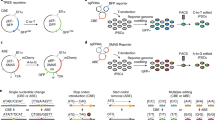
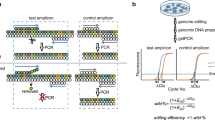
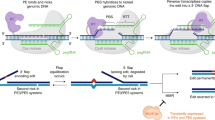
Abstract
Simple, efficient and well-tolerated delivery of CRISPR genome editing systems into primary cells remains a major challenge. Here we describe an engineered Peptide-Assisted Genome Editing (PAGE) CRISPR–Cas system for rapid and robust editing of primary cells with minimal toxicity. The PAGE system requires only a 30-min incubation with a cell-penetrating Cas9 or Cas12a and a cell-penetrating endosomal escape peptide to achieve robust single and multiplex genome editing. Unlike electroporation-based methods, PAGE gene editing has low cellular toxicity and shows no significant transcriptional perturbation. We demonstrate rapid and efficient editing of primary cells, including human and mouse T cells, as well as human hematopoietic progenitor cells, with editing efficiencies upwards of 98%. PAGE provides a broadly generalizable platform for next-generation genome engineering in primary cells.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The accession numbers for the RNA-seq dataset in this study is GSE223805(ref. 55). The GRCh38/hg38 human reference genome is publicly available. Key plasmids, Cas9-T6N and Cas12a-T8N have been deposited at Addgene (plasmid ID, 199604–199605). Source data are provided with this paper, including unprocessed Western blots.
References
Doudna, J. A. The promise and challenge of therapeutic genome editing. Nature 578, 229–236 (2020).
Hsu, P. D., Lander, E. S. & Zhang, F. Development and applications of CRISPR–Cas9 for genome engineering. Cell 157, 1262–1278 (2014).
Komor, A. C., Badran, A. H. & Liu, D. R. CRISPR-based technologies for the manipulation of eukaryotic genomes. Cell 168, 20–36 (2017).
June, C. H., O’Connor, R. S., Kawalekar, O. U., Ghassemi, S. & Milone, M. C. CAR T cell immunotherapy for human cancer. Science 359, 1361–1365 (2018).
Rosenberg, S. A., Restifo, N. P., Yang, J. C., Morgan, R. A. & Dudley, M. E. Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nat. Rev. Cancer 8, 299–308 (2008).
Stadtmauer, E. A. et al. CRISPR-engineered T cells in patients with refractory cancer. Science 367, eaba7365 (2020).
Atsavapranee, E. S., Billingsley, M. M. & Mitchell, M. J. Delivery technologies for T cell gene editing: applications in cancer immunotherapy. EBioMedicine 67, 103354 (2021).
Yin, H., Kauffman, K. J. & Anderson, D. G. Delivery technologies for genome editing. Nat. Rev. Drug Discov. 16, 387–399 (2017).
Chen, Z. et al. In vivo CD8+ T cell CRISPR screening reveals control by Fli1 in infection and cancer. Cell 184, 1262–1280 (2021).
Dong, M. B. et al. Systematic immunotherapy target discovery using genome-scale in vivo CRISPR screens in CD8 T cells. Cell 178, 1189–1204 (2019).
Wei, J. et al. Targeting REGNASE-1 programs long-lived effector T cells for cancer therapy. Nature 576, 471–476 (2019).
Huang, H. et al. In vivo CRISPR screening reveals nutrient signaling processes underpinning CD8+ T-cell fate decisions. Cell 184, 1245–1261 (2021).
LaFleur, M. W. et al. A CRISPR–Cas9 delivery system for in vivo screening of genes in the immune system. Nat. Commun. 10, 1668 (2019).
Zuris, J. A. et al. Cationic lipid-mediated delivery of proteins enables efficient protein-based genome editing in vitro and in vivo. Nat. Biotechnol. 33, 73–80 (2015).
Ramakrishna, S. et al. Gene disruption by cell-penetrating peptide-mediated delivery of Cas9 protein and guide RNA. Genome Res. 24, 1020–1027 (2014).
Staahl, B. T. et al. Efficient genome editing in the mouse brain by local delivery of engineered Cas9 ribonucleoprotein complexes. Nat. Biotechnol. 35, 431–434 (2017).
Erazo-Oliveras, A., Muthukrishnan, N., Baker, R., Wang, T. Y. & Pellois, J. P. Improving the endosomal escape of cell-penetrating peptides and their cargos: strategies and challenges. Pharmaceuticals (Basel) 5, 1177–1209 (2012).
Heitz, F., Morris, M. C. & Divita, G. Twenty years of cell-penetrating peptides: from molecular mechanisms to therapeutics. Br. J. Pharmacol. 157, 195–206 (2009).
Varkouhi, A. K., Scholte, M., Storm, G. & Haisma, H. J. Endosomal escape pathways for delivery of biologicals. J. Control. Release 151, 220–228 (2011).
Frankel, A. D. & Pabo, C. O. Cellular uptake of the tat protein from human immunodeficiency virus. Cell 55, 1189–1193 (1988).
Wadia, J. S., Stan, R. V. & Dowdy, S. F. Transducible TAT-HA fusogenic peptide enhances escape of TAT-fusion proteins after lipid raft macropinocytosis. Nat. Med. 10, 310–315 (2004).
Wherry, E. J. T cell exhaustion. Nat. Immunol. 12, 492–499 (2011).
Wherry, E. J. & Kurachi, M. Molecular and cellular insights into T cell exhaustion. Nat. Rev. Immunol. 15, 486–499 (2015).
Kurachi, M. et al. Optimized retroviral transduction of mouse T cells for in vivo assessment of gene function. Nat. Protoc. 12, 1980–1998 (2017).
Barber, D. L. et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 439, 682–687 (2006).
Sharma, P. & Allison, J. P. The future of immune checkpoint therapy. Science 348, 56–61 (2015).
Odorizzi, P. M., Pauken, K. E., Paley, M. A., Sharpe, A. & Wherry, E. J. Genetic absence of PD-1 promotes accumulation of terminally differentiated exhausted CD8+ T cells. J. Exp. Med. 212, 1125–1137 (2015).
Gier, R. A. et al. High-performance CRISPR–Cas12a genome editing for combinatorial genetic screening. Nat. Commun. 11, 3455 (2020).
Kleinstiver, B. P. et al. Engineered CRISPR–Cas12a variants with increased activities and improved targeting ranges for gene, epigenetic and base editing. Nat. Biotechnol. 37, 276–282 (2019).
DeWeirdt, P. C. et al. Optimization of AsCas12a for combinatorial genetic screens in human cells. Nat. Biotechnol. 39, 94–104 (2021).
Zetsche, B. et al. Cpf1 is a single RNA-guided endonuclease of a class 2 CRISPR–Cas system. Cell 163, 759–771 (2015).
Ren, J. et al. Multiplex genome editing to generate universal CAR T cells resistant to PD1 inhibition. Clin. Cancer Res. 23, 2255–2266 (2017).
Eyquem, J. et al. Targeting a CAR to the TRAC locus with CRISPR/Cas9 enhances tumour rejection. Nature 543, 113–117 (2017).
Weissman, I. L. & Shizuru, J. A. The origins of the identification and isolation of hematopoietic stem cells, and their capability to induce donor-specific transplantation tolerance and treat autoimmune diseases. Blood 112, 3543–3553 (2008).
Grevet, J. D. et al. Domain-focused CRISPR screen identifies HRI as a fetal hemoglobin regulator in human erythroid cells. Science 361, 285–290 (2018).
Frangoul, H. et al. CRISPR-Cas9 gene editing for sickle cell disease and beta-thalassemia. N. Engl. J. Med. 384, 252–260 (2021).
Bauer, D. E. et al. An erythroid enhancer of BCL11A subject to genetic variation determines fetal hemoglobin level. Science 342, 253–257 (2013).
Qin, K. et al. Dual function NFI factors control fetal hemoglobin silencing in adult erythroid cells. Nat. Genet. 54, 874–884 (2022).
Shmakov, S. et al. Diversity and evolution of class 2 CRISPR-Cas systems. Nat. Rev. Microbiol. 15, 169–182 (2017).
Rees, H. A. & Liu, D. R. Base editing: precision chemistry on the genome and transcriptome of living cells. Nat. Rev. Genet. 19, 770–788 (2018).
Anzalone, A. V. et al. Search-and-replace genome editing without double-strand breaks or donor DNA. Nature 576, 149–157 (2019).
Strecker, J. et al. RNA-guided DNA insertion with CRISPR-associated transposases. Science 365, 48–53 (2019).
Yarnall, M. T. N. et al. Drag-and-drop genome insertion of large sequences without double-strand DNA cleavage using CRISPR-directed integrases. Nat. Biotechnol. (in press).
Tou, C. J., Orr, B. & Kleinstiver, B. P. Precise cut-and-paste DNA insertion using engineered type V-K CRISPR-associated transposases. Nat. Biotechnol. (in press).
Durrant, M. G. et al. Systematic discovery of recombinases for efficient integration of large DNA sequences into the human genome. Nat. Biotechnol. (in press).
Pauken, K. E. et al. Epigenetic stability of exhausted T cells limits durability of reinvigoration by PD-1 blockade. Science 354, 1160–1165 (2016).
Gootenberg, J. S. et al. Nucleic acid detection with CRISPR-Cas13a/C2c2. Science 356, 438–442 (2017).
Cao, Z. et al. ZMYND8-regulated IRF8 transcription axis is an acute myeloid leukemia dependency. Mol Cell 81, 3604–3622 (2021).
Milone, M. C. et al. Chimeric receptors containing CD137 signal transduction domains mediate enhanced survival of T cells and increased antileukemic efficacy in vivo. Mol. Ther. 17, 1453–1464 (2009).
Dobin, A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 (2013).
Ritchie, M. E. et al. Limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 43, e47 (2015).
Clement, K. et al. CRISPResso2 provides accurate and rapid genome editing sequence analysis. Nat. Biotechnol. 37, 224–226 (2019).
Brinkman, E. K., Chen, T., Amendola, M. & van Steensel, B. Easy quantitative assessment of genome editing by sequence trace decomposition. Nucleic Acids Res. 42, e168 (2014).
Wu, Y. et al. Highly efficient therapeutic gene editing of human hematopoietic stem cells. Nat. Med. 25, 776–783 (2019).
Zhang, Z. et al. Efficient engineering of human and mouse primary cells using peptide-assisted genome editing. NCBI. https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE223805 (2023).
Acknowledgements
We thank M. Szurgot and R. Marmorstein (Department of Biochemistry and Biophysics, University of Pennsylvania) for sharing the protease ULP1 expression vector and purification protocol. We also thank the staff at the Flow Cytometry Core Laboratory of Children’s Hospital of Philadelphia. G.A.B. acknowledges NIH/NHLBI (R01-HL119479). R.M.K. acknowledges NIH (R01-GM138908). E.J.W. acknowledges support from the NIH (AI105343, AI082630, AI108545, AI155577, AI149680 and U19AI082630), funding from the Allen Institute for Immunology and the Parker Institute for Cancer Immunotherapy. Work in the Wherry lab is supported by the Parker Institute for Cancer Immunotherapy. S.L.B. acknowledges NIH/NCI (R35-CA263922). J.S. acknowledges NIH/NCI (R01-CA258904).
Author information
Authors and Affiliations
Contributions
Z.Z., E.J.W., S.L.B. and J.S. conceived and developed the Peptide-Assisted Genome Editing (PAGE) approach and designed the research. Z.Z., A.E.B., D.R., K.Q., Z.C., S.M., H.H., C.A.K., P.F.B. and J.B.P. performed experiments and analyzed the data. G.A.B., R.M.K., E.J.W., S.L.B. and J.S. supervised the research. Z.Z. and J.S. drafted the manuscript. Z.Z., A.E.B., G.A.B., R.M.K., E.J.W., S.L.B. and J.S. reviewed and edited the manuscript with input from all authors. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
Z.Z., A.E.B., Z.C., J.B.P., R.M.K., E.J.W., S.L.B. and J.S. through the University of Pennsylvania have filed a patent application on aspects of this work. E.J.W. is a member of the Parker Institute for Cancer Immunotherapy which supported this study. E.J.W. is an advisor for Danger Bio, Janssen, New Limit, Marengo, Pluto Immunotherapeutics Related Sciences, Santa Ana Bio, Synthekine and Surface Oncology. E.J.W. is a founder of and holds stock in Surface Oncology, Danger Bio and Arsenal Biosciences. R.M.K. is on the Scientific Advisory Board for Life Edit, Inc.
Peer review
Peer review information
Nature Biotechnology thanks Meisam Kararoudi and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–22.
Supplementary Tables
Table 1–Sequences of guide RNA used in this study; Table 2–Sequences of primers used in this study.
Source data
Source Data Fig. 1
Unprocessed Western Blots
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, Z., Baxter, A.E., Ren, D. et al. Efficient engineering of human and mouse primary cells using peptide-assisted genome editing. Nat Biotechnol 42, 305–315 (2024). https://doi.org/10.1038/s41587-023-01756-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41587-023-01756-1
This article is cited by
-
Engineering self-deliverable ribonucleoproteins for genome editing in the brain
Nature Communications (2024)
-
Delivering genome editing tools to primary cells
Nature Reviews Drug Discovery (2023)
-
A knockout run for CRISPRed cells
Nature Biomedical Engineering (2023)