146–3151 (2014).
Packey, C. D. et al. Molecular detection of bacterial contamination in gnotobiotic rodent units. Gut Microbes 4, 361–370 (2013).
Denton, P. W. et al. IL-2 receptor γ-chain molecule is critical for intestinal T-cell reconstitution in humanized mice. Mucosal Immunol. 5, 555–566 (2012).
Nochi, T., Denton, P. W., Wahl, A. & Garcia, J. V. Cryptopatches are essential for the development of human GALT. Cell Rep. 3, 1874–1884 (2013).
Allali, I. et al. A comparison of sequencing platforms and bioinformatics pipelines for compositional analysis of the gut microbiome. BMC Microbiol. 17, 194 (2017).
Azcarate-Peril, M. A. et al. An attenuated Salmonella enterica serovar Typhimurium strain and galacto-oligosaccharides accelerate clearance of Salmonella infections in poultry through modifications to the gut microbiome. Appl. Environ. Microbiol. 84, e02526-17 (2018).
Guadamuro, L., Azcarate-Peril, M. A., Tojo, R., Mayo, B. & Delgado, S. Use of high throughput amplicon sequencing and ethidium monoazide dye to track microbiota changes in an equol-producing menopausal woman receiving a long-term isoflavones treatment. AIMS Microbiol. 5, 102–116 (2019).
Caporaso, J. G. et al. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc. Natl Acad. Sci. USA 108, 4516–4522 (2011).
Mohsen, A., Park, J., Chen, Y. A., Kawashima, H. & Mizuguchi, K. Impact of quality trimming on the efficiency of reads joining and diversity analysis of Illumina paired-end reads in the context of QIIME1 and QIIME2 microbiome analysis frameworks. BMC Bioinformatics 20, 581 (2019).
Callahan, B. J. et al. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 13, 581–583 (2016).
Bokulich, N. A. et al. Optimizing taxonomic classification of marker-gene amplicon sequences with QIIME 2′s q2-feature-classifier plugin. Microbiome 6, 90 (2018).
Delecluse, H. J., Hilsendegen, T., Pich, D., Zeidler, R. & Hammerschmidt, W. Propagation and recovery of intact, infectious Epstein-Barr virus from prokaryotic to human cells. Proc. Natl Acad. Sci. USA 95, 8245–8250 (1998).
Kumar, R., Whitehurst, C. B. & Pagano, J. S. The Rad6/18 ubiquitin complex interacts with the Epstein-Barr virus deubiquitinating enzyme, BPLF1, and contributes to virus infectivity. J. Virol. 88, 6411–6422 (2014).
Whitehurst, C. B. et al. HIV co-infection augments EBV-induced tumorigenesis in vivo. Front. Virol. 2, 861628 (2022).
Wahl, A. et al. A cluster of virus-encoded microRNAs accelerates acute systemic Epstein-Barr virus infection but does not significantly enhance virus-induced oncogenesis in vivo. J. Virol. 87, 5437–5446 (2013).
Therneau, T. A package for survival analysis in R. R package version 3.2-11 https://CRAN.R-project.org/package=survival (2021).
Gray, R. J. cmprsk: Subdistribution analysis of competing risks. R package version 2.2-11 https://CRAN.R-project.org/package=cmprsk (2022).
Aalen, O. O. & Johansen, S. An empirical transition matrix for non-homogeneous Markov chains based on censored observations. Scand. J. Stat. 5, 141–150 (1978).
Gray, R. J. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann. Stat. 16, 1141–1154 (1988).
Acknowledgements
We thank current and former members of the Garcia and Wahl laboratories for technical assistance and technicians at the UNC National Gnotobiotic Rodent Resource Center, Microbiome Core Facility, Division of Comparative Medicine, and Animal Histopathology and Clinical Chemistry Core for technical support. We also thank M. Kane, S. Lemon, J. Turpin and N. Raab-Traub for helpful comments and discussions. Figure 1a was created using BioRender.com. This work was supported by funding from NIH grants AI123010 (A.W.), DK131585 (A.W., J.V.G. and R.B.S), 1UM1AI126619 (current award 1UM1AI164567; J.V.G), P40OD010995 (R.B.S. and A.R.R.), P30DK034987 (R.B.S), U19AI082637 (I.M.) and FIC D43TW009532 (J.D.T). The UNC CFAR Biostatistics Core is supported by NIH-funded program P30AI050410. UNC Animal Histopathology & Clinical Chemistry is supported in part by an NCI Center Core Support Grant (5P30CA016080-42). The UNC Microbiome Core is funded in part by the Center for Gastrointestinal Biology and Disease (P30 DK034987) and the UNC Nutrition Obesity Research Center (P30 DK056350).
Author information
Authors and Affiliations
Contributions
W.Y., B.L., C.R., M.C. and A.W. constructed BLT mice, necropsied mice, and performed flow cytometric analysis of peripheral blood and tissues. G.D. contributed to the flow cytometric analysis of tissues. W.Y. and A.W. performed the immunohistochemical analysis. A.W., A.F., K.S. and G.D. performed experiments with EBV-exposed BLT mice, and A.W. analyzed the data. C.B.W and J.S.P. contributed to the EBV studies. W.Y., C.R. and A.W. performed experiments with HIV-exposed BLT mice and analyzed data. L.L. assisted with rectal HIV exposures. F.L. and J.F. contributed to the rederivation of GF mice and microbial testing. M.A.A. contributed to the microbiome sequencing analysis. M.G.H assisted with statistical analyses and data presentation. A.R.R. and R.B.S contributed to the rederivation of GF mice, microbial testing and experimental design. I.M. contributed to the conceptualization of the study. C.A.F and J.D.T. contributed to data interpretation, and J.D.T also assisted with the supervision of B.L. J.V.G. and A.W. conceived and designed the study and experiments; supervised the work; and contributed to data interpretation, analysis, data presentation, and manuscript conceptualization and writing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Biotechnology thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
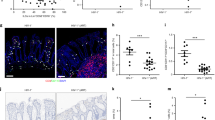
Extended Data Fig. 1 Fecal bacterial microbiome of CV-BLT mice.
The composition of the bacterial microbiome was analyzed by 16S amplicon sequencing in fecal pellets collected from CV-BLT mice (n = 10). The mean relative abundance at the a, phylum and b, genus levels are shown. Taxa with a mean relative abundance <2% were grouped together as other.
Extended Data Fig. 2 Human hematopoietic cells are present in lymphoid and non-lymphoid tissues of GF-BLT mice.
Immunohistochemical staining for a, human hematopoietic cells (hCD45+) including human dendritic cells (hCD11c+), myeloid cells (hCD68+), B cells (hCD20+) and T cells (hCD3+) in the spleen (n = 6 analyzed), lymph nodes (n = 5 analyzed), liver (n = 5 analyzed), and lung (n = 6 analyzed) of GF-BLT mice and b, human B cells (hCD20+) in the small intestine (n = 3 analyzed), cecum (n = 6 analyzed), and large intestine colon (n = 3 analyzed). Positive cells are stained brown. Scale bars, 100 um. c, Flow cytometric analysis of human T cell levels in the intraepithelial layer (IEL) and lamina propria layer (LPL) of the small intestine (S), cecum (C), and large intestine (L) of GF-BLT mice (n = 14 S IEL, C LPL; n = 13 S LPL, C IEL, L IEL; n = 12 L LPL). Horizontal and vertical lines represent the mean and standard error mean respectively.
Extended Data Fig. 3 CD21 expression on human splenic B cells in CV-BLT and GF-BLT mice.
CD21 expression was evaluated on human B cells isolated from the spleen of CV-BLT (n = 5, black) and GF-BLT (n = 5, red) mice using flow cytometry. The a, percent of human B cells expressing CD21 and the b, mean fluorescent intensity (MFI) of CD21 staining on CD21+ human B cells was compared between CV-BLT and GF-BLT mice with a two-sided Mann-Whitney test. Horizontal and vertical lines represent the mean and standard error mean respectively.
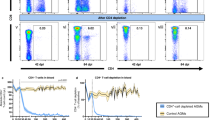
Extended Data Fig. 4 HIV replication is enhanced in the presence of resident microbiota following a systemic HIV exposure.
CV-BLT mice (n = 8) and GF-BLT mice (n = 8) were challenged systemically (via intraperitoneal injection) with HIV-1JRCSF. HIV RNA levels in peripheral blood plasma were monitored longitudinally by real-time PCR. Five weeks post-exposure, aviremic BLT mice were administered a second systemic dose of HIV. GF-BLT mice were housed in a gnotobiotic isolator for the duration of the study and their GF status monitored longitudinally. a, Peripheral blood plasma viral load (HIV RNA copies/ml) in CV-BLT mice (left panel, black) and GF-BLT mice (right panel, red) following HIV challenge. The limit of detection is shown with a dashed line. b, Percent HIV positive CV-BLT mice (black) and GF-BLT mice were compared with a two-sided log-rank Mantel Cox test based on the presence of HIV RNA and/or HIV DNA in peripheral blood and/or tissues. c, Peripheral blood plasma viral load of viremic CV-BLT mice (n = 7, black) and GF-BLT mice (n = 6, red). For mice that acquired HIV infection after the second challenge, week one represents the viral load one week after the second HIV exposure (6 weeks after the first exposure). Solid lines represent mean plasma viral loads. d, Mean and e, peak plasma viral load of viremic CV-BLT mice (n = 7, black) and GF-BLT mice (n = 6, red). f, HIV RNA levels in the bone marrow (BM), human thymus (THY), spleen (SPL), lymph nodes (LN), liver (LIV), and lung (LNG) of viremic CV-BLT mice (n = 6, black) and GF-BLT mice (n = 6, red). In d–f, horizontal and vertical lines represent the mean and standard error mean respectively. c–f, HIV RNA levels were compared with a two-sided Mann-Whitney test.
Extended Data Fig. 5 CD8+ T cell activation is higher in the intestinal tract in the presence of resident microbiota following rectal HIV acquisition.
Flow cytometric analysis of a, activated (HLA-DR+CD38+) human CD8+ T cells of viremic GF-BLT (red) and CV-BLT (black) mice at day 8 (GF, n = 4; CV n = 12), day 13 (GF, n = 4; CV n = 12), day 20 (GF, n = 4; CV n = 9), day 28 (GF, n = 4; CV n = 5) and day 35 (GF, n = 4; CV n = 4) post rectal HIV acquisition. Percent activated human CD8+ T cells in the b, PB, bone marrow (BM), spleen (SPL), lymph nodes (LN), human thymic organoid (THY), liver (LIV), and lung (LNG) as well as the c, small intestine (S), cecum (C), and large intestine (L) intraepithelial layer (IEL) and lamina propria layer (LPL) of aviremic (filled bars) and viremic (open bars) GF-BLT mice. Aviremic GF-BLT mice, n = 9 (PB, BM, SPL, LN, THY, LIV), n = 8 (LNG, S IEL, C IEL, C LPL, L IEL, L LPL), or n = 4 (S LPL). Viremic GF-BLT mice, n = 4 (PB, BM, SPL, LN, THY, LIV, LNG) or n = 3 (S IEL, S LPL, C IEL, C LPL, L IEL, L LPL). Percent activated human CD8+ T cells in the d, PB, BM, SPL, LN, THY, LIV, and LNG as well as the e, S IEL, S LPL, C IEL, C LPL, L IEL, and L LPL of aviremic (filled bars) and viremic (open bars) CV-BLT mice. Aviremic CV-BLT mice, n = 5 (PB, BM, SPL, LN, THY, LIV, LNG, S IEL, C IEL, C LPL, L IEL, L LPL), n = 3 (S LPL). Viremic CV-BLT mice, n = 8 (PB, BM, SPL, LN, LIV, LNG), n = 7 (S IEL, C IEL, C LPL, L IEL, L LPL), or n = 6 (THY, S LPL). SP, CD8+ single positive thymocyte. DP, CD4+CD8+ double positive thymocyte. b–e, Shown is the difference in percent activated CD8+ T cells between viremic and aviremic mice. Horizontal and vertical lines represent the mean and standard error mean respectively. a-e, Cell levels mice were compared with a two-sided Mann-Whitney test.
Extended Data Fig. 6 HIV infection mediated CD4+ T cell depletion is not impacted by the presence of resident microbiota.
Flow cytometric analysis of a, human CD4+ T cells in the peripheral blood (PB) of viremic GF-BLT (red) and CV-BLT (black) mice at day 8 (GF, n = 4; CV n = 12), day 13 (GF, n = 4; CV n = 12), day 20 (GF, n = 4; CV n = 9), day 28 (GF, n = 4; CV n = 5) and day 35 (GF, n = 4; CV n = 4) post rectal HIV acquisition. Percent CD4+ of human T cells in the b, PB, bone marrow (BM), spleen (SPL), lymph nodes (LN), human thymic organoid (THY), liver (LIV), and lung (LNG) as well as the c, small intestine (S), cecum (C), and large intestine (L) intraepithelial layer (IEL) and lamina propria layer (LPL) of aviremic (filled bars) and viremic (open bars) GF-BLT mice. Aviremic GF-BLT mice, n = 9 (PB, BM, SPL, LN, THY, LIV), n = 8 (LNG, S IEL, C IEL, C LPL, L IEL, L LPL), or n = 5 (S LPL). Viremic GF-BLT mice, n = 4 (PB, BM, SPL, LN, THY, LIV, LNG) or n = 3 (S IEL, S LPL, C IEL, C LPL, L IEL, L LPL). Percent CD4+ of human T cells in the d, PB, BM, SPL, LN, THY, LIV, and LNG as well as the e, S IEL, S LPL, C IEL, C LPL, L IEL, and L LPL of aviremic (filled bars) and viremic (open bars) CV-BLT mice. Aviremic CV-BLT mice, n = 5 (PB, BM, SPL, LN, THY, LIV, LNG, S IEL, C IEL, C LPL, L IEL, L LPL), n = 4 (S LPL). Viremic CV-BLT mice, n = 8 (PB, BM, SPL, LN, LIV, LNG), n = 7 (S IEL, C IEL, C LPL, L IEL, L LPL), or n = 6 (THY, S LPL). SP, CD4+ single positive thymocyte. DP, CD4+CD8+ double positive thymocyte. b–e, Shown is the difference in percent CD4+ T cells between viremic and avirmic mice. Horizontal and vertical lines represent the mean and standard error mean respectively. a-e, Cell levels mice were compared with a two-sided Mann-Whitney test.
Extended Data Fig. 7 Minimal impact of resident microbiota on human CD4+ T cell homeostasis in the peripheral blood and nonintestinal tissues.
Numbers of a, human hematopoietic cells (hCD45+), including b, T cells (hCD3+) and c, CD4+ T cells in the small intestine (S) and large intestine (L) intraepithelial (IEL) and lamina propria (LPL) layers of GF-BLT mice (GF; red bars; S IEL, n = 14; S LPL, n = 13; L IEL, n = 13; L LPL, n = 12) and CV-BLT mice (CV; black; S IEL, n = 11; S LPL, n = 9; L IEL, n = 11; L LPL, n = 11) were determined by flow cytometric analysis. Flow cytometric analysis of human hematopoietic cells (hCD45+) including T cells (hCD3+) and CD4+ T cells in the d, peripheral blood (GF, n = 16; CV, n = 11), e, bone marrow (GF, n = 14; CV, n = 11), f, thymic organoid (GF, n = 15; CV, n = 11), g, spleen (GF, n = 16; CV, n = 11), h, lymph nodes (GF, n = 15; CV, n = 10), i, liver (GF, n = 16; CV, n = 11), and j, lung (GF, n = 16; CV, n = 11) of GF-BLT (GF; red boxes) and CV-BLT (CV; black boxes). Cell counts are normalized to a-c, tissue length (cm) or f, g, i, j, weight (g). SP, CD4+ single positive thymocyte. DP, CD4+CD8+ double positive thymocyte. Horizontal and vertical lines represent the mean and standard error mean respectively. Cell levels between GF-BLT and CV-BLT mice were compared with a two-sided Mann-Whitney test. a and b, The exact p values shown as P<0.0001 for comparisons of human CD45+ and CD3+ T cell numbers in the S IEL are P = 0.000020 and P = 0.000043 respectively.
Extended Data Fig. 8 Resident microbiota regulate human CD8+ T cell homeostasis in the intestinal tract.
Levels of human a–c, B cells, d–f, myeloid cells, and g–i, CD8+ T cells in the peripheral blood (GF, n = 15; CV, n = 11), bone marrow (BM; GF, n = 14; CV, n = 11), thymic organoid (THY; GF, n = 15; CV, n = 11), spleen (SPL; GF, n = 16; CV, n = 11), lymph nodes (LN; GF, n = 15; CV, n = 10), liver (LIV; GF, n = 16; CV, n = 11), lung (LNG; GF, n = 16; CV, n = 11) and the small intestine (S), cecum (C), and large intestine (L) intraepithelial layer (IEL; GF, n = 13; CV, n = 11) and lamina propria layer (LPL; GF S LPL, n = 13; CV S LPL, n = 9 CV-BLT; GF C LPL, n = 14; CV CLPL, n = 11; GF LPL, n = 12; CV LPL, n = 11) of GF-BLT (GF, red boxes) and CV-BLT mice (CV, black boxes). Horizontal and vertical lines represent the mean and standard error mean respectively. Cell levels between GF-BLT and CV-BLT mice were compared with a two-sided Mann-Whitney test. e and i, The exact p values shown as P<0.0001 for comparisons of human myeloid cell numbers in the SPL and human CD8+ T cell numbers in the S IEL and L IEL are P = 0.000041, P = 0.000020, and P = 0.000077 respectively.
Supplementary information
Supplementary Information
Supplementary Figs. 1–2 and Tables 1–10.
Source data
Source Data Fig. 1
Graphed source data.
Source Data Fig. 2
Graphed source data.
Source Data Fig. 3
Graphed source data.
Source Data Fig. 4
Graphed source data.
Source Data Fig. 5
Graphed source data.
Source Data Extended Data Fig. 1
Graphed source data.
Source Data Extended Data Fig. 2
Graphed source data.
Source Data Extended Data Fig. 3
Graphed source data.
Source Data Extended Data Fig. 4
Graphed source data.
Source Data Extended Data Fig. 5
Graphed source data.
Source Data Extended Data Fig. 6
Graphed source data.
Source Data Extended Data Fig. 7
Graphed source data.
Source Data Extended Data Fig. 8
Graphed source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wahl, A., Yao, W., Liao, B. et al. A germ-free humanized mouse model shows the contribution of resident microbiota to human-specific pathogen infection. Nat Biotechnol (2023). https://doi.org/10.1038/s41587-023-01906-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41587-023-01906-5
This article is cited by
-
Gut microbiota in parasite-transmitting gastropods
Infectious Diseases of Poverty (2023)